Case Report, Clin Oncol Case Rep Vol: 6 Issue: 10
Complete Remission of Follicular Dendritic Cell Sarcoma with Adjuvant Pazopanib
Chalette Lambert-Swainston1*, Neilmegh Varada2 , Russell Gollard1,3
1Department of Internal Medicine, Kirk Kerkorian School of Medicine at UNLV, Las Vegas, NV, USA
2Department of Internal Medicine, Tulane University, New Orleans, LA, USA
3OptumCare Cancer Center, Las Vegas, NV, USA
*Corresponding Author: Chalette Lambert-Swainston, Department of Internal Medicine
Kirk Kerkorian School of Medicine at UNLV, Las Vegas, NV, USA
E-mail: Chalette.lambert-swainston@unlv.edu
Received: October 04, 2023; Manuscript No: COCR-23-115599
Editor Assigned: October 07, 2023; PreQC Id: COCR-23-115599 (PQ
Reviewed:October 16, 2023; QC No: COCR-23-115599 (Q)
Revised: October 18, 2023; Manuscript No: COCR-23-115599 (R)
Published: October 21, 2023; DOI: 10.4172/cocr.6(10).314
Citation: Swainston LC, Varada N, et al. Complete Remission of Follicular Dendritic Cell Sarcoma with Adjuvant Pazopanib Clin Oncol Case Rep 6:10
Abstract
Follicular Dendritic Cell Sarcoma (FDCS) is a rare neoplasm of non-hematopoietic mesenchymal origin, with less than 1000 cases documented worldwide. Pathogenesis and treatment for FDCS are not well defined. Typically, treatment involves surgical resection and radiotherapy but data on chemotherapy treatment is limited. We present a patient with FDCS of the neck who was treated surgically with adjuvant Pazopanib. The patient presented at 47 years old with a large asymptomatic neck mass and underwent surgical resection. Pathology of the mass was consistent with follicular dendritic cell sarcoma arising in association with hyaline vascular Castleman disease, with positive staining for CD21 and CD23 and variable staining for CD163 and CD68. The patient was successfully treated with adjuvant Pazopanib, a tyrosine kinase inhibitor that acts on multiple growth factor receptors. This case highlights the importance of targeting angiogenesis in the treatment of FDCS but also the importance of ascertaining the role of new immunotherapies in the targeting of soft tissue sarcomas. In the past, chemotherapy for soft tissue sarcoma has involved toxic agents such as adriamycin, gemcitabine with taxane and dacarbazine, but more research is needed into the differential sensitivity of soft tissue sarcomas to new immunotherapy treatments such as Pazopanib.
Keywords: Follicular Dendritic Cell Sarcoma; Adjuvant pazopanib; Non-hodgkin lymphoma
Introduction
Follicular Dendritic Cell Sarcoma (FDCS) is a rare neoplasm of non-hematopoietic mesenchymal origin. Follicular dendritic cells are found in primary and secondary lymphoid follicles and function in antigen presentation to B cells and typically stain positive for CD21, CD23, and CD35 [1]. They have also been found to express adhesion molecules, cytokines and chemokines for the retention of B and T cells in the germinal center. Tumors arising from these follicular dendritic cells are rare and the pathogenesis is not well understood. Associations have been made with Castleman disease, a benign lymphoproliferative disorder. FDCS has been shown to develop in the setting of hyaline-vascular Castleman disease, with sequential biopsies showing at first FDC proliferation in the follicles followed by interfollicular overgrowth and then cytologic atypia and mitotic activity [2-4]. Association of FDCS has also been made with EBV, though this pathogenesis is unknown. Case numbers for FDCS are less than 1000 worldwide, making data on management and treatment of FDCS difficult [2,5-7]. FDCS typically presents as an asymptomatic mass, though symptoms related to mass effect and systemic symptoms such as fever, fatigue and night sweats have also been reported [1,2]. The mainstay of treatment is surgical resection and radiotherapy, and due to the limited number of cases, data on adjuvant chemotherapy treatments is not well studied. Previous chemotherapy regimens for individual cases have included gemcitabine with a taxane, CHOP, ifosfamide, cyclophosphamide and etoposide, imatinib, rituximab, nivolumab and ipilimumab [5,8,9]. We present a case of FDCS successfully treated with surgical resection and adjuvant Pazopanib.
