Research Article, J Hydrogeol Hydrol Eng Vol: 7 Issue: 2
Assessment of Water Quality of Groundwater of Thar Desert, Sindh, Pakistan
Muhammad Yar Khuhawar1, Habib-ul-Rehman Ursani2, Taj Muhammad Jahangir Khuahwar1, Muhammad Farooque Lanjwani3*, Ali Asgar Mahaser4, Imran Aziz Tunio4, Abdul Ghaffar Soomro4, Imran Khan Rind1, Rafi-o-Zaman Brohi1, Aftab Hussain Khuhawar1, Shahzad Hussain Solangi5, Raheel Soomro5, Abdul Jabbar Kandhro1 and Agha Sarfaraz Pathan5
1Institute of Advanced Research Studies in Chemical Science, University of Sindh, Jamshoro, Sindh, Pakistan
2Sindh Barrages Improvement Project, Irrigation Department, Hyderabad, Sindh, Pakistan
3Dr. M. A. Kazi Institute of Chemistry, University of Sindh, Jamshoro, Sindh, Pakistan
4Sindh Barrages Improvement Project, irrigation Department Hyderabad, Sindh, Pakistan
5Agha Abdul Qayoom Associates, Sindh, Pakistan
*Corresponding Author : Muhammad Farooque Lanjwani
Dr M A Kazi Institute of Chemistry, University of Sindh Jamshoro, Sindh, Pakistan
Tel: +92 3473911562
E-mail: mfarooquechemist@yahoo.com
Received: October 22, 2018 Accepted: December 27, 2018 Published: January 05, 2019
Citation: Khuhawar MY, Ursani H, Khuahwar TMJ, Lanjwani MF, Mahessar AA, et al. (2019) Assessment of Water Quality of Groundwater of Thar Desert, Sindh, Pakistan. J Hydrogeol Hydrol Eng 7:2. doi: 10.4172/2325-9647.1000171
Abstract
The work examines water quality of Thar Desert, Sindh, Pakistan, by investigating 2193 dug wells. The samples were collected from August to December 2015. All the 2170 samples were divided in three categories on the basis of their (TDS). TDS above 3000 mg/L, TDS within 1500-3000 mg/L, TDS below 1500 mg/L. The 1247 (57.5%) were observed with TDS above 3000 mg/L, 320 (14.7%) were with TDS within 1500 – 3000 mg/L and 588 (27.1%) samples with TDS below 1500 mg/L. Among 588 samples suitable for drinking with TDS less than 1500 mg/L, chloride was above WHO permissible level in 91 (15.5%), alkalinity in 78 (13.3%) samples, sulphate in 85 (14.5%) samples, arsenic in 64 (10.9%) samples, and fluorides in 100 (17.0%) samples. The Tharparkar 27.1% samples which were considered safe and were within maximum permissible limits of WHO, indicated arsenic and fluoride contamination in the range of 11-17%.
Keywords: Thar desert; Drinkable water; Irrigation
Introduction
The clean and drinkable water is the gift of life. The drinkable water in Sindh province of Pakistan is based on the surface water, mostly based on the river Indus and groundwater. The surface water is subjected to microbiological contamination during its travel and should be treated according to the public health engineering rules before consumption as a source of drinking water. The groundwater has proved as a useful source of drinking water, particularly where there is no water supply schemes available or easy access to potable water [1]. It has also contributed significantly to growth in global irrigated areas [2]. Millions of farmers in Africa and Asia have significantly improved their livelihood and household food security. The groundwater boom has been driven by the supply-push factor, such as government subsidies and easy availability of an inexpensive pumps and drilling technologies.
District Tharparkar is located at south eastern part of Pakistan. The population depends for their livelihood mainly on limited agriculture, and rising animals, including goats, sheep, cattle and camels. The region contains a number of sand dunes with characteristic vegetation of desert. The areas between the sand dunes are suitable for irrigation, which is mainly dependent on the rainfall. The rainfall in the region is inconsistent mostly during monsoon season (June-September) each year. There are also cycles of drought in the region which effects the limited irrigation and socio – economic conditions of the inhabitants. The reoccurrence of the drought cycle results into the migration of a section of the population towards other parts of province of Sindh, to earn their livelihood. The population in the Thar is scattered in small villages, mostly around a source of water in the form of dug wells at the average depth of 10 to 100 meters. The water resources are located at the bottom of the sand dunes and may depend on annual rainfalls. The inhabitants of the District are generally poor with low education and poor health. However, the conditions are slightly improving because of the possible use of Thar coal for commercial purposes and for the development of the economy of Pakistan.
A number of studies have been carried out by different organisations to explore the potentials of groundwater resources, but the crisis of water at Thar still persists. A large area of the Thar has been examined geologically, through test-drilling for the geophysical monitoring of the coal under the program [3-7]. The north eastern parts of the Thar Desert has also been hydrologically investigated in a joint WAPDA-BGR venture [8,9] examined the status of drinking water quality in the western parts of the Thar Desert, Sindh, Pakistan. They observed that primary source of drinking water was dug well. Water available from these dug wells was suspected to be unfit for drinking. Water samples from the western parts of Thar Desert were analysed for anions, cations and trace metals. The data indicated elevated levels with possible hazardous effects on human health for the use as drinking water. They have recommended the supply of clean water to the inhabitants [10] reported an applied palae hydrological study in Cholistan Thar Desert, Pakistan. The study was carried out during 1986 to 1991 to explore fresh groundwater resources in the Thar Desert of Pakistan. Low rainfall, high rates of evapotranspiration, low groundwater recharge and absence of perennial streams were reported as general reasons for the scarcity of water in Cholistan, Tharparkar and upper Narra areas of their study. In the Thar Desert brackish to saline groundwater dominates. Hydro geological, geophysics, and isotope hydrological methods were used to locate fresh groundwater resources and to assess their origin and recharge [11] examined fluoride ion contamination in the groundwater of Mithi sub-district, the Thar Desert Pakistan. They collected groundwater samples from different locations of Mithi and analysed for fluoride ion together with other chemical parameters. The results indicated that collected water samples were contaminated with fluoride ion at concentration above the permissible limits of WHO for drinking water. The fluoride ion concentration ranged between 0.09 to 11.63 mg/L with mean value of 3.64 mg/L. Higher concentration of fluoride was reported in the vicinity of Islamkot and Mithi towns. Fluoride ion concentration was positively correlated with the concentrations of sodium, carbonate and pH. The Piper diagram indicated that all samples were sodium type and among anions, chloride ion dominated with a minor influence of bicarbonate and sulphate. Husain et al., [12] described arsenic and fluoride mobilisation mechanism in groundwater of Indus Delta and Thar Desert, Sindh, Pakistan. They reported salinity, microbial pollution and fluoride toxicity at groundwater of Thar Desert. Fluoride was found in the range of 0.96-2.74 mg/L in all the samples from Tharparkar district. The pH of groundwater was reported alkaline in the range of 7.38-8.59 which was accelerating the maximum dissolution of fluoride in the groundwater. Brahman et al., [13] evaluated high levels of fluoride, arsenic species and other physicochemical parameters in groundwater of Diplo and Chachro sub-districts of Tharparkar, Sindh, Pakistan. The concentrations of total arsenic, inorganic arsenic species, fluoride and other physicochemical parameters were reported in terms of basic statistical parameters, principal components analysis, cluster analysis, sodium adsorption ratio and saturation indices. The positive correlation of fluoride and arsenic species with sodium and bicarbonate indicated that the water with high salinity and alkalinity stabilised the arsenic species and fluoride in the groundwater. The positive correlation (r=0.640, p=0.671) was observed between total arsenic and its species with fluoride. They suggested that the groundwater of their study area were severely contaminated with arsenic and fluoride ions, which were higher than World Health Organization (WHO) provisional guideline values (0.01 mg/L and 1.5 mg/L). Rafique et al., [14] examined geochemical factors controlling the occurrence of high fluoride groundwater in the Nagar Parkar area, Sindh, Pakistan. The concentration of fluoride in groundwater near Nagar Parkar in the Thar Desert ranged from 1.13 to 7.85 mg/L, and roughly 78% of the samples contained fluoride concentration higher than the drinking water standard of 1.5 mg/L set by WHO. The groundwater was alkaline (pH 7.1 – 8.4), brackish (TDS 449 – 15933 mg/L), and classified as Na-Cl type water. Sample depth and water temperatures did not have a significant role in the distribution of fluoride in the groundwater.
A number of studies have been carried out to examine the quality of groundwater and their possible effects on the human health and irrigation from different countries [15-18] and from Pakistan [19-21].
The present work examines systematically the water quality of Thar Desert by collecting 2170 sample from dug wells, commonly used as a source of drinking water and compares the results with permissible limits of WHO. The work also examines the water samples with some better water quality for the possible use for irrigation.
Geography of Thar
The Thar Desert of Pakistan is part of a much larger desert extending to the north and east border of India. Thar Desert of Pakistan is bounded to the north, east and south by the Indian border and on the west to the irrigated Indus River flood plain. Most of the sand dunes of the Thar are stabilised by the vegetation and grasses. There is no drainage system developed and when the Manson rains fall, the water is immediately absorbed in the sand or rarely, during heavy downpours, flows very short distance down into the low lying areas where it is quickly absorbed [3]. Annual rainfall ranges from 200-300 mm [8]. A few roads have been constructed recently, but still four wheel vehicles are required to travel to different villages.
The Thar Desert is covered by sand dunes to an average depth of about 80 m. The only outcropping bed rock in the Thar Desert is found at Nager Parker, where tricking red granite basement rocks are visible above the surface of dunes. The basement rocks are not all granite, there are minor amounts of rhyolite and metamorphic rocks mixed in the granite area [3-5] based on seismic data suggested that the Thar Desert rests upon a structural platform where granitic basement is at shallower depths.
The granite basement has pre-Jurassic rifting, which caused flexure and the ultimate development of the basin. The basement shows rise towards southeast and deepening towards northwest, as a result of Paleozoic-Mesozoic divergent tectonics. Rehman et al., [22] based on the results of the geo-electric, drilling and geophysical/ geological log data indicated following four major divisions of litho logical sequences almost throughout the Thar Desert: (1) Dune Zone (Recent), (2) Oxidized Zone (Subrecent), (3) Coal-Bearing Formation (Mesozoic/tertiary), and (4) Basement Complex (Precambrian Basement).
Dune zone: The zone is based on eolian sand. The soil consists of about 8% clay and silt, near the surface, and 15% clay and slit in the subsoil [23]. The thickness of sand dunes is thinner in the northern part of the desert (about 5 to 15 m thick in Gadro – Khokhrapar area), but increases to about 40 to 93 m in the central and southern Thar in Chachro-Islamkot-Mithi area.
Oxidized zone (Sub-recent): It is based on compact and loose clays, silts and sands with ironstone. The thickness of this zone ranges from 11 to 209 m [3]. This sub-recent zone lies over the coal-bearing formation.
Coal-bearing formations: This zone is based on clay stones, slit stones, sandstones and lignite. There are some intercalations of siderite bands, nodules and granite –wash at places [24]. The thickness of this zone ranges from zero to 185 m. The cumulative lignite beds thicknesses are reported ranging from 0.5 to 34 m.
Basement complex: Granitic basement is encountered at the depth ranging from 112 to 279 m holes drilled in the east and southeast of Chachro [3]. The geoelectrical resistivity survey indicated that this basement complex had high resistivity of 50 to 150 ohmm (m), and was interpreted to be a deep fissured sandstone aquifer, bearing fresh water by [25] under the WAPWA – BGR Groundwater Exploration Project based on the results of the deep vertical electric sounding indicated two trends of apparent resistivity values in the area south of Chachro. One trend indicated massive granite basement and the other trend revealed the presence of layered Archered meta sediments.
Hydrology
The source of water generally within the area is at the contact of sub-recent deposits and overlying sand dunes. The water table varies between 52 and 93 m depth from the surface. Water column is within the range 0.61 and 7.62 m [26]. The drill hole geology shows the presence of three possible compact sand aquifer zones at varying depths. These are (1) above the coal zone, (2) within the coal zone, and (3) below the coal zone [27]. The available water above the coal zone is situated between the depth of 41.38 m above sea level and 40 m below mean sea level. The water bearing horizons are of medium to coarse sand with different thicknesses at the contact of the sub-recent deposits with the overlying sand dunes. Most of the dug wells are within this region of aquifer zone [11]. The sub-recent sediments hold saline aquifers with very limited yield. This unit has been considered non-water bearing zone. Aquifer within the coal zone has two to three perched aquifers consisting of compact sand horizons which vary in thickness [28]. All aquifers in the coal-bearing formation are under pressure. Aquifers below the coal zone are present between 94 and 174 m below the sea level and are present in all the drill holes in the area. It is based on coarse, gritty quartzitic sand/sandstone. This aquifer is reported as a source of water for most of the tube wells installed in the Thar region [11].
Materials and Methods
Description of the study area
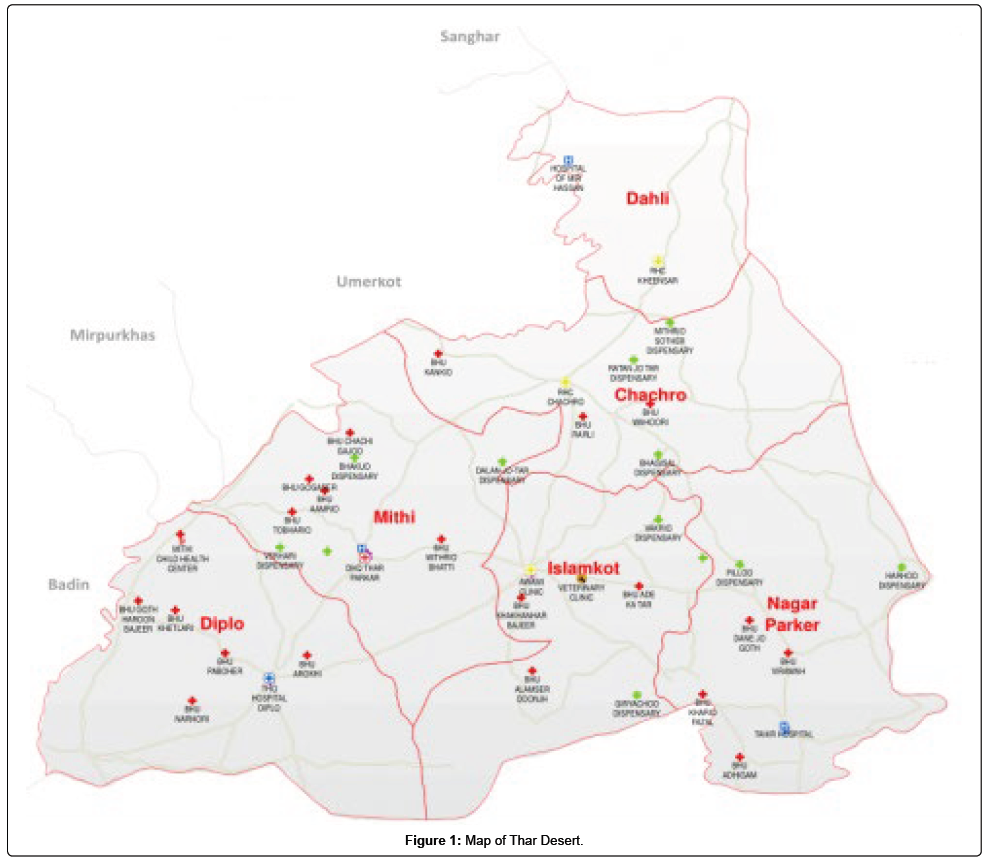
The study area was District Tharparkar, comprising mainly Chachro, Mithi and Diplo Sub-districts and some parts of Nangarparkar sub-district (Figure 1). Total area of the district is 19638 Sq. Kms., with total population of 1649661 persons, based on 2017 consensus. The expected average annual growth rate is about 3.1% (WWW. Pbs. gov.pk/content/districts-glance-tharparkar). The district population is based on urban 8.0% and rural 92.0%. The rural population comprises of scattered villages mostly around the source of water in the form of dug wells. The literacy is very low within the district 18.3%, particularly for females 6.91% (WWW. Pbs.gov.pk/ content/districts-glance-tharparkar). The study area has an arid and tropical desert climate, the temperature ranged from 9 C to 48 C. There are different sources of drinking water in Tharparkar, among those 93.9% from wells, 2.26% tube wells, 1.66% hand pumps, 0.69% pond water and 1.46% other sources of water [13]. The Tharparkar district is very rich in mineral resources like China clay, granite, coal and salts.
Chemicals and glassware
Double distilled water from all glass was used for preparing all the reagents, standards and finally washing all glass ware for measurements. All the chemicals and reagents EDTA, hydrochloric acid, nitric acid, sulphuric acid, silver nitrate, sodium carbonate, potassium hydroxide, sodium hydroxide, sodium bicarbonate, barium chloride, sodium nitrate, disodium hydrogen phosphate of reagent grade was from Merck (Darmstadt, Germany) or Fluka (Buchs, Switzerland) (Figure 2). The stock standard solution of arsenic (III) at the concentration of 1 mg/mL was prepared by dissolving of arsenic (III) oxide Merck (Darmstadt, Germany) in 1 M NaOH and adjusting the pH to 7 with HCl. The stock solutions of nitrate-nitrogen and phosphate-phosphorus (100 mL) containing 1 mg/mL each were prepared by dissolving appropriate amount of sodium nitrate and disodium hydrogen phosphate in distilled water.
Working standard solutions were prepared by stepwise diluting the stock solutions before use. All the glassware’s were kept overnight in 5 M nitric acid, and washed with distilled water.
Instruments
The pH measurement was made with calibrated Orion 420 A pH meter (Orion Inc. Boston, USA) with glass electrode and reference internal electrode. Electrical conductivity, salinity and total dissolved solids (TDS) were recorded with calibrated Orion 5 Star conductivity meter (Orion Inc. Boston, USA). Spectrophotometric studies were carried out on Perkin Elmer Lamda 35 UV/VIS (Perkin Elmer, Singapore) double beam spectrophotometer with dual 1- cm silica cuvettes, or double beam Hitachi 220 (Hitachi, Pvt, Tokyo, Japan) spectrophotometer with dual 1 cm silica cuvettes. The essential metal ions sodium, potassium, calcium and magnesium in 49 samples with TDS less than 1500 mg/L, selected randomly from 588 samples were analysed by – acetylene flame atomic absorption spectrophotometer (Perkin Elmer AA800, Singapore, spectrophotometer) with standard burner head. The analysis was carried out in triplicate with integration time 3 sec and delay time 3 sec. The analysis was carried out as recommended by the manufacturer. Some of the samples for arsenic were also analysed by atomic absorption spectrophotometer Perkin Elmer AA 800 (Singapore) connected with FIAS 100 flow injection system with quartz tube placed on the acetylene flame (Perkin Elmer, Singapore). The arsenic hydride was generated by 10% hydrochloric acid (reservoir) and 0.2% sodium borohydride. The nitrogen was used as carrier gas. The fluoride ions were determined by Hana multimeter connected with fluoride ion selective electrode. The equipment was calibrated and used for sample analysis as recommended by the manufacturer.
Sampling and pre-treatment
Groundwater water samples 2170 were collected from different villages, mainly from three sub-divisions Chachro, Mithi, and Diplo of district Tharparkar. Each of the well was assigned identification number (ID), the village where well was located, and the parameters of latitude and longitude on Global positioning system (GPS) were recorded. The total depth of the well and the depth of water available at the time of sampling were also recorded. The date and time of collection of sample were noted (Appendix 1). The groundwater samples were collected from wells directly above the outlet after withdrawing the sample several times from the water table with a stainless steel container tightened with fibre rope, calibrated in feet. The collected samples were kept cool, and delivered to laboratory on the same day. The water sub-samples from each site were mixed in a clean and washed plastic bracket to make composite sample of each well. The composite sample was filled in two 1.5 L plastic bottles, rinsed several times with sample, before filling the bottles with sample. One of the bottles was acidified with 1.5 mL of concentrated nitric acid to decrease the pH below to arrest the metal ions from precipitation or absorption on the surface of container and another bottle was kept for the analysis of anions and other physicochemical parameters determinations. All the samples were kept cool until analysis [29-31].
Physicochemical procedures
The physicochemical parameters were determined in the laboratory following standard procedures. The analytical procedures were appropriately standardised including blank determination before the sample analysis. Total hardness, alkalinity, chloride, sulphate, phosphate-phosphorus and nitrate-nitrogen were estimated as reported [32]. Electrical conductivity (EC), salinity, total dissolved salts (TDS), and pH were determined by Orion 5 Star conductivity meter (Orion Inc. Boston, MA, USA). The electrodes were calibrated before measurements as suggested by the manufacturer. Sulphate, phosphate and nitrate were determined by spectrophotometric methods. Fluoride ions were determined by ion selective electrode method. The arsenic was determined by MERCK test kit (low range 0.05-0.300 mg/L (Merck, Germany) using the procedure provided by the manufacturer. In order to check the results of arsenic determination, 5 to 10% samples observed with TDS below 1500 mg/L were analysed by atomic absorption spectrometry (AAS), connected with flow injection system using hydride generation technique [30] Sodium, potassium, calcium and magnesium were determined by air-acetylene flame atomic absorption spectrometry (Perkin Elmer AA-800 spectrophotometer). The graphical presentation of arsenic, fluoride and physicochemical parameters were made using Minitab software. The contour plots were developed by measuring field data (coordinates) collected through handpicked device Global Positioning System (GPS).
Statistical analysis
Basic statistics and correlation calculations were carried out to give initial information about the water quality data. The characteristics of the data are indicated as mean, minimum value, maximum value, 95% Confidence interval of the mean value and standard deviation. The multivariate statistical methods, including cluster analysis (CA) and principal component analysis are employed on the data set using SPSS 22 Statistical software.
Results and Discussion
The dug Wells 2170 of Thar Desert within district Tharparkar were examined for water quality of groundwater. The water samples based on their total dissolved solids (TDS) and electrical conductivity were divided into three parts. The samples with TDS greater than 3000 mg/L, higher than permissible limits of WHO and were not considered suitable for drinking purposes. The samples were analysed for pH, electrical conductivity, total dissolved salts (TDS), and salinity (Table 1). The samples with TDS within 1500 – 3000 mg/L, though with TDS values higher than permissible limits of WHO, were considered suitable for drinking purposes under stress conditions for limited time. The samples were analysed for pH, electrical conductivity (EC), TDS, salinity, chloride (Cl), total hardness as CaCO3, and alkalinity (P-alkalinity and M-alkalinity) measured as CaCO3. The third group of samples comprised of TDS below 1500 mg/L, and were considered within permissible limits of WHO under stress conditions. The samples were analysed for pH, electrical conductivity, TDS, salinity, chloride (Cl), total hardness (TH) as CaCO3, alkalinity (P-alkalinity and M-alkalinity) measured as CaCO3, nitrate (NO3), sulphate (SO4), total phosphate-phosphorus (PO4-P), fluoride (F), and arsenic (As). The Latitude, longitude, village, Population, well diameters, level meters, bottom meters and water depth meters were measured and is summarized in (Appendix 1. Additional data) It was considered useful to examine the samples with TDS below 1500 mg/L for their possible use of water for irrigation purposes. Forty nine samples among them were picked randomly and analysed for sodium (Na), potassium (K), calcium (Ca) and magnesium (Mg) contents, to calculate sodium adsorption ratio (SAR), sodium % (Na%) and related parameters critical for evaluation of the water for irrigation purposes. (Table 2) indicates the minimum, maximum, mean, standard deviation and 95% confidence interval of parameters of TDS greater than 3000 mg/L (n=1247). Table 3 indicates the 1500 – 3000 mg/L (n=320). The Table 4 indicates the TDS less than 1500 mg/L (n=588). pH is term used universally to express the intensity of the acid or alkaline condition of the solution. The pH affects the total ionic stability and solubility at lesser concentrations [33]. The pH of the samples with TDS higher than 3000 mg/L was within the range 6.7 – 9.88 with average value 8.09 (n=1247). The samples 186 indicated pH above the maximum permissible limit of 8.5 and the samples 1061 were within the permissible limit of 6.5 – 8.5. The samples with TDS within 1500 – 3000 mg/L indicated pH within the range 7.29 – 9.87 with average value of 8.11 (n=320). The samples 42 were observed above the permissible limit of 8.5. The samples with TDS less than 1500 mg/L were observed from slightly acidic to alkaline in nature with pH between 6.26 – 10.76. The average pH was observed 7.98 (n=588). The samples 48 indicated pH above the permissible limit of 8.5 and a sample indicated pH below the permissible limit of 6.5. Conductivity is a mathematical expression of waters ability to transmit electric current and is directly related to the concentration of the ionised substance in water and may also be related to the problems of excessive hardness and other minerals contamination [34]. The electrical conductivity of the samples with TDS higher than 3000 mg/L was observed within the range 3.8 to 108.6 mS/cm with average value of 10.57 mS/cm (n=1247). All the samples indicated electrical conductivity above the permissible limit for drinking water. The samples with TDS within 1500 – 3000 mg/L indicated conductivity within the range 2346 – 4660 uS/cm with an average value of 3090.4 uS/cm (n=320). All the samples indicated electrical conductivity more than the permissible limit for human consumption. The electrical conductivity of the samples with less than 1500 mg/L was observed within the range 170 – 2371 uS/cm with average of 1095.9 uS/cm (n=588). The standard deviation of the data set was calculated 588.7 uS/cm and indicates wide variation in conductivity among sampling stations. The samples 145 indicated conductivity above the permissible limit of 1500 uS/cm and 443 samples were within the permissible limit of WHO. In the ground water, total dissolved solids (TDS) consists mainly of inorganic salts such as carbonates, bicarbonates, chlorides, sulphates, phosphates and nitrates of calcium, magnesium, sodium, potassium and iron. A small amount of organic matter is also present. Higher concentration of dissolved salts imparts mineral taste to potable water and creates aesthetic problems for consumers such as undesirable salty bitter taste. To determine the suitability of groundwater of any purposes, the groundwater has been classified, depending upon their hydro chemical properties based on their TDS values [35,36]. Among 2170 samples of dug wells analysed, 1247 samples indicated TDS more than 3000 mg/L (Appendix 2), 320 samples were with TDS within 1500-3000 mg/L (Appendix 3) and 588 samples with TDS less than 1500 mg/L (Appendix 2) According to Davis et al., [35], 225 samples were observed with TDS less than 500 mg/L and are classified as desirable for drinking. The samples 457 were recorded with TDS less than 1000 mg/L and can be classified as permissible for drinking. The samples 905 were observed with TDS less than 3000 mg/L and may be classified useful for irrigation. According to Freeze et al., [36], the samples 456 with TDS less than 1000 mg/L may be classified as fresh water type. The samples 1566 with TDS within 1000 – 10000 mg/L may be classified as brackish water type and only samples 132 indicated TDS above 10000 mg/L, and can be classified as saline water type. Salinity is defined as total solids in water after all carbonates have been converted to oxides, all bromide and iodides have been replaced by chlorides.
| Variables | Abbreviation | Unit | Analytical Method |
|---|---|---|---|
| pH | Ph | pH | pH Meter |
| Electrical Conductivity | EC | µS/cm | Conductivity Meter |
| Salinity | Salinity | g/L | Conductivity Meter |
| Total Dissolved Solids | TDS | mg/L | Conductivity Meter |
| Total Hardness | TH | mg/L | Titrimetric Method |
| Chloride | Cl | mg/L | Titrimetric Method |
| Alkalinity | Alk | mg/L | Titrimetric Method |
| Total Phosphate-Phosphorus | T-PO4 | mg/L | Spectrophotometric |
| Sulphate | SO4 | mg/L | Spectrophotometric |
| Nitrate-Nitrogen | NO3-N | mg/L | Spectrophotometric |
| Fluoride | F | mg/L | Ion Selective Electrode |
| Arsenic | As | µg/L | Merck test kit/ASS |
| Sodium | Na | mg/L | FAAS |
| Potassium | K | mg/L | FAAS |
| Calcium | Ca | mg/L | FAAS |
| Magnesium | Mg | mg/L | FAAS |
Table 1: The water quality parameters associated with their abbreviation, units and analytical methods.
| S: No: | Parameters | Minimum | Maximum | Mean | Standard Deviation | 95% Confidence Interval |
|---|---|---|---|---|---|---|
| 1 | pH | 6.7 | 9.88 | 8.09 | 0.43 | 8.09 ± 0.84 |
| 2 | Conductivity (mS/cm) | 3.8 | 108.6 | 10.57 | 5.33 | 10.57 ± 10.39 |
| 3 | TDS (mg/L) | 3002 | 69504 | 6774.26 | 3415.23 | 6774.26 ± 6659.25 |
| 4 | Salinity (ppt) | 2.5 | 68.1 | 6.24 | 3.44 | 6.24 ± 6.708 |
Table 2: The results of statistical analysis for minimum, maximum, mean, standard deviation and 95% confidence interval for the parameters analysed for samples with TDS more than 3000 mg/L (n=1247).
| S: No: | Parameters | Minimum | Maximum | Mean | Standard Deviation | 95% Confidence Interval |
|---|---|---|---|---|---|---|
| 1 | pH | 7.29 | 9.87 | 8.11 | 0.40 | 8.11 ± 0.78 |
| 2 | Conductivity (µS/cm) | 2346 | 4660 | 3090.4 | 655.7 | 3090 ± 1278.6 |
| 3 | TDS (mg/L) | 1501 | 2982 | 2227.13 | 420.36 | 2227.13 ± 819.70 |
| 4 | Salinity (ppt) | 1.2 | 2 .8 | 1.85 | 0.38 | 1.85 ± 0.74 |
| 5 | Chloride (mg/L) | 124 | 1091 | 547.39 | 222.88 | 547.39 ± 434.61 |
| 6 | T Hardness (mg/L) | 100 | 1420 | 501.84 | 224.58 | 501.84 ± 437.93 |
| 7 | M Alkalinity (mg/L) | 125 | 1230 | 338.42 | 149.00 | 338.42 ± 290.55 |
Table 3: The results of statistical analysis for minimum, maximum, mean, standard deviation and 95% confidence interval values calculated for the samples with TDS within 1500-3000 mg/L (n=320).
| S: No: | Parameters | Minimum | Maximum | Mean | Standard Deviation | 95% Confidence Interval |
|---|---|---|---|---|---|---|
| 1 | pH | 6.24 | 10.76 | 7i.98 | 0.56 | 7.98 ± 1.095 |
| 2 | Conductivity (µS/cm) | 170 | 2371 | 1095.90 | 588.68 | 1059.90 ± 1147.92 |
| 3 | TDS (mg/L) | 109 | 1498 | 701.43 | 377.73 | 701.43 ± 736.56 |
| 4 | Salinity (ppt) | 0.1 | 1.5 | 0.55 | 0.31 | 0.55 ± 0.61 |
| 5 | Chloride (mg/L) | 3.5 | 528 | 110.56 | 106.28 | 110.56 ± 207.24 |
| 6 | T Hardness (mg/L) | 40 | 1050 | 264.58 | 137.94 | 264.58 ± 267.20 |
| 7 | M Alkalinity (mg/L) | 30 | 905 | 231.2 | 101.02 | 231.02 ± 196.99 |
| 8 | SO4 (mg/L) | 0 | 192 | 26.23 | 27.02 | 26.23 ± 52.69 |
| 9 | NO3 (mg/L) | 0 | 33.60 | 5.89 | 6.27 | 5.89 ± 12.22 |
| 10 | PO4 (mg/L) | 0 | 7.99 | 0.92 | 1.13 | 0.93 ± 2.17 |
| 11 | Arsenic (mg/L) | 0 | 0.25 | 0.016 | 0.02 | 0.02 ± 0.04 |
| 12 | Fluoride (mg/L) | 0.16 | 6.4 | 1.35 | 1.18 | 1.36 ± 2.30 |
Table 4: The results of statistical analysis for minimum, maximum, mean, standard deviation and 95% confidence interval calculated for the samples with TDS less than 1500 mg/L (n=588).
Total hardness (TH) is the total concentration of calcium and magnesium ions found in water expressed as CaCO3. Common cations are calcium, magnesium, but iron, strontium and manganese may also contribute to hardness. Calcium dissolves in water from rocks and soils. The TH of samples with TDS within 1500 – 3000 mg/L was observed within the range 100-1420 mg/L with an average value 501.8 mg/L (n=320). The samples 147(47%) indicated TH above the permissible limit (500 mg/L). Similarly TH for the samples with TDS less than 1500 mg/L indicated between 40-1050 mg/L with an average value of 364.6 mg/L (n=588). The samples 9 (1.5%) indicated TH above the permissible limit of WHO for human consumption.
Chloride is the main component of most waters. Natural waters usually contain chloride but its variable concentration depends upon the geological formation of the area. It includes the disintegration of sedimentary rocks, dissolution of salt deposit and then participation through the deposited suppressive in the aquifer. According to WHO standard maximum allowable limit for chloride in drinking water is 250 mg/L. Chloride is extremely soluble in water. It has been suggested that patients of hearts and kidney diseases should avoid drinking water having high chloride concentration. The chloride concentration of the samples with TDS within 1500-3000 mg/L was observed between 124-1091 mg/L with an average value 547.4 mg/L (n=320). The samples 291 (90%) indicated chloride concentration above the permissible limit for drinking water. Similarly the samples with TDS less than 1500 mg/L showed the chloride concentration in the range 3.5-528 mg/L with an average value of 110.6 (n=588). The samples 61(10.4%) indicated chloride concentration above the permissible limit for drinking water. The amount of a strong acid needed to neutralise the alkalinity is called total alkalinity, and is measured as mg/L CaCO3. The main cause of alkalinity is bicarbonate. Highly mineralized alkaline water may cause excessive drying of the skin due to the fact that they tend to remove normal skin oil. High alkalinity imparts a bitter test to water. The maximum permissible limits of carbonates and bicarbonates according to WHO guidelines are 30 mg/L and 500 mg/L respectively. The samples with TDS within 1500-3000 mg/L indicated alkalinity in the range of 125-1230 mg/L with an average value of 338.4 mg/L (n=320). The samples 11 (3.4%) indicated higher concentration of carbonate and 28 samples (8.8%) showed higher concentration of bicarbonate than the permissible limits. Similarly the samples with TDS less than 1500 mg/L indicated alkalinity in between 30-905 mg/L with an average value of 231.2 mg/L (n=588). The samples 12 (2.0%) had higher concentration of carbonate and 4 samples (0.7%) had higher concentration of bicarbonate than the permissible limits of alkalinity. Sulphate is present in all natural waters but excessive sulphate concentration in water (more than 250 mg/L) has a laxative effect and imparts an unpleasant taste of water and cause diarrhoeas and gastrointestinal disorders. The sulphate in the samples with TDS less than 1500 mg/L was observed within 0-192 mg/L with an average value of 26.2 mg/L. The sulphate in all the samples was observed within the permissible limit for drinking water (Appendix 4). Nitrate nitrogen is the extremely oxidised form and nitrate contamination in groundwater is one of the major issues in water quality studies [37,38]. The occurrence of high level of nitrate in ground water is a prominent problem in many part of the world. The concentration of nitrogen in groundwater is derived from the biosphere. Nitrogen is originally fixed from the atmosphere and mineralized by soil bacteria into ammonia. The high concentration of nitrate in drinking water is toxic and causes blue baby disease/methemoglobinaemia in children and gastric carcinomas [39]. Nitrate produces no colour or older in water and can cause cancer in humans when consumed over a long period of time [40]. The nitrate concentration in the samples with TDS less than 1500 mg/L was observed within the range below the limit of quantitation to 33.6 mg/L with an average value of 5.89 mg/L. A number of samples exceeded the WHO limit of 10 mg/L nitrate for drinking water. The presence of arsenic in water poses health hazards to humans, creates non-cancer effects such as hyper- and hypo-pigmentation, keratosis, black foot disease, hypertension, cardiovascular diseases and diabetes [41]. Inorganic arsenic species dissolved in drinking water are the most significant forms of natural exposure. The four main species of arsenic occur in water, inorganic forms such as arsenite, arsenate, and organic forms (methyl arsenic acid) and dimethyl arsenic acid. Arsenite is 60 times more toxic than arsenate [42]. Inorganic arsenicals belong to group I carcinogens, it causes cancers of the skin, lungs and bladder [43]. Inorganic arsenic contents were examined in samples with TDS less than 1500 mg/L, which is more drinkable for human consumption. The arsenic contents were observed in the range blow the limit of detection to 0.25 mg/L with an average value of 0.016 mg/L (n=588). The samples 173 (29.4%) indicated arsenic contents more than permissible limit of WHO (0.01 mg/L) for human consumption. The samples 64 (10.8%) indicated arsenic concentration 0.05 mg/L and above, which may be considered as toxic level for human consumption. The samples 14(2.4%) indicated arsenic concentration 0.1 mg/L and above. These samples may be considered as hazardous for their use as a source of drinking water. Fluoride is an essential micronutrient for human beings, serving to strengthen the apatite matrix of skeleton tissues and teeth at the concentration less than 1 mg/L. On the other hand, high fluoride concentration greater than 1.5 mg/L results into dental and skeletal fluorosis, renal, and neuronal disorders along with myopathy [44]. High concentrations of fluoride in groundwater are therefore expected in areas where fluoride bearing minerals are abundant in geologic substrate [45]. Endemic fluorosis has been reported from many parts of the world including China [46] and Pakistan [11-13]. The samples with TDS less than 1500 mg/L were analysed for the contents of fluoride. The amounts found were within the range 0.16 – 6.4 mg/L with an average value of 1.35 mg/L (n=588). The samples 165 (28%) crossed the permissible level of 1.5 mg/L and 46 samples (7.8%) crossed the hazardous level of 3.0 mg/L of fluoride in drinking water.
The samples 49 were selected randomly from 588 samples with TDS less than 1500 mg/L and were analysed for the contents of sodium, potassium, calcium and magnesium. The results of analysis are summarised in (Appendix 5). The results of statistical analysis with minimum, maximum, mean, standard deviation and confidence interval at 95% level are summarised in Table 5. Sodium is commonly found in biosphere and it is present in all natural waters. Sodium is helpful to maintain body fluids and is involved in nerve impulse transmission and muscle contraction. High intake of sodium can damage to the kidneys and may cause hypertension to the human. The sodium concentration found in the samples analysed (n=49) was in the range 10-327 mg/L with a mean value of 111.96 mg/L. The samples 7(14%) crossed the permissible limit of WHO (200 mg/L) for human consumption. The natural water generally contains lesser amount of potassium than sodium, calcium and magnesium.
| S: No: | Parameters | Minimum | Maximum | Mean | Standard Deviation | 95% Confidence Interval |
|---|---|---|---|---|---|---|
| 1 | Sodium (mg/L) | 10 | 327 | 111.96 | 74.47 | 111.96±145.21 |
| 2 | Potassium (mg/L) |
3.0 | 62 | 17.84 | 13.69 | 17.84±26.69 |
| 3 | Calcium (mg/L) |
22 | 104 | 56.23 | 19.53 | 56.23±38.08 |
| 4 | Magnesium (mg/L) |
18 | 141 | 49.29 | 28.11 | 49.29±54.81 |
Table 5: The results of statistical analysis for minimum, maximum, mean, standard deviation and 95% confidence interval for 49 samples analysed for major elements from TDS less than 1500 mg/L (n=588).
Potassium is one of the major positive ions found in the cell cytoplasm. Proper level of potassium in the body is essential for the regulation of heart beat and normal muscle function. The quantity of potassium in human body ranged between 110 – 140 g and it depend upon muscles strength. However, if ingested in excess may behave as a laxative. The concentration of potassium in the samples analysed (n=49) was observed within 3 – 62 mg/L with an average value of 17.8 mg/L. Among the samples analysed for the contents of potassium 24 (48.9%) crossed the permissible limit of WHO (12 mg/L) for drinking water. Calcium is abundantly present in earth crust and in natural waters. It is most abundant element in the human body and is one of the important dietary mineral. It helps in bone and teeth strengthening. Calcium is also vital for muscle contractions, in maintaining membrane activity, in transfer of nerve impulses and to stimulate the secretions of neurotransmitter from synaptic vesicles. The permissible dose for oral consumption of calcium is 5-50 mg/kg weight of the body. The calcium concentration found in the samples analysed (n=49) was observed within 22-104 mg/L with a mean value of 56.2 mg/L. The results of analysis indicated that all the samples analysed for calcium were within permissible limit of WHO (175 mg/L) for drinking water.
Magnesium is one of the dietary mineral of all organisms including insects. The human body contains approximately 25 g of magnesium. The magnesium present in the water more than 125 mg/L may promote laxative effect [47]. The magnesium in the samples analysed (n=49) was observed in the range of 18 – 141 mg/L with a mean value of 49.3 mg/L. The samples 4 (8.2%) indicated magnesium concentration higher than permissible limit of WHO (75 mg/L).
Data analysis using correlation matrix
The results of coefficient of correlation matrix (Table 6) were obtained by inserting the results of physicochemical analysis for 49 samples indicated in Table 7 in SPSS version 16.0 software. A strong correlation between two variables is exhibited by a high correlation coefficient (near +1 or -1), and its value around zero means no relation between them. Alternatively, it can be expressed that the parameters indicating correlation coefficient higher than 0.7 are considered to be strongly correlated, whereas value between 0.5 and 0.7 shows moderate correlation. Conductivity has strong correlation with TDS, salinity and chloride, sodium with chloride. A moderate correlation is indicated by sodium with conductivity, TDS and salinity, fluoride with conductivity and TDS.
| pH | Cond | TDS | Salinity | Na | K | Mg | Ca | SO4 | Cl | HCO3 | NO3 | PO4 | As | F | |
| pH | 1.000 | ||||||||||||||
| Cond | 0.169 | 1.000 | |||||||||||||
| TDS | 0.158 | 0.999 | 1.000 | ||||||||||||
| Salinity | 0.053 | 0.930 | 0.931 | 1.000 | |||||||||||
| Na | 0.076 | 0.629 | 0.632 | 0.656 | 1.000 | ||||||||||
| K | 0.164 | -0.003 | 0.004 | -0.039 | 0.163 | 1.000 | |||||||||
| Mg | 0.166 | 0.440 | 0.445 | 0.417 | 0.128 | 0.252 | 1.000 | ||||||||
| Ca | -0.094 | 0.496 | 0.495 | 0.433 | 0.281 | 0.067 | 0.264 | 1.000 | |||||||
| SO4 | 0.324 | 0.390 | 0.399 | 0.284 | 0.223 | 0.046 | 0.153 | 0.061 | 1.000 | ||||||
| Cl | 0.079 | 0.721 | 0.726 | 0.719 | 0.924 | 0.112 | 0.196 | 0.358 | 0.278 | 1.000 | |||||
| HCO3 | -0.078 | 0.373 | 0.369 | 0.475 | 0.179 | 0.086 | 0.401 | 0.186 | -0.153 | 0.159 | 1.000 | ||||
| NO3 | 0.452 | 0.000 | 0.000 | -0.083 | 0.134 | 0.272 | 0.126 | -0.086 | 0.001 | 0.093 | -0.139 | 1.000 | |||
| PO4 | -0.239 | -0.031 | -0.026 | -0.027 | -0.170 | 0.281 | -0.036 | 0.117 | -0.137 | -0.113 | 0.299 | -0.101 | 1.000 | ||
| As | -0.426 | -0.232 | -0.235 | -0.095 | 0.019 | -0.059 | -0.081 | -0.149 | -0.305 | -0.055 | 0.232 | -0.144 | 0.188 | 1.000 | |
| F | -0.167 | 0.547 | 0.548 | 0.746 | 0.346 | -0.009 | 0.395 | 0.257 | 0.139 | 0.371 | 0.554 | -0.323 | 0.023 | 0.125 | 1.000 |
Table 6: Linear Pearson correlation coefficient matrix of different Physicochemical parameters for 49 samples indicated.
| Rotated Component Matrixa | |||||
|---|---|---|---|---|---|
| Component | |||||
| 1 | 2 | 3 | 4 | 5 | |
| Ca | 0.895 | 0.112 | 0.007 | 0.051 | 0.056 |
| Cl | 0.891 | 0.155 | 0.088 | 0.157 | 0.041 |
| TDS | 0.700 | 0.486 | 0.318 | 0.329 | -0.050 |
| Cond | 0.687 | 0.461 | 0.356 | 0.220 | -0.058 |
| Salinity | 0.675 | 0.491 | 0.300 | 0.356 | -0.094 |
| NO3 | 0.626 | -0.165 | -0.161 | -0.216 | -0.099 |
| HCO3 | 0.076 | 0.811 | -0.178 | 0.052 | 0.194 |
| F | 0.307 | 0.794 | -0.109 | 0.053 | -0.065 |
| K | -0.020 | 0.604 | 0.291 | 0.363 | 0.111 |
| pH | -0.094 | 0.000 | 0.786 | 0.021 | 0.011 |
| SO4 | 0.258 | 0.145 | 0.705 | -0.109 | -0.031 |
| As | -0.070 | 0.247 | -0.633 | -0.242 | 0.043 |
| TH | -0.048 | 0.167 | 0.062 | 0.813 | -0.099 |
| Na | 0.296 | 0.081 | -0.009 | 0.811 | 0.132 |
| Mg | 0.026 | 0.001 | 0.168 | 0.020 | 0.858 |
| Po4 | -0.067 | 0.129 | -0.223 | -0.007 | 0.743 |
| Initial Eigenvalues | 5.436 | 1.938 | 1.827 | 1.287 | 1.095 |
| Variance % | 33.977 | 12.115 | 11.420 | 8.045 | 6.846 |
| Cumulative % | 33.977 | 46.093 | 57.513 | 65.558 | 72.404 |
Table 8: Rotated Component Matrixa.
Principal components analysis
Principal components analysis (PCA) is a statistical method to reduce the dimensions of multi-index data [48]. The analytical data obtained for 49 samples collected randomly with TDS below 1500 mg/L, were used as input for PCA. The computer program SPSS 22 (SPSS Inc., Chicago, IL, USA) was used. Sixteen parameters were introduced and five principal components (PC) were identified with eigenvalues more than 1. The total cumulative % of five PCs was calculated 72.40. The PC 1 accounted for variance% of 33.977. Ca and Cl had strong correlation (0.891-0.895), TDS, EC, salinity and NO3 moderate correlation (0.626-0.700) and F, SO4, and Na low correlation (0.258-0.307). PC 2 corresponded for variance% 12.115 and HCO3 and F indicated strong correlation (0.811-0.794) and K (0.604). The results indicated that an increase in the concentration of HCO3 had positive effect on the concentration of F. PC 3 indicated the variance % of 11.420 and showed that pH and SO4 are positively correlated (0.786-0.705) and negatively correlated with As (-0.633). PC 4 showed variance % 8.045 and TH and Na are strongly correlated (0.813-0.811) and weakly correlated with TDS and EC (0.318-0.329). PC 5 indicated variance % 6.846 and Mg and PO4 were correlated (0.858-0.743).
Contour diagram
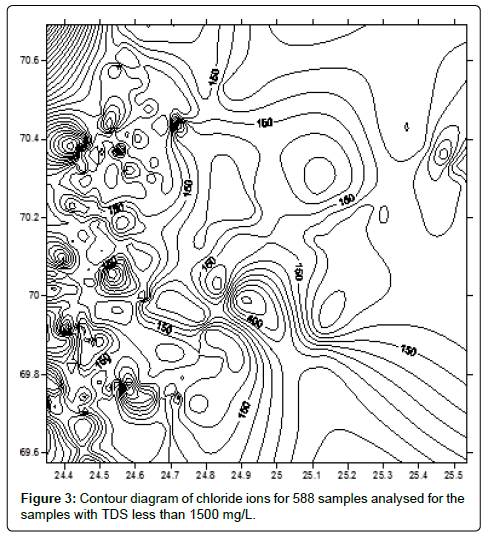
The contour diagrams for chloride ions were drawn for 588 samples with TDS below than 1500 mg/L by inserting GPS readings of latitude and latitude of all the samples against the concentrations of chloride ions in Surfer 8 software (Figure 3). M-alkalinity contour diagram drawn for 588 (Surfer version 8 software) (Figure 3). (Additional data). The contour plots for sodium and calcium by inserting the readings of longitude and latitude of the samples analysed from the GPS against the concentrations of sodium and calcium in the Militab software. The black spots indicate sampling locations and the sheds as concentrations of metal ions.
Hierarchical cluster analysis
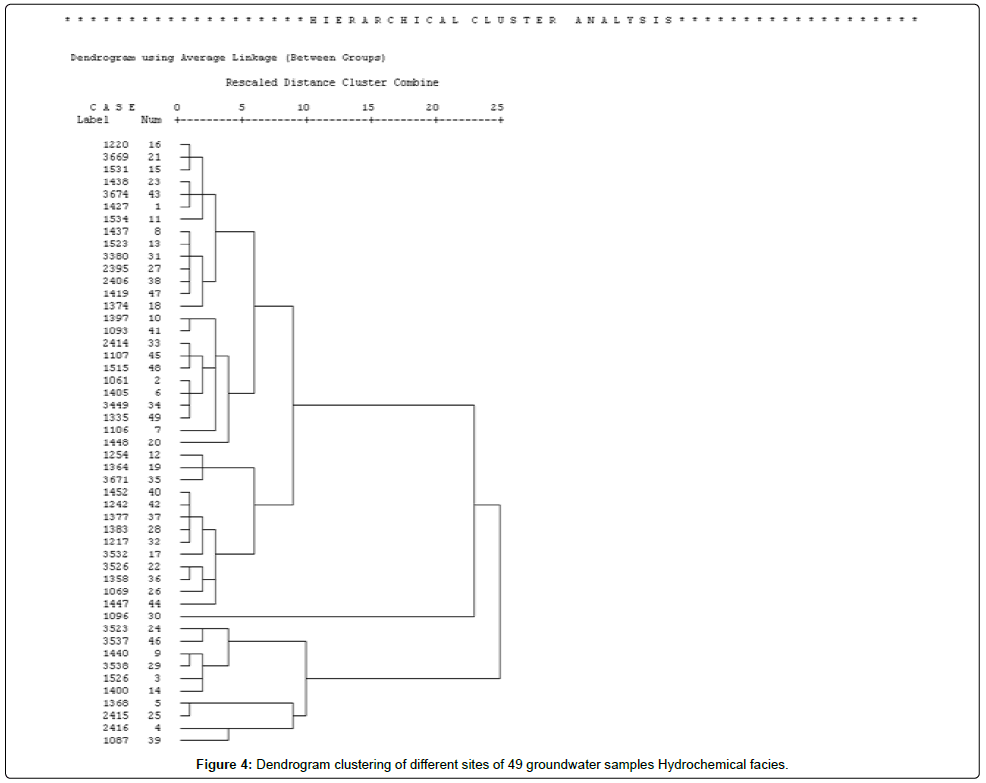
This is a classification method which enables the grouping of similar stations on the basis of distance criteria and of a specific aggregative algorithm in order to create a topology which will characterise the elements to be classified [49]. It groups the objects into the classes or clusters on the basis of similarities within a class and dissimilarities between different classes. The results of cluster help in interpreting the data and indicate patterns. In hierarchical clustering, clusters are formed sequentially by starting with the most similar pair of objects and forming higher clusters step by step. Hierarchical agglomerative clusters are performed on the normalised data set (average values) by means of Wards method using squared Euclidean distances as a measure of similarity. Cluster analysis was applied to the 49 groundwater samples for water quality data with a view to group similar sampling sites spread in the region.
The samples were observed to be grouped into three clusters as observed in dendrogram (Figure 4). Each of the group is again divided into two groups (a, b). Group 1a is based on 14 samples (16, 21, 15, 23, 43, 1, 11, 8, 13, 31, 27, 38, 47, 18). The cluster 1b comprises of 11 samples (10, 41, 33, 45, 48, 2, 6, 34, 49, 7, 20). Group 2a is based on 3 samples (12, 19, 35) and 2 b on 11 samples (40, 42, 37, 28, 32, 17, 22, 36, 26, 44, 30). The cluster 3a comprises of 6 samples (24, 46, 9, 29, 3, 14) and 3 b on 4 samples (5, 25, 4, 39). It is observed that group 3 samples have higher values for the parameters than group 1 and 2. Similarly group 2 has higher values than group 1.
The results indicate that clusters analysis is useful in offering reliable classification of water resources in the study area and will make it possible to design future spatial sampling strategy [50].
Hydro chemical facies
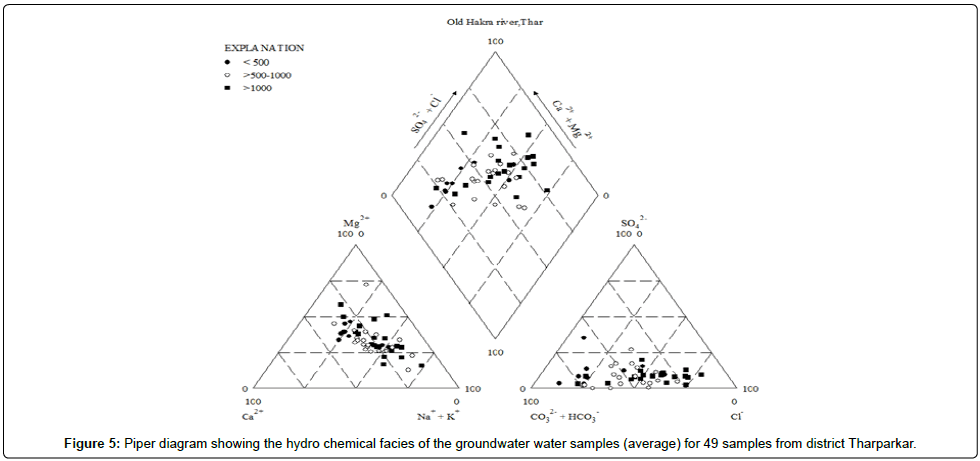
The hydro chemical facies analysis describes the chemical processes operative in a certain lithological environment and under specific geochemical conditions [51]. Piper has developed a form of trilinear diagram, which is an effective tool in segregating analysis data with respect to sources of the dissolved constituents in groundwater, modifications in the character of water as it passes through an area and related geochemical problems. In the cations triangle Na and K are somewhat dominant than Ca and Mg. More samples are in mixed type zone than Na and K zone. For anion triangle Cl and HCO3 are dominant than other anions. The diamond shape indicates groundwater of diverse origin. Major portion is within mixed zone, followed by Na and K, and SO4 and Cl zones. Some of the samples are also in Ca and Mg zone. The major hydro chemical species in the 49 samples are examined are Na, Ca, Cl, HCO3 and Na, Ca, Mg, SO4 and Cl according to their order of dominance (Figure 5). The majority of the groundwater samples exhibit the characteristics of NaCl, Ca, Mg, HCO3, SO4 and Na, Ca, Cl species.
Scatter graphical plots
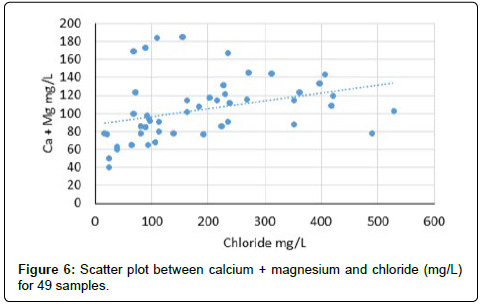
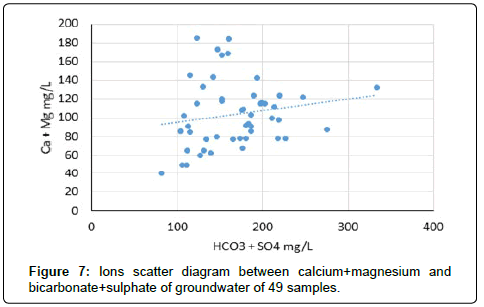
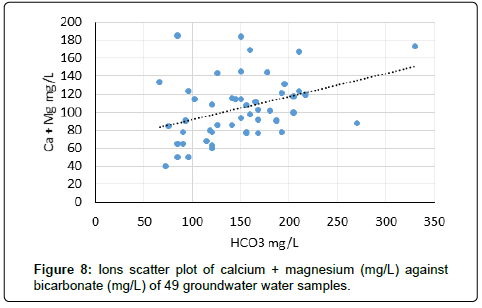
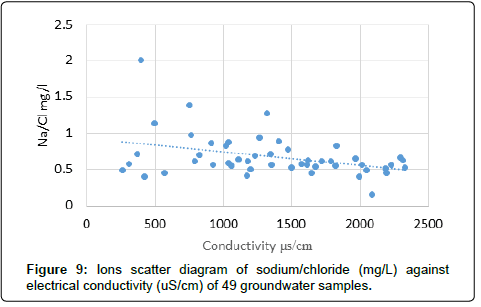
The scatter graphical plots can reveal the types of various hydro geochemical processes taking place in the Tharparkar area. The scatter diagram (Figure 6) of Ca+Mg versus Cl indicates that the concentrations of two items are increasing as Ca and Mg increase with salinity, which could be an indication of occurrence of ion exchange process in the clay. The scatter diagram of Ca+Mg versus HCO3+SO4 (Figure 7) indicates the graph somewhat increases with salinity and suggest that these ions have resulted from the weathering of carbonate and sulphate minerals. Moreover the calcium and magnesium may be originated from carbonate and silicate weathering and an excess of Ca and Mg may be derived from other processes such as reverse ion exchange reactions. The scatter diagram Ca+Mg mg/L against bicarbonate (mg/L) (Figure 7) also increases with salinity in groundwater. The Ca and Mg originate from the dissolution of carbonates in the aquifer materials and from the weathering of the accessory materials. The scatter plot of Na/Cl ratio versus electrical conductivity is indicated (Figure 7) in groundwater samples. The evaporation process is not only common in surface water but also in groundwater system. Na/Cl ratio can be used to indicate the evaporation process in groundwater. Evaporation will increase the electrical conductivity in ground water, and the Na/Cl ratio will remain the same and is one of the indicative factors of evaporation. If the evaporation is dominant process, Na/Cl ratio may be constant when electrical conductivity rises [52]. The electrical conductivity (EC) versus Na/Cl ratio of scatter plot indicates that the trend line is inclined, and Na/Cl ratio decreases with increasing EC, which may be the removal of sodium by ion exchange reaction. This observation may indicate that evaporation may not be the major geochemical process controlling the chemistry of groundwater in this study area or ion exchange reaction may be dominating over evaporation.
Criteria of groundwater suitability for irrigation
The composition and concentration of dissolved salts in water depends upon its quantity for irrigation purposes. A number of factors affect water quality for irrigation, mainly total concentration of soluble salts and relative amounts of sodium to calcium and magnesium. The mineral constituent of water affects both plants and soil for the suitability for agricultural purposes [48]. The concentration of the salt in the soil may affect soil structure, permeability and aeration, which can affect plant growth. Thus some of the parameters have been set to test the suitability of groundwater for irrigation.
Statistical calculation on the suitability of 49 groundwater samples with TDS less than 1500 mg/L for irrigation were examined for SAR=Sodium Adsorption Ratio, Na %=Sodium Percentage, KI=Kelly’s Index, RSC=Residual Sodium Carbonate, PI=Permeability Index, CA1=Chloro Alkaline Indices 1, Cl/SO4=Chloride Sulphate ratio, Cl/ HCO3=Chloride bicarbonate ratio and are summarized in (Appendix 6).
Sodium adsorption ratio (SAR): Sodium hazard in groundwater is expressed in the form of sodium adsorption ratio (SAR). SAR is calculated from the ratio of sodium to calcium and magnesium. The presence of calcium and magnesium in the groundwater generally counter the effect of sodium. The use of groundwater with high SAR values for longer time affects the physical structure of the soil. The soil changes to hard and compact when dry and prevents the penetration of water. The extent to which irrigation water could enter into cation exchange reaction in soil is expressed as SAR. The SAR is considered important parameter for the suitability of groundwater for irrigation, because it is responsible for sodium hazards. SAR is calculated using following relation:
SAR= Na/{(Ca+Mg)/2}1/2.
Where all concentrations are expressed as meq/L. The analytical data calculated for 49 samples for SAR values indicates that 2 samples (4.0%) exceed the permissible limit of 6 for SAR and 96% samples are within permissible limits, suitable for irrigation. The 4% samples which exceed the permissible limit of 6 for SAR, are still within acceptable limit of 10 for SAR with low conductivity. Thus the samples can be used for irrigation in almost all types of the soil with little danger of exchangeable sodium.
Sodium percentage (Na %): The groundwater containing high amounts of sodium can be a source of concern, owing to the effects of the sodium on the soil and may cause sodium hazards [53]. Hence it is considered essential to calculate sodium % by the following relation:
Na=Na+K/(Ca+Mg+Na+K) X 100
where all the concentrations are expressed in meq/L. On the basis of the analyses of 49 samples 4 samples (8.2%) are classified as excellent with Na% less than 20%, 20 samples (40.8%) are considered as good with sodium % within 20 – 40%, 22 samples (44.8%) are counted as permissible with sodium % within 40 – 60%, and 4 samples (8.2%) are classified as doubtful with sodium % within 60 – 80%. No sample was observed unsuitable with Na % more than 80%.
Kelly’s index (KI): Kelly’s index is used to classify the groundwater for irrigation. Sodium is measured against calcium and magnesium [54]. The values more than 1 for Kelly’s index show an excess level of sodium in groundwater. Therefore the groundwater with Kelly's index less than 1 are considered suitable for irrigation. The Kelly's index is calculated using following relation.
KI=Na/(Ca+Mg).
Where all the concentrations are expressed as meq/L. Among the 49 samples the calculated values for KI shows that 11 samples (22.4%) indicate values more than 1 and 38 samples (77.6%) have values less than 1. Hence, on the basis of KI also 77.6% samples are considered suitable for irrigation.
Residual sodium carbonate (RSC): When total concentration of carbonates is higher than calcium and magnesium, the quality of groundwater for irrigation may deteriorate. When the concentration of carbonate is in excess than calcium and magnesium, the carbonate combines the calcium and magnesium to form solid material, which can settle out of water. The relative concentrations of sodium, calcium, magnesium and carbonate and bicarbonate may influence the suitability of groundwater for irrigation. The residual sodium carbonate (RSC) is calculated using following relation:
RSC=(CO3+HCO3) - (Ca+Mg)
where all the concentrations are included as meq/L. The values of RSC less than 1.25 are considered safe for irrigation, the values between 1.25-2.5 meq/L are considered marginal and values above 2.5 meq/L are unsuitable for irrigation. All the negative values are considered as suitable [55]. Not a single sample was observed with RSC value above 2.5 meq/L, which is considered unsuitable for irrigation.
ermeability index (PI): The permeability of the soil is affected by long term use of groundwater for irrigation. Sodium, calcium, magnesium and bicarbonate contents influence the permeability of the soil. The permeability index is calculated using following equation:
PI=Na+HCO3/(Ca+Mg+Na) x 100
Where the concentrations of all the ions is expressed in meq/L. The PI values above 75% indicate (Class 1) excellent quality of groundwater for irrigation. If the values of PI are between 75 and 50%, they indicate (Class II) as good quality of groundwater for irrigation. However if the values of PI are less than 50% (Class III), they indicate unsuitable groundwater for irrigation. The results of analysis for 49 random samples indicate that 35 samples (71.4%) are in (Class I) with excellent water quality for irrigation. The samples 12 (24.4%) reflect (Class II) with good quality of groundwater for irrigation. However, only samples 2 (4.0%) are within (Class III) with unsuitable groundwater quality for irrigation.
Chloro alkaline indices (CAI – 1): The chemical composition of the groundwater changes during its travel in the subsurface, due to the ion exchange between the groundwater and host environment. Chloro alkaline indices are used to understand the phenomenon [56,57]. The chloro alkaline indices -1 is calculated by using following relation:
CAI – 1=[Cl – (Na+K)]/Cl
If CAI is negative then there is Base Exchange between sodium and potassium with calcium and magnesium in rocks. If the ratio is positive there is no base exchange. The results of analysis of 49 samples indicate that 27 samples (55%) are negative and 22 samples (45%) are positive (Figure 8).
Chloride-sulphate ratio (Cl/SO4): Chloride – sulphate ratio was calculated for 49 samples with TDS less than 1500 mg/L and was observed in the range (minimum-maximum) of 0.32 to 95.2 with an average value of 10.86. The standard deviation of the data set was calculated 14.2. The results support the Piper diagram that chloride is dominant as compared to sulphate ions.
Chloride – bicarbonate ratio (Cl/CO3): Chloride to bicarbonate ratio was calculated for 49 samples and was found in the range (minimum – maximum) of 0.11 to 5.1 with an average value of 1.2. The standard deviation of the results was calculated to 0.96. The results support the Piper diagram (Figure 9) that the bicarbonate ions are second dominant anions after chloride in the groundwater system of the area.
Conclusion
It is observed that most of the inhabitants of district Tharparkar are using groundwater for human consumption, based on dug wells. The dug wells are within sand dunes region, which varies in depth between 15 to 93 m. The groundwater mostly in the region is recharged by seasonal rainfall in monsoon (June – September).
It may be roughly concluded that 57.5 populations of Talukas mainly Mithi, Chachro, and Diplo of district Tharparkar are consuming groundwater with TDS greater than 3000 mg/L, which is much higher than permissible limit of WHO for human consumption and may be one of the reason for the poor health of the inhabitants. Similarly 14.7% population of the areas are exposed to relatively less harmful as a source of drinking water with TDS within 1500 – 3000 mg/L. The population 27.1 are consuming groundwater with TDS less than 1500 mg/L, which is within permissible limit of WHO under stress conditions. The groundwater samples 27.1% which are considered relatively safe for human consumption are contaminated up to 17% with arsenic, fluoride and other cations and anions above the permissible limits of WHO.
The accuracy of the analysis was checked by cations – anions balance (meq/L) for 44 representative samples and relative deviation among the results was obtained with an average value within acceptable limit of 5.6%.
The results of analysis indicate the presence of cations and anion on the average in groundwater in the following decreasing order: Na>Ca>Mg>K, and Cl>CO3>SO4.
Some of the samples with TDS less than 1500 mg/L were examined for their suitability for irrigation and the results indicated that 77.6 – 96.0% samples were within safe limit for their use for irrigation.
References
- Sener S, Sener E, Davraz A (2017) Assessment of groundwater quality and health risk in drinking water basin using GIS. J Water Health 15: 112-132.
- Islam MA, Rahman MM, Bodrud-Doza M, Muhib MI, Shammi M, et al. (2018) A study of groundwater irrigation water quality in south-central Bangladesh: A geo-statistical model approach using GIS and multivariate statistics. Acta Geochimica 37: 193-214.
- Fassett JE, Durrani NA (1994) Geology and coal resources of the Thar coal field, Sindh Province, Pakistan, U.S. Geological Survey, Virginia, USA.
- Boyd JT (1994) Preliminary assessment of the lignite resources and surface mining potential, Thar Coal Deposit, Thar Parkar District, Sindh Province, Pakistan. Mining and Geological Engineers, Pittsburgh, Pennsylvania, USA.
- Zaigham NA, Ahmed MA (1996) Thar rift and its impact on the coal bearing horizons in the Tharparkar region of southeastern Pakistan.
- Zaigham NA, Ahmed MA (2000) Experimental resistivity scanning approach to delineate coal zones and the basement in Thar desert of Sindh, Pakistan. ACTA Mineralogica Pakistanica11: 129-136.
- Kazmi AH (1985) Geology of the Thar desert, Pakistan. ACTA Mineralogica Pakistanica1: 64-68.
- Ploethner (1992) Groundwater investigations in desert areas of Pakistan.Federal Institute for Geosciences and Natural Resources (BGR) 2: 84-135.
- Danishwar S, Shah MT, Leghari A (1995) Status of drinking water quality in western part of the Thar desert, Sindh, Pakistan. Geol Bull Univ 28: 39-47.
- Geyh MA, Ploethner D (1995) An applied palaeohydrological study in Cholistan, Thar Desert, Pakistan. Application of Tracers in Arid Zone Hydrology 232.
- Rafique T, Naseem S, Bhanger MI, Usmani TH (2008) Fluoride ion contamination in the groundwater of Mithi sub-district, the Thar Desert, Pakistan. Environ Geol56: 317-326.
- Husain V, Nizam H, Arain GM (2012) Arsenic and fluoride mobilization mechanism in groundwater of Indus Delta and Thar Desert, Sindh, Pakistan. Int J Econ Env Geol 3: 15-23.
- Brahman KD, Kazi TG, Afridi HI, Naseem S, Arain SS, et al. (2013) Evaluation of high levels of fluoride, arsenic species and other physicochemical parameters in underground water of two sub districts of Tharparkar, Pakistan: A multivariate study. Water Res47: 1005-1020.
- Rafique T, Naseem S, Usmani TH, Bashir E, Khan FA, et al. (2009) Geochemical factors controlling the occurrence of high fluoride groundwater in the Nagar Parkar area, Sindh, Pakistan. J Hazard Mater171: 424-430.
- Jareda G, Mahapatra SP, Dhekne PY (2018) Water quality index, heavy metal pollution index and seasonal variation correlation of groundwater of bailadila iron ore mine area and its peripherals: Dantewada district, Chhattisgarh, India. Desalin Water Treat 101: 7-16.
- Dinka MO (2016) Quality composition and irrigation suitability of various surface water and groundwater sources at Matahara Plain. Water Resour 43: 677-689.
- Esmaeili-Vardanjani M, Rasa I, Amiri V, Yazdi M, Pazand K (2015) Evaluation of groundwater quality and assessment of scaling potential and corrosiveness of water samples in Kadkan aquifer, Khorasan-e-Razavi Province, Iran. Environ Monit Assess 187: 18.
- Varol S, Davraz A (2014) Evaluation of the groundwater quality with WQI (Water Quality Index) and multivariate analysis: a case study of the Tefenni plain (Burdur/Turkey). Environ Earth Sci 73: 1725-1744.
- Naseem S, McArthur JM (2018) Arsenic and other water-quality issues affecting groundwater, Indus alluvial plain, Pakistan. Hydrol Process 32: 1235-1253.
- Khan A, Qureshi FR (2018) Groundwater quality assessment through Water Quality Index (WQI) in New Karachi Town, Karachi, Pakistan. Asian Journal of Water Environment and Pollution 15: 41-46.
- Naseem S, Bashir E, Ahmed P, Rafique T, Hamza S, et al. (2018) Impact of seawater intrusion on the geochemistry of groundwater of gwadar district, balochistan and its appraisal for drinking water quality. Arab J Sci Eng 43: 281-293.
- Rehman MU, Zaigham NA, Nizamani MA, Ahmad M, Huda QU (1993) Geophysical logging for coal exploration in Tharparkar, Sind, Pakistan.
- Qadri SMA (1993) Thar desert: situation paper, The Council.
- Zaigham NA (2002) Strategic sustainable development of groundwater in Thar Desert of Pakistan. Science Vision7: 61-74.
- Schildknecht F (1991) Geoelectrical investigations in Tharparkar. Federal Institute for Geosciences and Natural Resources (BGR)19.
- Mir M, Naseem S (2005) Thar coal: geology and its potential for power generation. Mafraq, Jordan.
- Jaleel A, Alam GS, Hussain MT (2002) Coal resources of four blocks in Thar coalfield, Sindh, Pakistan. Geol Surv Pak Recs115: 74.
- Abbas G, Atique M (2005) A brief on coal deposits of Sindh, Pakistan. Geological Survey of Pakistan27.
- Gong Z, Lu X, Ma M, Watt C, Le X (2002) Arsenic speciation analysis. Talanta58: 77-96.
- American Water Works Association-Water Environment Federation (1998) Standard methods for the examination of water and wastewater. American Public Health Association (APHA), American Water Works Association (AWWA) and Water Environment Federation (WEF).
- Kazi T, Arain M, Jamali M, Jalbani N, Afridi H, et al. (2009) Assessment of water quality of polluted lake using multivariate statistical techniques: A case study. Ecotox Environ Safe72: 301-309.
- Kazi TG, Katz SA, Jenniss SW (1987) Spectrophotometry determination of nitrate nitrogen and nitrite nitrogen in sewage sludges. Spectrosc Lett20: 509-517.
- Smith AE (1973) A study of the variation with pH of the solubility and stability of some metal ions at low concentrations in aqueous solutions. Part II Analyst 98: 209-212.
- Alam M, Rais S, Aslam M (2012) Hydro chemical investigation and quality assessment of ground water in rural areas of Delhi, India. Environ Earth Sci 66: 97-110.
- Davis SN, Wiest De (1966) Hydrogeology. Wiley, New York, USA.
- Freeze RA, Cherry JA (1979) Groundwater. Prentice –Hall, New Jersey, USA.
- Schilling KE, Walter CF (2007) A GIS-based groundwater travel time model to evaluate stream nitrate concentration reduction from land use change. Environ Geol 53: 433-443.
- Raju NJ, Ram P, Dey S (2009) Groundwater quality in the lower Varuna River basin, Varanasi district, Uttar Pradesh, India. Journal of Geological Society of India 73: 178-192.
- Gilli G, Carro G, Ravioli S (1984) Concentration of nitrate in drinking water and incidence of carcinomas. First descriptive study of the Piemonate Region, Italy. Sci Total Environ 34: 35-37.
- Jahed KGR, Dehghani MH, Mahvi AH, Rafati L, Tavanafar E (2008) Concentration of nitrate and nitrite in groundwater resources of Hamdan Province, Iran. Res J Chem Environ 12: 56-58.
- Milton H, Hassan Z, Shahidullah SM, Sharmin S, Jakariya MD, et al. (2004) Association between nutritional status and arsenicosis due to chronic arsenic exposure in Bangladesh. Int J Environ Heal R 14: 99-108.
- Fazal MA, Ichion E (2001) Extent and severity of groundwater arsenic contamination in Bangladesh. Water Int 26: 370-379.
- Mandal BK, Suzuki KT (2002) Arsenic round the world, a review. Talanta 58: 201-235.
- Ayoob S, Gupta AK (2006) Fluoride in drinking water a review on the status and stress effects. Crit Rev Env Sci Tec 36: 433-487.
- Naseem S, Rafique T, Bashir E, Bhanger MI, Leghari A, et al. (2010) Lithological influences on occurrence of high – fluoride groundwater in Nagar Parkar area, Thar Desert, Pakistan. Chemosphere 78: 1313-1321.
- Guo Q, Wang Y, Ma T, Ma R (2007) Geochemical processes controlling the elevated fluoride concentrations in groundwater of the Taiyuan Basin, Northern China. J Geochem Explor 93: 1-12.
- Missailididis S, Anastassopoulou J, Polissiou M, Tarantilis P (1992) Theophanides the metal ions in biology and medicine. Jhon Eurotext Pairis 351-362.
- Wilcox LV (1955) Classification and use of irrigation water. United States Department of Agriculture, Circular, Washington, USA.
- Danielsson A, Cato I, Carman R, Rahm L (1999) Spatial clustering of metals in the sediments of the Skagerrak/Kattegat. Applied Geochemistry 14: 689-706.
- Simeonova P, Simeonov V, Andreev G (2003) Environmetric analysis of the Struma river water quality. Cent Eur J Chem 2: 121-126.
- Ophori DU, Goth J (1989) Patterns of groundwater chemistry, Ross Creek basin, Alberta, Canada. Ground Water 27: 20-26.
- Jankowski J, Acworth RI (1997) Impact of depris-flow deposits on hydrogeochemical processes and the development of dry land salinity in the Yass River catchment, New South Wales. Aust Hydrogeology J 5: 71-88.
- Rao NS (2006) Seasonal variation of groundwater quality in a part of Guntur district, Andhra Pradesh, India. Environ Geol 49: 413-429.
- Kelly WP (1940) Permissible composition and concentration of irrigated waters. In: Proceeding of the ASCF 66: 607.
- Haritash K, Gaur S, Gary S (2016) Assessment of water quality and suitability analysis of river Ganga in Rishikesh, India. Appl Water Sci 6: 383-392.
- Schoeller H (1977) Geochemistry of groundwater. In: Groundwater studies an international guide for research and practice. UNESCO, Paris, France.
- Rizvi Y, Ahsan SN, Ali SP, Khan MD (2002) Preliminary petrological studies of basement rocks, Thar coal basin and its comparison with Nagar Parkar massif complex, Thar Parkar District, Sindh, Pakistan.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi