Case Report, Clin Oncol Case Rep Vol: 5 Issue: 3
Brain Metastasis of Primary Dedifferentiated Liposarcoma. A Case Report and Review of Literature
Emmanuel KE*, Lawal HO, Okponobi S, Sangha L, and Cinicola JT
*Corresponding Author:
Emmanuel KE
Resident Physician, UPMC, USA
E-mail: emmanuelek@upmc.edu
Received: March 09, 2022, Manuscript No: COCR-22-56583;
Editor assigned: March 12, 2022, PreQC No: P-56583;
Reviewed: March 29, 2022; QC No: Q-56583;
Revised: March 31, 2022, Manuscript No: R-56583;
Published: April 03, 2022, DOI: 10.4172/cocr.5(3).223
Citation: Emmanuel KE, Lawal HO, Okponobi S, Sangha L, and Cinicola JT (2022) Brain Metastasis of Primary Dedifferentiated Liposarcoma. A Case Report and Review of Literature. Clin Oncol Case Rep 5:3
Abstract
Liposarcomas are rare tumors representing less than 1% of all malignancies. It is the second most common soft tissue malignancy diagnosed in adults. It is divided into various groups based on histological differences including well differentiated liposarcoma (WDL), dedifferentiated liposarcoma (DDL), myxoid liposarcoma and polymorphic liposarcoma.
This case focuses on dedifferentiated type (DDL) which is described as WDL with abrupt transition into high grade, non-lipogenic tumor. DDLs are known to mostly occur within the retroperitoneum and have a low rate of metastasis. The lungs are the most common site of metastasis while brain metastasis is rare.
Due to the rarity of this case, a literature review was done which revealed five similar cases showing brain metastasis. Primary sites for these metastases ranged from extremities including digit to the heart. The cases reviewed featured men above 50 years of age however age ranged from 17 to 63 years old. There was notable variability in the presenting neurological symptoms based on the site of the distant metastasis and brain metastasis was noted to be a poor prognostic factor.
It is therefore imperative to have a high index of suspicion for metastasis in DDL and more research is needed to understand the pattern of metastasis to better guide management and ensure early intervention.
Keywords: Liposarcoma; Dedifferentiated liposarcoma; Brain metastasis; Chemotherapy; Radiation
Introduction
Liposarcoma is a rare cancer of the soft tissue and represents less than 1% of all neoplasms [1]. It is second to malignant fibrous histocytiocytoma as the most diagnosed soft tissue sarcoma in adults, and accounts for 15%-25% of all soft tissue sarcomas [2]. There are different variants of liposarcoma, including well-differentiated (low grade), dedifferentiated, myxoid, and pleomorphic. The well-differentiated and dedifferentiated variants are the most common variants. Although they share morphological and cytogenetic similarities, they exhibit significantly different biological behavior [3]. Dedifferentiated Liposarcomas [DDLS] are high grade, non-lipogenic sarcomas that may either arise de-novo or in a background of pre-existing well-differentiated liposarcoma [4]. They are histologically pleomorphic sarcomas with frequent occurrence within the retroperitoneum. Genetic aberrancy is the bedrock in the pathogenesis of all liposarcomas, Having a previous history of cancer is also a risk factor as dedifferentiated liposarcoma co-occurs with other types of tumors [5-6]. Imaging with either computed tomography or magnetic resonance imaging plays a crucial role in the diagnosis of liposarcomas and is often the first line in the diagnostic spectrum [7-9]. However, histopathology remains the gold standard in making a final diagnosis [10].
Although fatal, with a high rate of local recurrence, the incidence of metastasis of dedifferentiated liposarcoma is low when compared to other pleomorphic sarcomas and varies widely from 1%-18% [11]. The mechanism of metastasis of dedifferentiated liposarcoma is not well understood, although hematogenous dissemination and contiguous spread have been the suggested mechanisms of distant metastasis [12]. In the event of metastasis, the lung has been reported as the most obvious site [13]. On the other hand, brain metastasis is extremely rare; to our knowledge, there have been only five reported cases of dedifferentiated liposarcoma with brain metastasis. A report by Shweikeh and colleagues described nine cases of liposarcoma with metastasis to the brain, however the liposarcoma sub-type was not specified [14]. This report describes the case of 60-year-old male who was diagnosed with brain metastasis of dedifferentiated liposarcoma. The primary tumor was a dedifferentiated liposarcoma of the retroperitoneum which had been resected three years prior to his presentation. We also review reports of similar cases, with the aim of describing the biological characteristics of liposarcomas, and the diagnosis, management, and prognosis of dedifferentiated liposarcomas with metastasis to the brain.
Background
Liposarcomas are groups of mesenchymal tumors with variable expression ranging from indolent to aggressive rapidly fatal tumors. They are the second most common soft-tissue malignancy in adults, comprising 10-20% of soft-tissue sarcomas and less than 1% of all malignancies [15]. Although Liposarcomas can occur at any site, they are most commonly seen in deep seated fascial planes of the retroperitoneum,extremities and spermatic cord. They make up about 24% and 45% of extremity and retroperitoneal soft tissue sarcomas respectively [16]. The biological behavior of liposarcomas varies due to their unique genetics and histologic heterogenicity [17]. According to the World Health Organization classification of tumours of soft tissue and bone, [18] there are four main liposarcoma subtypes that are characterized by distinct morphologies and unique genetic findings. The subtypes are atypical lipomatous tumor/Well Differentiated Liposarcoma (WDLS) including adipocytic or lipoma-like, sclerosing inflammatory and spindle cell variant dedifferentiated liposarcoma, myxoid liposarcoma and pleomorphic liposarcoma [18]. Dedifferentiated liposarcoma and pleomorphic liposarcoma are high grade aggressive tumors with metastatic potential while well differentiated and myxoid liposarcoma are low grade tumors that follow a more indolent clinical course. However, a subset of myxoid liposarcoma with >5% hypercellularity are classified as high grade tumors that are extremely aggressive. Well differentiated liposarcoma and dedifferentiated liposarcoma occur mostly in the retroperitoneum while primary myxoid liposarcoma are almost never found in the retroperitoneum but occur most often in the lower limbs [19].
Well differentiated liposarcoma and dedifferentiated liposarcoma represent the most common biological groups of liposarcomas; both groups are known to have an amplification of chromosome 12q13-15, which contain oncogenes MDM2, HMGA2 and CDK4 [20-21]. Co-amplification of MDM2 and CDK4 is a known feature of WDLS, and is also the initiating factor in fat tumorigenesis. This results in proliferation through combined effects on P53 (by inactivating TP53) and the cell cycle (by RB1 phosphorylation) respectively. Similar to well differentiated liposarcoma, dedifferentiated liposarcoma is characterized by the presence of supernumerary ring and/or giant rod chromosomes containing amplified segments from the 12q13-15 regions [22]. This chromosomal region reportedly contains several oncogenes including MDM2, CDK4, HMGA2, TSPAN31 (SAS) YEATS4, miR-26a-2, CPM, OS1, OS9, CHOP and GLI1 [20-22].
The term ‘de-differentiation was first used in 1971 by Dahlan and Beabout to characterize the morphological progression of low-grade tumor to a less differentiated neoplasm with a more aggressive behavior [23]. The term was then used to characterize a liposarcoma for the first time by H.L Evans in 1979. He described a liposarcoma containing a well differentiated component juxtaposed to areas of high-grade nonlipogenic sarcomathat occured from the well-differentiated liposarcoma after several years [24]. Dedifferentiated liposarcoma it consists of a combination of atypical lipomatous tumor components and cellular non lipogenic sarcomatous areas that have significant mitotic activity [6,17,25]. There have been other descriptions of dedifferentiated liposarcoma which focused on its origin, characteristics, and behavior. It has been described as a well-differentiated liposarcoma with rapid and sudden transition to high grade, non-lipogenic sarcoma, either in a primary or recurrent well differentiated liposarcoma or [3] Other authors have characterized it as the coexistence of well-differentiated and poorly differentiated areas in the same tumor.
Most dedifferentiated liposarcomas arise de novo (90%), with a few arising as secondary tumors from previously diagnosed well differentiated liposarcoma [26]. It is still unclear whether dedifferentiated liposarcoma and well differentiated liposarcoma originate from two different cellular clones or whether there is a process of progressive evolution from one to the other. However, it is known that while well differentiated liposarcoma is a locally aggressive non-metastasizing cancer composed of mature adipocytes while dedifferentiated liposarcoma represents a more aggressive local disease with significant metastatic potential. It is essential to understand that Dedifferentiated Liposarcoma is a more aggressive neoplasm compared to well differentiated liposarcoma. On the other hand, it is typically a much less aggressive tumor when compared to other types of high-grade pleomorphic sarcoma [27-28].
There has been limited research in predictive risk of metastasis. One recent study evaluated the predictors of outcome in patients with primary, localized, retroperitoneal dedifferentiated liposarcoma after surgery. It was seen that metastases occurred in 30% of patients, with lung, liver, and bone as the most frequent sites of metastases [29]. Although there have been reported cases of metastasis to the retroperitoneum and bone, the lung is the organ that is most commonly involved. Metastasis to the central nervous system has been rarely reported. Liposarcoma with metastasis to the brain is more likely to occur with increased recurrence of the primary tumor. Recurrence and distant spread are related to histologic type, grade, location of the primary tumor, and completeness of surgical excision. [30] Local recurrence rates from 57% to 78% [6,18,31] are seen with liposarcomas from all locations. Histology of the liposarcoma is an important factor affecting metastasis, with myxoid and well-differentiated varieties demonstrating a lesser tendency to recur [31-33]. Hematogenous spread is seen with sarcomas, and this is the proposed method of spread to the central nervous system as well. Surgical excision with wide margins, with or without chemoradiation, is the standard of care for retroperitoneal liposarcomas [33].
Case Presentation
Our patient is a 60-year-old Caucasian male with a medical history of coronary artery disease and retroperitoneal sarcoma who was brought to the hospital after a syncopal episode. He was driving a forklift at his workplace when he had sudden onset nausea and generalized body weakness. He fell to the ground and did not remember anything after the fall. His coworkers called the ambulance and he was brought to the hospital. The patient had no previous history of seizures, cerebrovascular accident, or syncope. His surgical history is significant for a left nephrectomy three years prior to his retroperitoneal liposarcoma. His home medication includes aspirin 81mg daily for secondary prevention of cardiovascular disease.
On presentation, vitals were temperature 36.8-degree Celsius, and blood pressure 126/72 mmHg. His heart rate was 80 bpm, respiratory rate was 16 cycles per minute, and his SpO2 was 94% on room air. His physical examination was remarkable for 4/5 strength on his right hand and a slight left pronator drift; he had no other neurological deficits.
While he was being examined in the emergency room, the patient had an episode of witnessed seizure for which he received one dose of 4mg lorazepam, which terminated the seizure. He was hemodynamically stable at the time. Laboratory data on presentation showed: white blood cell count of 5.4 k/Ul, hemoglobin 14.5 g/Dl, hematocrit 42.9%, platelets 133 k/Ul, red blood cell counts 4.39 M/Ul, sodium 139 mmol/L, potassium 4.4 mmol/L, chloride 104 mmol/L, bicarbonate 24 mmol/L, blood urea nitrogen 25 mg/dl, creatinine 1.13 mg/dl, EGFR 71 ML/MIN/1.73 SQM, calcium 8.8 mg/dl, and magnesium 1.8 mg/dl.
The patient had an additonal episode of generalized tonic clonic senure while he was in the CT room for a computed tomography of the head without contrast. He received another 4 mg dose of lorazepam followed by a loading dose of levetiracetam to abort the seizure. His seizure was successfully aborted and a scheduled dose of levetiracetam 500 mg every twelve hours was started for seizure prophylaxis. His CT of the head showed a large right frontal lobe intra-axial mass with associated vasogenic edema and subfalcine herniation (Figure 1).
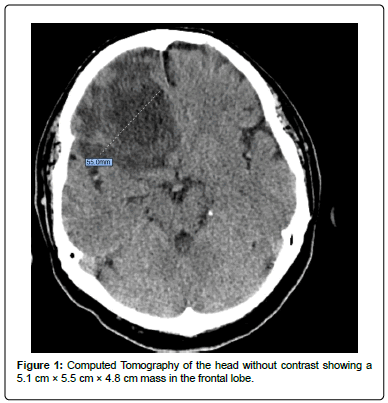
Figure 1: Computed Tomography of the head without contrast showing a 5.1 cm x 5.5 cm x 4.8 cm mass in the frontal lobe.
A computed tomography of the chest was done which was negative for any masses or abnormlitied (Figure 2). The patient was then admitted to the hospital. Levetiracetam 500 mg every 12 hrs was continued for seizure prevention, and dexamethasone was started for reduction of the intracranial vasogenic edema.
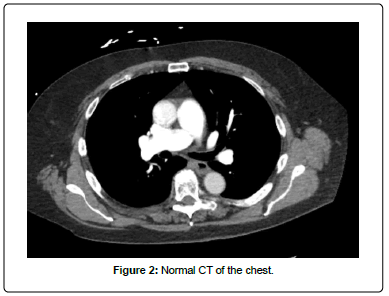
Figure 2: Normal CT of the chest.
Shortly after admission, the patient was evaluated by both a neurologist and a neurosurgeon, and an MRI was obtained for further evaluation (Figure 3).
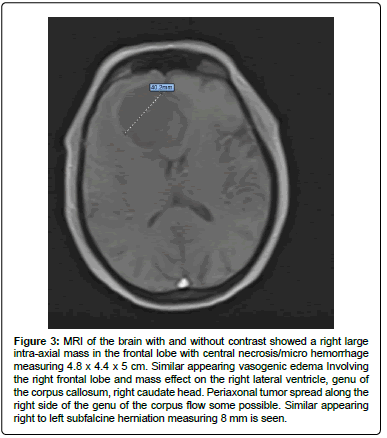
Figure 3: MRI of the brain with and without contrast showed a right large intra-axial mass in the frontal lobe with central necrosis/micro hemorrhage measuring 4.8 x 4.4 x 5 cm. Similar appearing vasogenic edema Involving the right frontal lobe and mass effect on the right lateral ventricle, genu of the corpus callosum, right caudate head. Periaxonal tumor spread along the right side of the genu of the corpus flow some possible. Similar appearing right to left subfalcine herniation measuring 8 mm is seen.
The patient remained hemodynamically stable and seizure free. On hospital day 3, he had a successful craniotomy with mass evacuation. He had an uneventful post-operative course, initially in the intensive care unit followed by a downgrade to the general medicine floor. His only post-operative complaint was headache and pain at the site of incision. Both complaints were adequately managed with analgesics.
An evacuated tissue sample measuring 6 cm × 5.5 cm × 2.5 cm in aggregate was sent to pathology, which revealed a dedifferentiated liposarcoma (Figure 4). The case and samples were reviewed by a second pathologist, with concurrence of the diagnosis.

Figure 4: Immunohistochemical stains show spindle cell tumor with cellular atypia, areas of necrosis and increased mitotic activity. In between some of the spindle cells, adipose tissue is seen. The tumor cells stain negative for cytokeratin AE1/AE3, S-100, SOX10, glial fibrillary acidic protein, EMA, smooth muscle actin, desmin, PAX8, CD117. The tumor cells stain positive for vimentin, BCL-2, and CD34.
He was discharged on hospital day twelve to an acute rehabilitation facility. His discharge instructions included follow up appointments with the neurosurgeon and the oncologist.
Literature Review
We searched Google, Google Scholar, and PubMed using the terms ‘liposarcoma,’ ‘softtissue tumors,’ ‘dedifferentiated liposarcoma,’ ‘metastatic spread of dedifferentiated liposarcoma,’ and ‘brain metastasis of dedifferentiated liposarcoma.’ Relevant articles describing case reports and clinical references were selected and reference lists from those articles were reviewed.
Articles that reported liposarcoma but did not specify de differentiated liposarcoma subtype were not included. Articles that reported dedifferentiated liposarcoma but did not have a direct spread to the brain were excluded. Articles whose primary tumor was in the peripheral nervous system were also excluded.
A total of five cases of primary dedifferentiated liposarcoma with metastasis to the brain or dedifferentiation liposarcoma at the site of metastasisthat was characterized as secondary dedifferentiation were identified. Two cases had metastasis to the brain alone, one case had metastasis to the brain and lung, one case had metastasis to the brain and the orbit, one case had metastasis to the brain, sub mandible and the skin.
Of the five cases, only one had a primary re-occurrence at the time of metastasis while the other four had no reoccurrence of the tumor at the primary site (Table 1).
Patient characteristics
The age range for these patients was 17 to 63 years old, with 75% of the cases being in patients older than 50. There was a predominance of males, who represented 75% of the reported cases. Patients had varying medical histories ranging from no medical history as seen in one case to a history of diabetes, hypertension and congestive heart failure which was reported in the other four cases combined.
Presenting symptoms
In all reviewed cases, the neurological symptoms at the time of diagnosis depended on the location of the distant metastasis of the primary tumor. Sung et al, reported a case presenting with left eye drooping and swelling for a primary tumor which had been in the thigh [33]. The case reported by Balasubramanyam, et al had cough as the primary presenting symptom for a primary thigh tumor which had metastasized to the lungs [34]. Left lower extremity weakness was reported by Bailey for dedifferentiated liposarcoma of the digit which had spread to the brain [15]. Multiple cutaneous lesions were reported by Ben Salha; in this case, brain metastasis was an incidental finding [17]. The last case was brain metastasis that was diagnosed late in the course of the disease, the patient was diagnosed with primary cardiac tumor which had metastasized to the brain after she presented with shortness of breath [35]. The most common presenting symptoms for the primary tumors are abdominal pain and fullness. This is because the majority of the DDLS occur primarily in the retroperitoneum. This was seen in the cases presented by Ben Salha et al. [17]. Primary tumors located outside the abdominal cavity showed variable presenting symptoms that ranged shortness of breath to painless extremity masses.
Management
Management of intracranial metastasis was similar in these cases: craniotomy for mass resection using the gamma knife stereotactic radiosurgery, [36] followed by chemotherapy, chemoradiation and/or whole brain radiation. The role of both neoadjuvant and adjuvant chemotherapy in the management of DDLS, especially as it pertains to survival rate, is still debated. Despite this, most patients with DDLS receive either chemotherapy, radiation therapy, or both after tumor resection. This practice is supported by the fact that although chemoradiation may have no proven survival benefit, it can improve local control [37-38] Soft tissue sarcomas have variable response to chemotherapy agents. The degree of response is determined by the histological type. De-differentiated liposarcoma has a poor response to systemic chemotherapy and so far, the use of chemotherapy is mostly for palliative purposes in this patient population [39].
Prognosis
Dedifferentiated liposarcomas show a characteristic pattern of differentiation by the time they are diagnosed, typically resembling malignant histiocytoma. The degree of differentiation is an important prognostic factor. Brain metastasis is a marker of poor prognosis despite advances in cancer care. From the review of reported cases, location of the primary tumor was the most important prognostic factor. Retroperitoneal tumors had a significantly worse survival than tumors found in other sites because they had a comparatively early metastasis. Despite its typically high-grade morphology and metastatic tendency, the high mortality of dedifferentiated liposarcoma is often related to uncontrolled local reoccurrences rather than metastatic spread [6]. Local recurrence is most often seen in tumors located deep into the retroperitoneum. Additonally, recurrence can be attributed to the incomplete excision of the primary tumor due to its location.
In the five cases identified, patients died from the complications of the tumor spread. The average length of time from diagnosis to death was between 1-3 years. In our case, the patient has been alive for three months since his diagnosis of metastatic brain DDLS. He is currently continuing his treatment with the oncology team.
Discussion
Metastatic brain tumors are not uncommon; as a result, they are seen most commonly seen with lung and breast cancers. On the other hand, bone and soft tissue tumors account for only a small percentage of them [2]. Dedifferentiated Liposarcoma can occur anywhere in the body however, the most common site of occurrence is in the retroperitoneum. It is usually seen in patients in their 60’s and 70’s. Unlike other biological groups of liposarcoma, Dedifferentiated Liposarcoma is a high-grade tumor with a much worse prognosis because of its propensity to metastasize. According to the literature, dedifferentiated liposarcoma metastases account for 12.5% of all soft tissue metastases and occur most commonly in the retroperitoneum. The time from diagnosing a primary tumor to the time of metastasis is usually three to five years, with an average of 3.3 years [14]. Metastasis can occur with or without resection of the primary tumor. To the best of our knowledge, this is the sixth reported case of dedifferentiated liposarcoma metastasizing to the brain. Our patient had metastasis to the right frontal lobe, which is the second most common site of intracranial spread. When comparing the specific region of the brain to which dedifferentiated liposarcoma spreads to, the most common site is the temporo-parietal region which accounts for 44% of the region for brain metastasis. The frontal region accounts for 22%, parafalcine and the skull base both accounts for 11% each and the rest of the brain accounts for the remaining 12% [14,39]. The lung is the most common area of metastasis; hence, it is important to obtain a chest computed tomography in all patients with dedifferentiated liposarcoma. The role of surveillance with interval computed tomography of the lungs for early detection of metastases is debated and poses an area of further research.
The management of metastatic dedifferentiated liposarcoma to the brain consists of craniotomy with mass resection with or without chemo-radiation. Our patient had craniotomy with mass resection. In some cases, patients may receive neo-adjuvant chemotherapy for tumor shrinkage. Our patient received systemic steroids to reduce vasogenic edema. Craniotomy for tumor resection is a common procedure which is highly tolerated in all patient populations. In the literature review, there were no reported major complications with this procedure. In the reported cases, patients had adjuvant chemotherapy with or without radiation for local control. There have been some reports on successful disease control with chemotherapy [39]. The patient in our case is still alive and undergoing chemotherapy; however, survival forecast following brain metastasis remains uncertain. Prognosis depends on several factors. Tumor size and anatomic location of the primary tumor are crucial factors because they affect resectability. Complete surgical resection with wide tumor free marginsis important to reduce the recurrence at the site of primary tumor and metastasis rate. However, for tumors that are very bulky and located deep in the retroperitoneum, this is often difficult to achieve.
Conclusion
Dedifferentiated liposarcoma to the brain is exceedingly rare and can be a challenging diagnosis. Brain metastasis occurs late in the disease and is a marker of overall poor prognosis. Treatment options such as surgical resection, chemotherapy, brain radiation should be offered to patients who are assessed to have good preoperative functional
status and minimal comorbidities. Despite the advances in medicine, there is poor prognosis for this disease process. More research is needed in the areas of surveillance, early detection of metastasis and more therapeutic options in this patient population.
References
- Liles JS, Tzeng CW, Short JJ, Kulesza P, Heslin MJ (2009) Retroperitoneal and intraabdominal sarcoma. Curr Probl Surg 46: 445-503.
Google Scholar Cross Ref - Arepally G, Kenyon LC, Lavi E (1996) Late onset of isolated central nervous system metastasis of liposarcoma-A case report. Am J Clin Oncol 19: 351-355.
Google Scholar Cross Ref - Nascimento AG (2001) Dedifferentiated liposarcoma. Semin Diagn Pathol 18: 263-266.
Google Scholar - Huang HY, Brennan MF, Singer S, Antonescu CR (2005) Distant metastasis in retroperitoneal dedifferentiated liposarcoma is rare and rapidly fatal: A clinicopathological study with emphasis on the low-grade myxofibrosarcoma-like pattern as an early sign of dedifferentiation. Mod Pathol 18: 976-984.
Google Scholar Cross Ref - Garber JE, Offit K (2005) Hereditary cancer predisposition syndromes. J Clin Oncol 23: 276-292.
Google Scholar Cross Ref - Matthyssens LE, Creytens D, Ceelen WP (2015) Retroperitoneal liposarcoma: Current insights in diagnosis and treatment. Front Surg 2: 4.
Google Scholar Cross Ref - Zhu Z, Zhao XM, Zhao YF, Yang L, Zhao J, et al. (2014) Evaluation of CT findings for the differentiation of benign from malignant primary retroperitoneal tumors. Chin Med J 127: 114-119.
Google Scholar Cross Ref - ESMO (2012) Soft tissue and visceral sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 23: 892-910.
Google Scholar Cross Ref - Thomas JM (2007) Retroperitoneal sarcoma. Br J Surg 94: 1057-1810.
Google Scholar Cross Ref - Mastrangelo G, Coindre JM, Ducimetière F, Dei TAP, Fadda E, et al. (2012) Incidence of soft tissue sarcoma and beyond. Cancer 118: 5339-5348.
Google Scholar Cross Ref - Ghadimi MP, Al-Zaid T, Madewel J (2011) Diagnosis, management, and outcome of patients with dedifferentiated liposarcoma systemic metastasis. Ann Surg Oncol 18: 3762-3770.
Google Scholar Cross Ref - Postovsky S, Ash IN, Ramu (2003) Central nervous system involvement in children with sarcoma. Oncology 65: 118-124.
Google Scholar Cross Ref - Huang HY, Brennan MF, Singer S, Antonescu CR (2005) Distant metastasis in retroperitoneal dedifferentiated liposarcoma is rare and rapidly fatal: A clinicopathological study with emphasis on the low-grade myxofibrosarcoma-like pattern as an early sign of dedifferentiation. Mod Pathol 18: 976-984.
Google Scholar Cross Ref - Faris S, Laura B, Kashif S, Reem S, Aarushi S, et al. (2014) Brain metastasis in bone and soft tissue cancers: A review of incidence, interventions, and outcomes. Sarcoma 2014: 475175.
Google Scholar Cross Ref - Bailey SC, Bailey B, Smith NT, Van Tassel P, Thomas Jr, et al. (2001) Brain Metastasis from a primary liposarcoma of the digit. American J Clin Oncol 24: 81-84.
Google Scholar - O'Regan KN, Jagannathan J, Krajewski K (2011) Imaging of liposarcoma: Classification, patterns of tumor recurrence, and response to treatment. AJR Am J Roentgenol 197: W37-W43.
Google Scholar Cross Ref - Ben Salha I, Zaidi S, Noujaim J (2016) Rare aggressive behavior of mdm2-amplified retroperitoneal dedifferentiated liposarcoma, with brain, lung and subcutaneous metastases. Rare Tumors 8: 6282.
Google Scholar Cross Ref - Henricks WH, Chu YC, Goldblum JR, Weiss SW (1997) Dedifferentiated liposarcoma: a clinicopathological analysis of 155 cases with a proposal for an expanded definition of dedifferentiation. Am J Surg Pathol 21: 271-281.
Google Scholar - Tap WD, Eilber FC, Ginther C, Dry SM, Reese N, et al. (2011) Evaluation of well-differentiated/de-differentiated liposarcomas by high-resolution oligonucleotide array-based comparative genomic hybridization. Genes Chromosomes Cancer 50: 95-112.
Google Scholar Cross Ref - Italiano A, Bianchini L, Gjernes E, Keslair F, Ranchere-Vince D, et al. (2009) Clinical and biological significance of CDK4 amplification in well-differentiated and dedifferentiated liposarcomas. Clin Cancer Res 15: 5696-5703.
Google Scholar Cross Ref - Louis-Brennetot C, Coindre JM, Ferreira C, Perot G, Terrier P, et al. (2011) The CDKN2A/CDKN2B/CDK4/CCND1 pathway is pivotal in well-differentiated and dedifferentiated liposarcoma oncogenesis: An analysis of 104 tumors. Genes Chrom Can 5: 896-907.
Google Scholar Cross Ref - Lee SE, Kim YJ, Kwon MJ, Choi DI, Lee J, et al. (2014) High level of CDK4 amplification is a poor prognostic factor in well-differentiated and dedifferentiated liposarcoma. Histol Histopathol 29: 127-138.
Google Scholar Cross Ref - Dahlin DC, Beabout JW (1971) Dedifferentiation of low-grade chondrosarcomas. Cancer 28: 461-466.
Google Scholar Cross Ref - Evans HL (1979) Liposarcoma: A study of 55 cases with a reassessment of its classification. Am J Surg Pathol 3: 507-523.
Google Scholar - Coindre JM, Pédeutour F, Aurias A (2010) Well-differentiated and dedifferentiated liposarcomas. Virchows Archiv 456: 167-179.
Google Scholar Cross Ref - Goldblum JR (2014) An approach to pleomorphic sarcomas: Can we subclassify, and does it matter? Modern Path 27: S39-S46.
Google Scholar Cross Ref - Dei Tos AP (2013) Liposarcomas: Diagnostic pitfalls and new insights. Histopathology 64: 38-52.
Google Scholar Cross Ref - Zagars GK, Ballo MT, Pisters PW, Pollock RE, Patel SR, et al. (2003) Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: An analysis of 225 patients. Cancer 97: 2530-2543.
Google Scholar Cross Ref - Toulmonde M, Bonvalot S, Meeus P (2014) Retroperitoneal sarcomas: Patterns of care at diagnosis, prognostic factors and focus on main histological subtypes: A multicenter analysis of the French Sarcoma Group. Ann Oncol 25: 735-742.
Google Scholar Cross Ref - Mariño-Enríquez A, Fletcher CD, Dal Cin P, Hornick JL (2010) Dedifferentiated liposarcoma with homologous lipoblastic (pleomorphic liposarcoma-like) differentiation: Clinicopathologic and molecular analysis of a series suggesting revised diagnostic criteria. Am J Surg Pathol 34: 1122-1131.
Google Scholar Cross Ref - Utsunomiya A, Kinouchi H, Kayama T, Yoshimoto T (1999) Distant metastasis of liposarcoma to the dura and skull: A case report. Brit J Neuro 13: 520-522.
Google Scholar Cross Ref - Crago AM, Singer S (2011) Clinical and molecular approaches to well differentiated and dedifferentiated liposarcoma. Curr Opin Oncol 23: 373-378.
Cross Ref - Sung EK, Ji HK, Yang SW (2020) Dedifferentiated transformation in metastatic liposarcoma of orbit and brain. Orbit 39: 437-440.
Cross Ref - Sadhana B, Joud H (2021) Case Report: Metastatic dedifferentiated liposarcoma presenting as hypereosinophilia in an adolescent. J Immun Prec Oncol 4: 21-25.
Google Scholar Cross Ref - Shen J, Fang Z, Zhang Y (2019) Primary cardiac dedifferentiated liposarcoma in a middle-aged female: A case report. J Cardiothorac Surg 14: 156.
Google Scholar Cross Ref - Bonvalot S, Raut CP, Pollock RE, Rutkowski P, Srauss DC, et al. (2012) Technical considerations in surgery for retroperitoneal sarcomas: Position paper from E-Surge, a master class in sarcoma surgery, and EORTC-STBSG. Ann Surg Oncol 19: 2981-2991.
Google Scholar Cross Ref - Beane JD, Yang JC, White D, Steinberg SM, Rosemary SA, et al. (2014) Efficacy of adjuvant radiation therapy in the treatment of soft tissue sarcoma of the extremity: 20-year follow-up of a randomized prospective trial. Ann Surg Oncol 21: 2484-2489.
Google Scholar Cross Ref - Krikelis D, Judson I (2010) Role of chemotherapy in the management of soft tissue sarcomas. Expert Rev Anticancer Ther 10: 249-260.
Google Scholar Cross Ref - Harold Haft, George CW (1988) Metastatic liposarcoma of the brain with response to chemotherapy: Case report. Neurosurgery 23: 777–780.
Google Scholar
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 