Research Article, Clin Dermatol Res J Vol: 8 Issue: 1
Adverse Drug Reactions Awareness among Sample of People in Northern Region, Saudi Arabia
Fawwaz Freih Alshammarie1*, Yasmeen Ali Muraizeq2, Rahaf Turki Aldhaban2, Asma Mohammed Almutairi2 and Monerah Thaar Alshammari2
1Department of Dermatology, College of Medicine, University of Hail, Hail, Saudi Arabia
2Department of Medical Sciences, University of Hail, Hail, Saudi Arabia
*Corresponding Author: Fawwaz Freih Alshammarie
Department of
Dermatology, College of Medicine, University of Hail, Hail, Saudi Arabia
Tel: +966569595500
E-mail: fawwzf@yahoo.com
Received date: 14 November, 2022, Manuscript No. CDRJ-22-79639;
Editor assigned date: 17 November, 2022, PreQC No. CDRJ-22-79639 (PQ);
Reviewed date: 01 December, 2022, QC No. CDRJ-22-79639;
Revised date: 13 February, 2023, Manuscript No. CDRJ-22-79639 (R);
Published date: 20 February, 2023, DOI: 10.4172/2576-1439.1000194
Citation: Alshammarie FF, Muraizeq YA, Aldhaban RT, Almutairi AS, Alshammari MT (2023) Adverse Drug Reactions Awareness among Sample of People in Northern Region, Saudi Arabia. Clin Dermatol Res J 8:1.
Abstract
Background: Drug reactions are a fundamental issue in many branches of medicine. Adverse drug reactions result in high mortality and morbidity globally. There is always the risk of unwanted side effects associated with the use of any substance that has a therapeutic effect. The safe use of drugs remains a critical issue for all health care professionals.
Objective: The current study investigates the level of drug reaction awareness among the public in Northern Saudi Arabia. Materials and methods: This was a questionnaire based cross-sectional study. Data were collected using Google forms, which were coded and processed using Microsoft excel and SPSS version 23.
Results: Altogether 475 people participated in this study including 382 women (80.4%) and 93 men (19.6%). Of the 475 participants, 83.6% were aware of drug reactions, 40.4% believed drug reactions are hereditary, 50.9% had received a previous skin allergy test, and 21.3% had received a previous blood test for an allergy.
Conclusion: The public in Northern Saudi Arabia are aware of drug reactions. We found that only half of the participants in our study had received a skin allergy test. It is the responsibility of physicians to provide the community with a valuable database of information on the adverse effects of drugs. We recommend that governmental and private institutions in Saudi Arabia carry out more studies with a larger sample to improve the understanding and awareness of drug reactions.
Keywords: Adverse drug reaction; Knowledge; Awareness; North region; Saudi Arabia
Introduction
The safe use of drugs remains a critical issue for all health care professionals include physicians, pharmacists and nurses as well as public.
• Drug reaction is defined by World Health Organization (WHO) as a
response to a drug which is harms and unintended, occurs at normal
doses used in human for the prophylaxis, diagnosis or therapy of
disease [1].
• Multi-type reactions can result, including Stevens-Johnson
syndrome, toxic epidermal necrolysis, and eosinophilia associated
with drug reactions, or acute generalized exanthemata's pustulosis.
• Some other prevalent adverse reactions include epidermal eruptions,
vomiting, diarrhoea, and gastric ulcerations. Death and perpetual
disabilities can be caused by adverse drug reactions. It is important
to recognize such reactions because they warn of future risks from a
particular drug(s) and may require precautionary measures,
treatment, dosage changes, or cancellation of drug license.
• The current study investigates the level of drug reaction awareness
among the public in Northern Saudi Arabia [2].
Materials and Methods
Study design and sample
It was a questionnaire based cross-sectional study processed using Microsoft excel and the software Statistical Package for the Social Science (SPSS) version 23 [3]. This research study focused on public who lives in North region, Saudi Arabia.
Data collection
The data were collected using google forms service, it took 10 months, at beginning of January 2020 to October 2020.
Data analysis
The data were collected using Google forms service, coded and processed using Microsoft excel and the software Statistical Package for the Social Science (SPSS) version 23. Descriptive statistics including frequencies and percentages were used to describe the items and the study variables [4]. Chi-square tests were conducted to test the differences of the nominal data. The p values at 0.05 were considered statistically significant [5].
Results
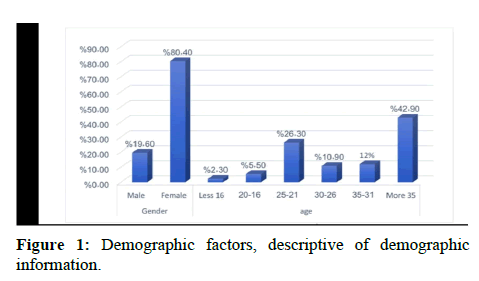
As shown in Figure 1 demographic factors, 475 people participated in this study including 382 female (80.4%) and 93 males (19.6%) (p<0.05). Their age ware ranged between less than 16 and more than 35 with advantage for age group more than 35 (42.90%) [6].
As shown in Table 1 drug reactions only 43 (9.1%) had drug allergy (p<0.01) [7-9]. The participants were asked about the medication kinds that they have been used with the reaction which show the highest for antibiotics (53.5%), other show less like: NSAI drugs (2.3%), antiepilepsy (0%), analgesics (18.6%), vitamins (7%), insulin (2.3%), aspirin (11.6%), herbal (16.3%) and other (14%).
| Statement | Frequency | Percentage | Chi-square/p value | ||
|---|---|---|---|---|---|
| Drug allergy exists | No | 432 | 90.90% | 318.57**/0.000 | |
| Yes | 43 | 9.10% | |||
| What kind of medication do you take? | Antibiotics | No | 20 | 46.50% | 0.209 (ns)/0.65 |
| Yes | 23 | 53.50% | |||
| NSAI | No | 42 | 97.70% | 39.093**/0.000 | |
| Yes | 1 | 2.30% | |||
| Antiepilepsy | No | 43 | 100% | Na | |
| Yes | 0 | 0 | |||
| Analgesia | No | 35 | 81.40% | 16.953**/0.000 | |
| Yes | 8 | 18.60% | |||
| Vitamins | No | 40 | 93% | 31.837**/0.000 | |
| Yes | 3 | 7% | |||
| Insulin | No | 42 | 97.70% | 39.093**/0.000 | |
| Yes | 1 | 2.30% | |||
| Aspirin | No | 38 | 88.40% | 25.326**/0.000 | |
| Yes | 5 | 11.60% | |||
| Herbal | No | 36 | 83.70% | 19.558**/0.000 | |
| Yes | 7 | 16.30% | |||
| Other | No | 37 | 86% | 22.349**/0.000 | |
| Yes | 6 | 14% | |||
| What symptoms do you have? | Rash | No | 22 | 51.20% | 0.023 (ns)/0.88 |
| Yes | 21 | 48.80% | |||
| Skin heat | No | 32 | 74.40% | 10.256**/0.001 | |
| Yes | 11 | 25.60% | |||
| Itching | No | 21 | 48.80% | 0.023/0.88 | |
| Yes | 22 | 51.20% | |||
| Fever | No | 42 | 97.70% | 39.093**/0.000 | |
| Yes | 1 | 2.30% | |||
| Cough | No | 31 | 72.10% | 8.395**/0.004 | |
| Yes | 12 | 27.90% | |||
| Shortness of breath | No | 41 | 95.30% | 35.372**/0.000 | |
| Yes | 2 | 4.70% | |||
| Anaphylaxis | No | 32 | 74.40% | 10.256**/0.001 | |
| Yes | 11 | 25.60% | |||
| Swelling (mouth, lips, tongue, eyes) | No | 39 | 90.70% | 28.488**/0.000 | |
| Yes | 4 | 9.30% | |||
| Tears | No | 39 | 90.70% | 28.488**/0.000 | |
| Yes | 4 | 9.30% | |||
| Runny nose | No | 30 | 69.80% | 6.721*/0.01 | |
| Yes | 13 | 30.20% | |||
| Lightheadedness | No | 41 | 95.30% | 35.372**/0.000 | |
| Yes | 2 | 4.70% | |||
| Other | No | 36 | 83.70% | 19.558**/0.000 | |
| Yes | 7 | 16.30% | |||
| When do symptoms start? | Immediately after taking the drug | No | 26 | 60.50% | 1.884 (ns)/0.17 |
| Yes | 17 | 39.50% | |||
| Hours | No | 28 | 65.10% | 3.93*/0.07 | |
| Yes | 15 | 34.90% | |||
| Days | No | 33 | 76.70% | 12.302**/0.000 | |
| Yes | 10 | 23.30% | |||
| Months | No | 42 | 97.70% | 39.093**/0.000 | |
| Yes | 1 | 2.30% | |||
| Medical intervention | No | 12 | 27.90% | 8.395(ns)/0.004 | |
| Yes | 31 | 72.10% | |||
| Number of health interventions | Once | No | 27 | 62.80% | 2.814 (ns)/0.09 |
| Yes | 16 | 37.20% | |||
| Twice | No | 36 | 83.70% | 19.558**/0.000 | |
| Yes | 7 | 16.30% | |||
| Three times | No | 42 | 97.70% | 39.093**/0.000 | |
| Yes | 1 | 2.30% | |||
| More than three times | No | 36 | 83.70% | 19.558**/0.000 | |
| Yes | 7 | 16.30% | |||
| **p<0.01; *p<0.05; ns: not significant; na: not available | |||||
Table 1: Descriptive analysis of drug reactions (n=475).