Case Presentation
Initially, a 47 year old male with no past medical history presented to an otolaryngologist with an asymptomatic neck mass. CT imaging of the neck revealed a mass measuring 63 mm x 79 mm. Biopsy of the mass was taken by ENT and initial pathological review indicated presence of small blue cells so the patient was referred to oncology. Of note, the patient reported a social history of sexual contact with males so initial concern was for HIV related NonHodgkin Lymphoma. Upon testing, the patient was found to be HIV negative. The patient underwent surgical excision of the mass and pathology was consistent with follicular dendritic cell sarcoma arising in association with hyaline vascular Castleman disease with positive staining for CD21 and CD23 and variable staining for CD163 and CD68 (Figure 1 and Figure 2).
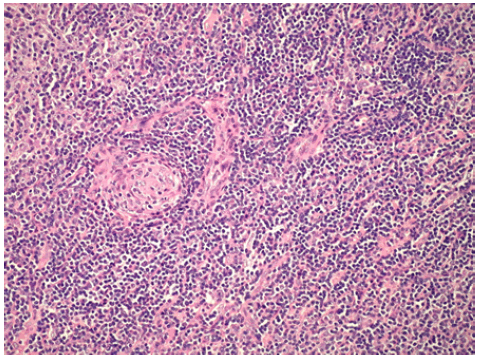
Figure 1: Histopathology of the patient’s neck mass showing hyaline vascularization characteristic of Castleman disease.
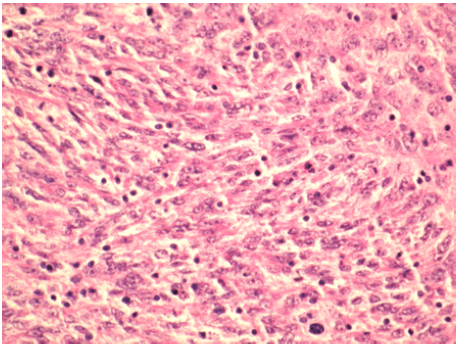
Figure 2: Histopathology of the patient’s neck mass showing ovoid to spindled cells with distinct nucleoli characteristic of Follicular Dendritic Cell Sarcoma.
Initial treatment for this patient was AIM therapy (Adriamycin, Ifosfamide, and Mesna). This course was deemed a treatment failure with no change in mass size. Following treatment failure, the patient underwent one course of Pazopanib and demonstrated reduction in mass size both clinically and confirmed with CT imaging. The patient did not experience any adverse effects with Pazopanib use and was confirmed to have remained in remission at a four year follow up appointment.
This 47 year old male, while asymptomatic, showed complete response to the checkpoint inhibitor. Though recurrence in the first year after successful treatment is common in FDCS, this patient successfully remained in remission for at least four years with no increase in mass size.
Discussion
Given the rare nature of FDCS, treatment regimens are not well established and current therapies have not shown significant disease-free survival [10]. While surgical excision is the gold standard of treatment, adjuvant therapies with chemotherapy and radiation often do not induce long term remission. Previous immunotherapy treatment has included imatinib, rituximab, nivolumab and ipilimumab without a definitive target because the pathogenesis of FDCS is not well understood. The success of Pazopanib and other checkpoint inhibitors may help shed light on the process by which follicular dendritic cells develop neoplastic potential.
Follicular Dendritic Cells (FDCs) are a type of antigen-presenting cells that are largely found in lymphoid follicles. FDCs are unique in terms of typical hystiocytic and dendritic cells in that they are mesenchymal in origin. Though thought to be similar in development to low grade sarcoma, the process by which FDCs develop neoplastic is not yet clearly delineated. Genetic and molecular analysis of FDCS has shown evidence of tumor suppressor driven genomic alterations, including NF-kB, as well as gain of function mutations in BRAF (V600E) [11-13]. FDCS, as well as Castleman disease, as is relevant in the case of our patient, have also been shown to upregulate EGFR [14]. Pazopanib is a multitarget tyrosine kinase inhibitor that has been shown to inhibit VEGFR, PDGFR, FGFR, c-kit, Itk, Lck, and c-Fms used in treatment for renal cell carcinoma and soft tissue sarcoma. Given the success of pazopanib in our patient, it is likely that growth factor activation is one mechanism in which malignant potential of FDCs develop. It is possible that the progression from overgrowth to atypia seen in Castleman disease also involves protein kinase activation resulting in possible anti-apoptotic effects, loss of checkpoint inhibition, angiogenesis and metastasis.
Conclusion
This case demonstrates long-term remission of FDCS with tyrosine kinase inhibitor use. This illustrates potential pathogenesis of FDCS to include growth factor inhibition in the progression from benign Castleman disease to malignancy. More research is needed into the use of tyrosine kinase and checkpoint inhibitors as first line agents after surgical resection given their demonstrated effect in achieving successful remission.
Acknowledgement
The publication fees for this article were supported by the Kirk Kerkorian School of Medicine at UNLV Medical Library Open Article Fund.
References
- Chen T, & Gopal P (2017). Follicular dendritic cell sarcoma. Archives of Pathology & Laboratory Medicine, 141(4), 596-599. [Google Scholar] [Cross Ref]
- Saygin C, Uzunaslan D, Ozguroglu M, Senocak M, & Tuzuner N, et al. (2013). Dendritic cell sarcoma: a pooled analysis including 462 cases with presentation of our case series. Critical reviews in oncology/hematology, 88(2), 253-271. [Google Scholar] [Cross Ref]
- Chan ACL, Chan KW, Chan JKC, Au WY, Ho WK, et al. (2001). Development of follicular dendritic cell sarcoma in hyalineâ?vascular Castleman’s disease of the nasopharynx: tracing its evolution by sequential biopsies. Histopathology, 38(6), 510-518. [Google Scholar] [Cross Ref]
- Chan JK, Fletcher CD, Nayler SJ, & Cooper K. (1997). Follicular dendritic cell sarcoma: clinicopathologic analysis of 17 cases suggesting a malignant potential higher than currently recognized. Cancer, 79(2), 294-313. [Google Scholar] [Cross Ref]
- Pang J, Mydlarz WK, Gooi Z, Waters KM, Bishop J, et al. (2016). Follicular dendritic cell sarcoma of the head and neck: case report, literature review, and pooled analysis of 97 cases. Head & neck, 38(S1), E2241-E2249. [Google Scholar] [Cross Ref]
- Jain P, Milgrom SA, Patel KP, Nastoupil L, Fayad L, et al. (2017). Characteristics, management, and outcomes of patients with follicular dendritic cell sarcoma. British journal of haematology, 178(3), 403-412. [Google Scholar] [Cross Ref]
- Perkins SM, & Shinohara ET. (2013). Interdigitating and follicular dendritic cell sarcomas: a SEER analysis. American journal of clinical oncology, 36(4), 395-398. [Google Scholar] [Cross Ref]
- Azim HA, Ehab E, Hatem AA. "Imatinib in the treatment of follicular dendritic sarcoma: a case report and review of literature." Oncology Research and Treatment 30.7 (2007): 381-384. [Google Scholar] [Cross Ref]
- Lee MY, Bernabe RC, Ramirez DC, & Maki RG. (2020). Follicular dendritic cell sarcoma and its response to immune checkpoint inhibitors nivolumab and ipilimumab. BMJ Case Reports CP, 13(4), e234363. [Google Scholar] [Cross Ref]
- Facchetti F, Simbeni M, & Lorenzi L. (2021). Follicular dendritic cell sarcoma. Pathologica, 113(5), 316. [Google Scholar] [Cross Ref]
- Andersen EF, Paxton CN, O'Malley DP, Louissaint JA, Hornick JL, et al. (2017). Genomic analysis of follicular dendritic cell sarcoma by molecular inversion probe array reveals tumor suppressor-driven biology. Modern Pathology, 30(9), 1321-1334. [Google Scholar] [Cross Ref]
- Go H, Jeon YK, Huh J, Choi SJ, Choi YD, et al. (2014). Frequent detection of BRAFV 600E mutations in histiocytic and dendritic cell neoplasms. Histopathology, 65(2), 261-272. [Google Scholar] [Cross Ref]
- Griffin GK, Sholl LM, Lindeman NI, Fletcher CD, Hornick JL , et al. (2016). Targeted genomic sequencing of follicular dendritic cell sarcoma reveals recurrent alterations in NF-κB regulatory genes. Modern Pathology, 29(1), 67-74. [Google Scholar]
- Sun X. "Epidermal growth factor receptor expression in follicular dendritic cells: a shared feature of follicular dendritic cell sarcoma and Castleman’s disease." Human pathology 34.9 (2003): 835-840. [Google Scholar] [Cross Ref]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 