Antibiotics reported insignificant (p>0.05) and antiepilepsy not used [10]. The rest kinds only ranged between 8 (18.6%) to one (2.3%) of using (p<0.01). Also, the symptoms that might participants have been stated such as rash, skin heat, itching, fever, cough, shortness of breath, anaphylaxis, swelling (mouth-lips-tongue-eyes), eye tears, runny nose, light headiness and others, it was clear that they did have that much symptoms as it ranged between only 13 (30.2%) for itchiness to 22 (51.2%) for fever (p<0.01), rash and itching were insignificant (p>0.05) [11].
Starting immediately after taking drug was insignificant (p>0.01), 15 (34.9%) indicated that starts hours, to 1 (2.3%) starts in months (p<0.01), although the symptoms were not much among the participants, however, it is clear that starts short time more than long time [12].
The participants reported their time of a health intervention, ranged between once 16 (37.2%) which is the highest to 3 times 1 (2.3%) (p<0.05).
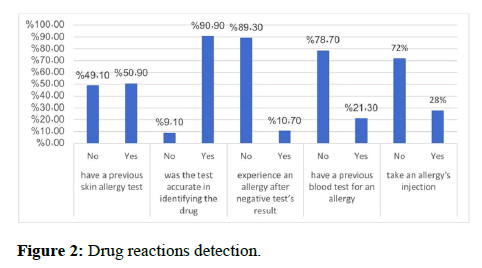
As shown in Table 2 and Figure 2 knowledge, 83.6% of participants were aware, 56.2% agreed that all the body is affected in drug reaction (p<0.05), then face (34.3%) follows by lips (14.1%). Tongue is lowest one with 6.7% (p<0.05) [13]. It is clear that the family history of drug reaction is very small ranged between 8.8% for children and 1.9% for father (p<0.05). 59.6% of participants did not think that drug reaction is hereditary (p<0.05), however 48.1% thought that drug reaction leads to death (p>0.05). As shown in Figure 2 drug reactions detection, 50.9% had previous skin general allergy test (p>0.05), and 90.9% reported that they test accurate in identifying the drug (p>0.01). Only 10.7% had experience an allergy after negative test’s result (p<0.05). 21.3% had a previous blood test for an allergy (p<0.01) and just 28% took an allergy's injection vaccine (p<0.01) [14].
| Statement | Frequency | Percentage | Chi-square/p value | ||
|---|---|---|---|---|---|
| Awareness | No | 78 | 16.40% | 214.234**/0.000 | |
| Yes | 397 | 83.60% | |||
| Which part of the body is affected? | Face | No | 312 | 65.70% | 46.739**/0.000 |
| Yes | 163 | 34.30% | |||
| Lips | No | 414 | 87.20% | 262.335**/0.000 | |
| Yes | 61 | 12.80% | |||
| Eyes | No | 408 | 85.90% | 244.802**/0.000 | |
| Yes | 67 | 14.10% | |||
| Tongue | No | 443 | 93.30% | 355.623**/0.000 | |
| Yes | 32 | 6.70% | |||
| Hand | No | 418 | 88% | 274.360**/0.000 | |
| Yes | 57 | 12% | |||
| All the body | No | 208 | 43.80% | 7.328**/0.007 | |
| Yes | 267 | 56.20% | |||
| Other | No | 456 | 96% | 402.040**/0.000 | |
| Yes | 19 | 4% | |||
| Don’t know | No | 428 | 90.10% | 305.602**/0.000 | |
| Yes | 47 | 9.90% | |||
| Family history of drug reactions | Father | No | 466 | 98.10% | 439.682**/0.000 |
| Yes | 9 | 1.90% | |||
| Mother | No | 450 | 94.70% | 380.263**/0.000 | |
| Yes | 25 | 5.30% | |||
| Sibling | No | 443 | 93.30% | 355.623**/0.000 | |
| Yes | 32 | 6.70% | |||
| Children | No | 433 | 91.20% | 321.855**/0.000 | |
| Yes | 42 | 8.80% | |||
| None | No | 108 | 22.70% | 141.223**/0.000 | |
| Yes | 367 | 77.30% | |||
| Do you think drug reactions are hereditary? | No | 283 | 59.60% | 17.434**/0.000 | |
| Yes | 192 | 40.40% | |||
| Do you think drug reactions lead to death? | No | 246 | 51.90% | 0.684 (ns)/0.408 | |
| Yes | 228 | 48.10% | |||
| **p<0.01; *p<0.05; ns: not significant | |||||
Table 2: Descriptive analysis (n=475).
Discussion
Out of 475 surveys distributed to public, the majorities of the participants were female (80.4%) while male (19.6%). This was consistent with reports in the literature, as most ADRs were reported by female patients [15].
The highest percent of age among participants having (42.9%) more than 35 years, followed by (26.3%) in age between 21 to 25. In our study when we asked public about medications use related to drug reaction, (53.5%) P>0.05 mentioned the antibiotics cause drug reaction when used. Additionally, at a South Korean tertiary care hospital when ask said antibiotics have been reported to be major causes of adverse drug reactions. Sulfonamides followed by penicillin were the most common causative antibiotics in a study that only included outpatients [16]. Nearly 39.5% of the respondents developed adverse reaction immediately after taking the drug, whereas the reminder did within few hours, days or within months as in 34.9%, 23.3% and 2.3% respectively. The majority in this study had reported adverse drug reaction in form of itching and skin rashes in 51.2%, and 48.8% respectively. Furthermore, some of them developed runny nose, cough, and anaphylaxis in 30.2%, 27.9%, and 25.6% respectively. Conversely, another study had found gastrointestinal illnesses were the most common adverse drug reactions.
We found that 72.1% needed medical intervention it is just ranged between once (37.2%) to 3 times (2.3%). In our survey 83.6%, of participants think to have knowledge about adverse drug reactions. The whole body is the most sites (56.2%) affected by adverse reactions to drugs in the present study followed by the face 34.3%, eyes 14.1%, lips 12.8%, hand 12% and tongue 6.7%. Most participants have no family history of adverse drug reactions. On the other hand, findings showed the higher number of cases found in children 8.8%. The proportion of participants who believe in adverse drug reaction is hereditary was 40.4%, but more than half of them disagreed with that. An individual's gene profile can affect their susceptibility to adverse drug reactions in a dose dependent or dose-independent manner. The confusion was observed between the participants regarding if drug reaction leads to death 48.1% was agreed while 51.9% disagree about that. According to previous international studies have reported the adverse drug reactions are the sixth leading cause of death worldwide. Most severe adverse cutaneous reactions to drugs are Stevens-Johnson syndrome and toxic epidermal necrolysis. In our study found that 50.9% of people had a previous general skin allergy test, which 90.0% of them show that the test was accurate in determining the drug. The use of a skin allergy test in diagnosing a person how have a drug reaction is not suitable all time and there is no uniform for it. So mostly depend on the symptoms appeared on him. According to our survey show that 89.3% have no allergy after a negative result of skin allergy test, and only 10.7% have allergy even when the result is negative, which give us a clue of non-specification of skin allergy test sometimes. Another option for diagnosing a drug reaction is a blood test which found in our study that 78.7% have never had this test. Blood test measures IgE against specific allergen and immunoassay is the most commonly methods used. Adverse drug reaction also it can diagnose by different and several tests and have more specification. About 72% have not taken allergy injection (vaccine), which is used to adverse the effect of drug reaction. The main idea of allergy injection “allergy immunotherapy” is to reduce response to allergic triggers and inflammatory response which sometimes produce a chronic condition.
Conclusion
We can conclude the general public in north region are aware of drug reactions. Our findings that people who did a skin allergy test is half of the participants in our study. The results showed contrasting perceptions about if drug reaction leads to deaths in the current opinion of participants more than half denied that, whereas others believe the drug reaction can lead to deaths. As a final note, it is the responsibility of physicians to provide the public with valuable information about pharmaceutical adverse effects.
Limitations
This was a single region study involving a limited number of people. Therefore, the study results cannot be generalized directly to people living in the north region who did not participate in this study or those in other regions of Saudi Arabia.
Acknowledgement
We thank the patients who were all participated in and contributed samples to the study.
Conflicting Interest
We declare that there are no conflicts of interests.
Financial Support and Sponsorship
Nil.
Informed Consent
Written informed consent was obtained from all individual who participate in the study.
Ethical Approval
The study was approved by the medical ethics committee of Hail university (ethical approval number: H-2020-256).
References
- Kaur M, Kosey S, Mehra N, Kumar R (2015) Knowledge and awareness of adverse drug reactions. Int J Pharm Sci Res 6:4601-4610.
- Khan SA, Goyal C, Chandel N, Rafi M (2013) Knowledge, attitudes, and practice of doctors to adverse drug reaction reporting in a teaching hospital in India: An observational study. J Nat Sci Biol Med 4:191-196.
[Google Scholar] [PubMed]
- Teo YX, Walsh SA (2016) Severe adverse drug reactions. Clin Med (Lond) 16:79-83.
[Crossref] [Google Scholar] [PubMed]
- Thomas F, Abiri OT, Komeh JP, Conteh TA, Bah AJ, et al. (2022) Inconsistent country-wide reporting of adverse drug reactions to antimicrobials in Sierra Leone (2017–2021): A wake-up call to improve reporting. Int J Environ Res Public Health 19:3264.
[Crossref] [Google Scholar] [PubMed]
- Coleman JJ, Pontefract SK (2016) Adverse drug reactions. Clin Med (Lond) 16:481–485.
[Crossref] [Google Scholar] [PubMed]
- Gomes D, Herdeiro MT, Ribeiro-Vaz I, Ferreira PL, Roque F (2022) Adverse drug reactions and potentially inappropriate medication in older patients: Analysis of the Portuguese pharmacovigilance database. J Clin Med11:2229.
[Crossref] [Google Scholar] [PubMed]
- Dubrall D, Just KS, Schmid M, Stingl JC, Sachs B (2020) Adverse drug reactions in older adults: A retrospective comparative analysis of spontaneous reports to the German federal institute for drugs and medical devices. BMC Pharmacol Toxicol 21:25.
[Crossref] [Google Scholar] [PubMed]
- Sales I, Aljadhey H, Albogami Y, Mahmoud MA (2017) Public awareness and perception toward adverse drug reactions reporting in Riyadh, Saudi Arabia. Saudi Pharm J 25:868-872.
[Crossref] [Google Scholar] [PubMed]
- Jung IY, Kim JJ, Lee SJ, Kim J, Seong H, et al. (2017) Antibiotic-related adverse drug reactions at a tertiary care hospital in South Korea. Biomed Res Int 2017:4304973.
[Crossref] [Google Scholar] [PubMed]
- Almubark RA, Aljadani RH, Alqahtani AS, Alshammari TM, BinDhim NF (2020) National cross-sectional study of community based adverse drug reactions in Saudi Arabia. Drugs Real World Outcomes 7:161-170.
[Crossref] [Google Scholar] [PubMed]
- Pirmohamed M, Park BK (2001) Genetic susceptibility to adverse drug reactions. Trends Pharmacol Sci 22:298-305.
[Crossref] [Google Scholar] [PubMed]
- Wolf R, Orion E, Marcos B, Matz H (2005) Life-threatening acute adverse cutaneous drug reactions. Clin Dermatol 23:171-181.
[Crossref] [Google Scholar] [PubMed]
- Brockow K, Przybilla B, Aberer W, Bircher AJ, Brehler R, et al. (2015) Guideline for the diagnosis of drug hypersensitivity reactions. Allergo J Int 24:94-105.
[Crossref] [Google Scholar] [PubMed]
- Siles RI, Hsieh FH (2011) Allergy blood testing: A practical guide for clinicians. Cleve Clin J Med 78:585.
[Crossref] [Google Scholar] [PubMed]
- Rive CM, Bourke J, Phillips EJ (2013) Testing for drug hypersensitivity syndromes. Clin Biochem Rev 34:15-38.
[Google Scholar] [PubMed]
- Huggins JL, Looney RJ (2004) Allergen immunotherapy. Am Fam Physician 70:689-696.
[Google Scholar] [PubMed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi