Research Article, J Blood Res Hematol Dis Vol: 5 Issue: 2
COVID-19: Neutrophils “Unfriendly Fire†Imbalances Proteolytic Cascades Triggering Clinical Worsening and Viral Sepsis. Potential Role Explanation for Convalescent Plasma as “Firehoseâ€
Pier Maria Fornasari*Regen Health Solutions Research Unit, Bologna, Italy
*Corresponding Author : Pier Maria Fornasari
Regen Health Solutions Research
Unit, Bologna, Italy
Tel: 393286853075
E-mail: fornasaripiermaria@gmail.com
Received date: May 27, 2020; Accepted date: June 12, 2020; Published date: June 20, 2020
Citation: Fornasari PM (2020) COVID-19: Neutrophils “Unfriendly Fire” Imbalances Proteolytic Cascades Triggering Clinical Worsening and Viral Sepsis. Potential Role Explanation for Convalescent Plasma as “Firehose”. J Blood Res Hematol Dis 5:2. doi: 10.37532/jbhrd.2020.5(2).120
Abstract
Based on COVID-19 Chinese CDCP report on, 14% of patients presented severe disease and 5% critical conditions. The average case-fatality rate was 2,3%, but mortality was as high as 49% in patients with critical illness. Serious life threatening thromboembolic complications have been found in 71,4% of non-survivors and micro/macro angiopathic coagulopathy has been found, also at autopsy, with highly increased neutrophil number, fibrinogen, concentrations of D-dimer and FDPs and NETs, ATIII decrease and normal number of platelets. A cytokine storm and interaction between inflammation and coagulation has been advocated as explanation of hypercoagulability. The paper shows that SARS-CoV-2 infection of alveolar cells induces recruitment of innate responder neutrophils, which release proteases and NETs inducing endothelial damage/ endotheliopathy and imbalance of the four major proteolytic cascades (coagulation, complement, fibrinolysis and kallikrein) with prevalence of activators over inhibitors and consequent thrombotic complications. Platelets adhesion to damaged endothelium and the presence of ULVWF multimers, due to decreased ADAMTS13, contribute to the state of hypercoagulability. Neutrophil innate unfriendly fire response can be identified as the trigger of a proteolytic storm, responsible for subsequent well known prothrombotic condition and cytokine storm, explaining also the pathology of recently described systemic Kawasaki Disease like vasculitis in Covid-19 young ill patients.
Keywords: Neutrophils; Proteolytic cascades; Convalescent plasma
Introduction
According to the largest current report from the Chinese Center for Disease Control and Prevention with 72 314 cases, 58 574 patients (81%) were classified as mild, 10 124 (14%) were classified as severe, and 3616 (5%) were considered critical (respiratory failure, septic shock, and/or multiple organ failure) [1]. Among 201 patients in Wuhan, Wu et al. reported that risk factors associated with development of acute respiratory distress syndrome and death included older age, neutrophilia, organ dysfunction, coagulopathy and elevated D-dimer levels [2].
As of May 19, 2020, John Hopkins Covid-19 dashboard has documented a total of 4.820.000 cases with over 319.000 deaths worldwide.
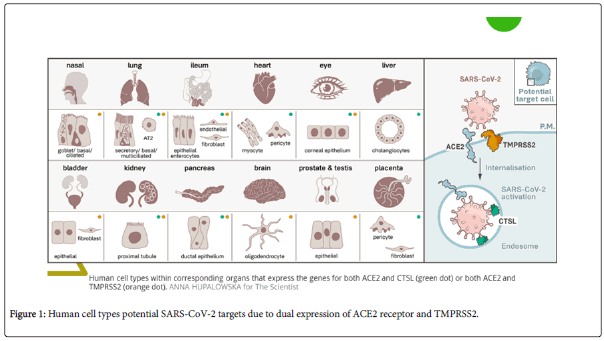
The strategy used by SARS-CoV-2 for cellular entry is the same in all the tissues presenting dual-positive ACE2+TMPRSS2+ cells, employing its S protein, previously primed by TMPRSS2 protease and cathepsin B/L, binding to ACE2 receptor [3]. ACE2, the viral receptor, and one of its entry-associated proteases, TMPRSS2, are expressed in nasal goblet cells, in lung goblet, multiciliated and AT2 cells and gut epithelial enterocytes.
Dual-positive ACE2+TMPRSS2+cellsare also present in pancreatic ductal cells, bladder, testis, prostate, kidney epithelial cells, cholangiocytes, oligodendrocytes, inhibitory enteric neurons, heart fibroblasts/pericytes, fibroblasts and pericytes in multiple other tissues. In line with the kidney’s role in the renin-angiotensin-aldosterone system, dual-positive cells are enriched in the proximal tubular cells and in principal cells of the collecting duct [4] (Figure 1).
Respiratory Tract Infection, Neutrophilia, Proteolytic Cascades and Platelets Activation Resulting in Inflammatory Endotheliopathic Micro Angiopathy
Focusing on the areas of the respiratory tract involved and based on the cells that are likely infected, COVID-19 can be divided into three phases that correspond to different clinical stages of the disease.
Stage 1: Asymptomatic state (Initial 1-2 days of infection): inhaled virus SARS-CoV-2 likely binds to epithelial cells in the nasal cavity and starts replicating. The viral burden may be low.
Stage 2: Upper airway and conducting airway response (Next few days): virus propagates and migrates down the respiratory tract along the conducting airways.
Stage 3: Alveolar phase. The disease COVID-19 is clinically manifest. About 19% of the infected patients will progress to stage 3 diseases and will develop pulmonary infiltrates. The virus infects alveolar type II cells. SARSCoV-2 propagates within type II cells, large numbers of viral particles are released and the cells undergo apoptosis and die.
The alveolar phase is evolving in 2 different patterns:
3a) Moderate with absent or minor endothelial leakage,
3b) Severe with alveolar collapse due to surfactant loss, fluid filling of interstitium, engulfing protein-rich fluid with neutrophils release products like NETs, reduced gas exchange, endothelial lesion, through which SARS-CoV-2 virus can enter into the bloodstream and induce viral sepsis.
SARS-CoV-2 nsp9 and nsp10 target NKRF to facilitate IL-8/IL-6 production, while, based on a recent paper, low levels of IFN-I and -III are likely produced and thus the response is imbalanced versus activation of the adaptive immune response [5].
Viral infected epithelial cells are a major source of beta and lambda interferons, chemokines belonging to neutrophil chemotaxis, HMGB1 and the classic neutrophil chemo attractants of the CXCL family [6]. SARS-Cov-2 infected lung cells also over expressed complement activation genes, involved in neutrophil degranulation, with deposits of terminal complement components C5b-9, C4d and MASP2 attacking the host ECs and causing trans membrane channel formation on the endothelium and inducing endotheliopathy, involved in ARDS like syndrome with systemic inflammation and lung neutrophilia [7]. A similar pattern has been shown in purpuric skin lesions due to a pauciinflammatory thrombogenic vasculopathy, with co-localization of COVID-19 spike glycoproteins and deposition of C5b-9 and C4d in both grossly involved and normally-appearing skin and in lung interalveolar septa. In severe COVID-19 lung infection, a catastrophic micro vascular injury syndrome is caused by activation of complement and coagulation pathways, inducing an associated procoagulant state [7].
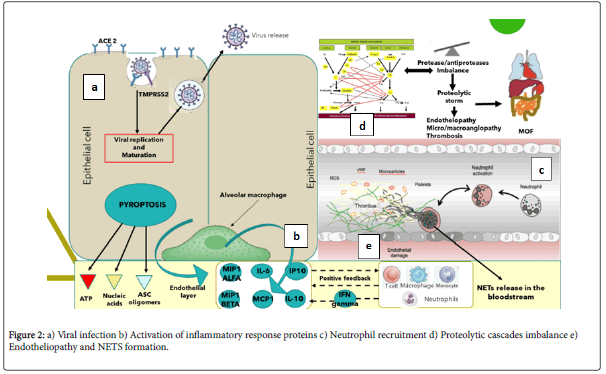
Platelets also play a critical role in lung innate defense response, the lung being a primary site for platelet biogenesis: activated platelets engulf virions and secrete anti-viral molecules (a-granules) to destroy virions, HMGB18 and express surface P-selectin enabling the initial attachment of neutrophils from the bloodstream (Figure 2).
The Neutrophils “Friendly fire”, the NETosis and the Proteolytic Storm. Dangerous not Inhibited Partnership of Platelets, Complement and Coagulation Cascades for Endotheliopathy and Thrombogenesis
The lung alveoli are thus the last defense line against SARS-Cov-2 dissemination through blood stream and viral sepsis and thus innate immunology is highly involved in fighting. In severe COVID-19, neutrophils, together with other mononuclear, are the first cells of the immune system to migrate to the infected alveoli, recruited by interferon’s, IL-1β9 and IL-610, where they attack SARS-CoV-2, but in addition to direct virus-inflicted pathologies, their exaggerated “unfriendly fire” responses, resulting in a “proteolysis storm” and, inducing alveolar capillary endotheliopathy, contribute to disease severity and subsequent viral dissemination.
The mechanisms that neutrophils undertake for host defense are phagocytosis, degranulation, cytokine production and neutrophil extracellular traps (NETs) release, known as NETosis.
NETs are DNA structures released including histones and over 30 components of primary and secondary granules, such as elastase, myeloperoxidase, cathepsin G, lactoferrin, pentraxin3, gelatinase, proteinase 3, LL37 and peptidoglycan-binding proteins.
Three models for NETosis are known to date: suicidal (NETs release and neutrophil lysis), vital NETosis (triggered by TLRs stimuli, platelet glycoprotein Ib, complement activation, after release neutrophils are stillable to phagocytose pathogens and have a normal lifespan) and mitochondrial DNA is released instead of nuclear DNA [8-11].
Platelet HMGB1 protein (passively released extracellularly as a prototypical DAMP from dying cells or stressed or activated cells present in any tissue) is the major endogenous inducers of NETs formation. NET chromatin disrupts epithelial lining, induces platelet aggregation [12] and activates further neutrophils recruitment. NETs, via electrostatic interactions, activate the contact pathway of coagulation19 and, through tissue factor, the intrinsic pathway [13]. NETs form a scaffold for thrombus formation by promoting platelet adhesion and by concentrating coagulation factors involved in clotting. Thrombus-resident neutrophils are strategic for thrombi extension by binding factor XII and supporting its activation through NETosis [14].
The endothelial cells damage (endotheliopathy), induced by SARSCoV- 2 virus and neutrophil elastase, triggers the activation of two independent endothelial pathways (inflammatory and microthrombotic), through release of inflammatory cytokines (interleukin IL-1, IL-6, tumor necrosis factor-α, and others) and activation of the platelet and endothelial exocytosis of ULVWF, mediating micro thrombogenesis via “activation of microthrombotic pathway ” . In parallel endothelial damage inhibits ADAMTS13 biosynthesis, while Neutrophil Elastase proteolytically cleaves and significantly decreases its plasma level [15]. FXIa and α-thrombin remove C-terminal domain of ADAMTS13, blocking its ability to cleave VWF on the endothelial cell surface and increase the release of ULVWF by endothelial cells, resulting in persistence of ULVWF strands and causing an increase in platelet adhesion under flow conditions [16].
This pathological chain of events has been described also for multi systemic vasculitis in Kawasaki Disease, characterized by platelet stimulation with increase in the shedding of P-selectin, translocating at the surface and externalized with subsequent hyper activation and the detection of circulating platelets–neutrophils aggregates. Neutrophils recruited by platelets P-selectin and PSGL-1 (vascular adhesion molecules playing an important role in the inflammatory response by mediating the interaction of leucocytes with stimulated endothelium and platelets bound in the vicinity of vascular injury) contribute, through Toll-like receptor 4, to NETs formation. NETs cause platelet activation and aggregation, thus linking inflammation and thrombosis to support the relevance of this mechanism in the pathogenesis of Kawasaki disease.
Simultaneously, serine proteases released by neutrophils and present in NETs cleave coagulation inhibitors such as tissue factor pathway inhibitor and anti thrombin [17].
On the damaged endothelial surface NETs, ULVWF multimers, platelets and activated not inhibited clotting and complement pathways initiate thrombogenesis within the microvasculature, leading to microthrombi enriched also by leukocytes recruited in the P-selectin dependent manner [18]. The microthrombi can become sufficiently large to be released from endothelial cells into the circulation, resulting in embolism [19].This condition can be defined “TTP-like syndrome”.
Blood circulation action and mechanical stress (like forced ventilation) may be sufficient to physically disrupt the fragile structure of NETs in the bloodstream, releasing NET fragments. Mechanically disrupted NETs augment NETosis and NETosis propagates inflammatory response.
TLR inhibitors may reduce inflammation, specifically by preventing NET-induced NETosis.
Intravascular NETosis is thus responsible for initiation, dissemination and local accretion of thrombotic events in arteries, veins and in the microvasculature, with end-organ damage in lungs, heart, kidneys and other organs [20].
Through endothelial lesions and NETs, SARS-CoV-2 virus activates its viremic phase and in each interested organ follows the same cellular entry strategy and catastrophic micro vascular injury syndrome, causing final MOF.
The mechanisms that neutrophils undertake for host defense are phagocytosis, degranulation, cytokine production and neutrophil extracellular traps (NETs) release, known as NETosis.
NETs are DNA structures released including histones and over 30 components of primary and secondary granules, such as elastase, myeloperoxidase, cathepsin G, lactoferrin, pentraxin 3, gelatinase, proteinase 3, LL37 and peptidoglycan-binding proteins.
Three models for NETosis are known to date: suicidal (NETs release and neutrophillysis), vital NETosis (triggered by TLRs stimuli, platelet glycoprotein Ib, complement activation, after release neutrophils are still able to phagocytose pathogens and have a normal lifespan) and mitochondrial DNA is released instead of nuclear DNA [11].
Platelet HMGB1 protein (passively released extracellularly as a prototypical DAMP from dying cells or stressed or activated cells present in any tissue) is the major endogenous inducers of NETs formation. NET chromatin disrupts epithelial lining, induces platelet aggregation [12] and activates further neutrophils recruitment. NETs, via electrostatic interactions, activate the contact pathway of coagulation19 and, through tissue factor, the intrinsic pathway [13]. NETs form a scaffold for thrombus formation by promoting platelet adhesion and by concentrating coagulation factors involved in clotting. Thrombus-resident neutrophils are strategic for thrombi extension by binding factor XII and supporting its activation through NETosis [14].
The endothelial cells damage (endotheliopathy), induced by SARSCoV- 2 virus and neutrophil elastase, triggers the activation of two independent endothelial pathways (inflammatory and micro thrombotic), through release of inflammatory cytokines (interleukin IL-1, IL-6, tumor necrosis factor-α, and others) and activation of the platelet and endothelial exocytosis of ULVWF, mediating micro thrombogenesis via “activation of micro thrombotic pathway”. In parallel endothelial damage inhibits ADAMTS13 biosynthesis, while Neutrophil Elastase proteolytically cleaves and significantly decreases its plasma level [15]. FXIa and α-thrombin remove C-terminal domain of ADAMTS13, blocking its ability to cleave VWF on the endothelial cell surface and increase the release of ULVWF by endothelial cells, resulting in persistence of ULVWF strands and causing an increase in platelet adhesion under flow conditions [16].
This pathological chain of events has been described also for multi systemic vacuities in Kawasaki Disease, characterized by platelet stimulation with increase in the shedding of P selectin, translocating at the surface and externalized with subsequent hyper activation and the detection of circulating platelets–neutrophils aggregates. Neutrophils recruited by platelets P-selectin and PSGL-1 (vascular adhesion molecules playing an important role in the inflammatory response by mediating the interaction of leucocytes with stimulated endothelium and platelets bound in the vicinity of vascular injury) contribute, through Toll-like receptor 4, to NETs formation. NETs cause platelet activation and aggregation, thus linking inflammation and thrombosis to support the relevance of this mechanism in the pathogenesis of Kawasaki disease.
Simultaneously, serine proteases released by neutrophils and present in NETs cleave coagulation inhibitors such as tissue factor pathway inhibitor and anti thrombin [17].
On the damaged endothelial surface NETs, ULVWF multimers, platelets and activated not inhibited clotting and complement pathways initiate thrombogenesis within the micro vasculature, leading to micro thrombien riched also by leukocytes recruited in the P-selectin dependent manner [18]. The microthrombi can become sufficiently large to be released from endothelial cells into the circulation, resulting in embolism [19].This condition can be defined “TTP-like syndrome”.
Blood circulation action and mechanical stress (like forced ventilation) may be sufficient to physically disrupt the fragile structure of NETs in the bloodstream, releasing NET fragments. Mechanically disrupted NETs augment NETosis and NETosis propagates inflammatory response.
TLR inhibitors may reduce inflammation, specifically by preventing NET-induced NETosis.
Intravascular NETosis is thus responsible for initiation, dissemination and local accretion of thrombotic events in arteries, veins and in the microvasculature, with end-organ damage in lungs, heart, kidneys and other organs [20].
Through endothelial lesions and NETs, SARS-CoV-2 virus activates its viremic phase and in each interested organ (Figure 1) follows the same cellular entry strategy and catastrophic micro vascular injury syndrome, causing final MOF.
Proteolytic Cascades Balance between Activators and Inhibitors is Imbalanced in Covid-19
If NETs induce hyper coagulability, the significant increase in neutrophil numbers and the released proteolytic enzymes (mainly elastase) contribute to a consumption of proteases inhibitors, with an unbalance of physiologic conditions and establishment of the “proteolytic storm”.
The activators/inhibitors balance in proteolytic cascades is essential for homeostasis and, due to this, in normality the inhibitor plate is largely superior to activator one.
The innate serine protease system has four major columns, coagulation, fibrinolysis, kallikrein and complement. These systems are strictly correlated, interconnected and their physiological maintenance is the result of a rigorous balance. Complement directly enhances coagulation and, in addition, inhibits anti-coagulant factors, while certain coagulation enzymes activate complement components. The inter play between complement and coagulation is crucial to understand the clinical implications in Covid-19, in which complement-coagulation interactions contribute to the development of life-threatening complications [7].
The contact system, also named as plasma kallikrein-kinin system, consists of three serine proteinases: coagulation factors XII and XI, plasma pre kallikrein and high molecular weight kininogen. Once activated by NETs, this system is prothrombotic by activating intrinsic pathway and pro inflammatory by producing bioactive peptide bradykinin.
Extrinsic and intrinsic pathways of blood coagulation induce simultaneous activation of the complement and fibrinolysis cascades, with an extensive cross talk mutually fine-tuning their activation status.
Main family of the serine protease inhibitors (SERPINs) is formed by SERPINA1 (ɑ1-antitrypsin) protecting lung tissue from neutrophil elastase, SERPINA5 (Protein C inhibitor), SERPINC1 (anti-thrombin) controls coagulation proteases, SERPIND (Heparin cofactor II), SERPINE1 (plaminogen activator inhibitor 1), SERPING1 (C1 inhibitor) regulates complement, callicrein and contact phase activation and SERPINF2 (ɑ-2- antiplasmin) inhibits plasmin and regulates fibrinolysis. Complement activation is inhibited also by Decay-accelerating factor (DAF) and Factor H (alternative pathway). Alpha 2 macroglobulin acts as an anti-protease for a variety of proteases like plasmin, kallikrein and thrombin. A delicate balance between serine proteases and their serpin inhibitors is crucial for normal functioning of biological pathways.
Proteases/anti-proteases balance is present also at the endothelial surface, where thrombo modulin, forming complexes with thrombin, induces protein C activation to suppress blood coagulation, while TNF-alpha and IL-1 beta, inducing TF and PAI-1, down-regulate the expression of thrombo modulin. Pro coagulant TF upregulation with down regulation of the anticoagulant TM/Protein C system converts the normal anticoagulant endothelium into a prothrombotic endothelium.
Lastly, NETs triggered significant platelet aggregation.
A proteases/anti-proteases balance is present also at alveolar space, where SERPINA1 strongly and specifically inhibits neutrophil elastase. When the inhibitor concentration is sufficient to block released elastase, no lesion happens nor in alveolar epithelium nor in alveolar endothelial wall and this corresponds with the moderate Covid-19 clinical condition.
Otherwise, if SERPINA1 is over helmed or is absent/deficient, as in homo/heterozygous patients (about 4% European population), imbalance results between elastase and anti-elastase activity and free elastase causes progressive damage of both alveoli and endothelium, inducing endotheliopathy and thrombogenic state previously described.
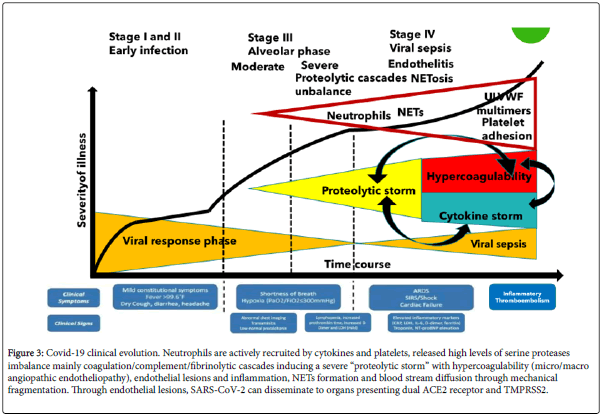
The hypercoagulability, as imbalance of proteases/antiproteases cascades, the decrease of ADAMTS13, the endotheliopathy, the increased platelet activation, the ULVWF multimers and the NETosis together create a severe thromboembolic environment, similar to Thrombotic Thrombocytopenic Purpura/HUS conditions and Acute Promyelocytic Leukemia activation of clotting systems with secondary hyper fibrinolysis (Figure 3).
Figure 3: Covid-19 clinical evolution. Neutrophils are actively recruited by cytokines and platelets, released high levels of serine proteases imbalance mainly coagulation/complement/fibrinolytic cascades inducing a severe “proteolytic storm” with hypercoagulability (micro/macro angiopathic endotheliopathy), endothelial lesions and inflammation, NETs formation and blood stream diffusion through mechanical fragmentation. Through endothelial lesions, SARS-CoV-2 can disseminate to organs presenting dual ACE2 receptor and TMPRSS2.
Critical SARS-Cov-2 Infection: The Feasible Role of Convalescent Plasma as a First-Line Therapy
Convalescent Plasma (CP) use as a therapy of untreatable infectious diseases has been extensively but an edoctically documented, including Spanish Influenza A (H1N1) infections in 1915 to 1917, severe acute respiratory syndrome (SARS) in 2003, pandemic 2009 influenza A (H1N1), avian influenza A (H5N1) and several hemorrhagic fevers such as Ebola. Based on studies, showing convalescent plasma antibodies can limit the virus reproduction, CP has been considered for critically sick COVID‐19 patients.
In SARS-CoV and MERS, CP was shown to provide Nabs binding to spike1-receptor binding protein (S1-RBD), S1-N-terminal domain and S2, thus blocking entry and containing viral amplification.
Very recently, Cochrane Database of Systematic Reviews published a rapid review on convalescent plasma or hyper immune immunoglobulin for people with COVID-1932, including eight anedoctical studies from China and South Korea (uncontrolled studies, 7 case series and 1 prospectively registered single-arm intervention study) describing 32 critically ill participants.
Other47 ongoing studies (21 randomized) evaluating CP and one ongoing study evaluating hyper immune immunoglobulin were identified.
For the primary outcomes, the included studies reported that all participants were alive at the end of follow, thus concluding that plasma therapy is safe and improves patient outcome, but there are significant limitations to each of these studies, due to small number of treated patients and absence of a control group. Two studies reported adverse events that were potentially grade 3 and grade 4, of which one was serious.
Fortunately, more extensive studies are now in the pipeline to provider obust evidence either for or against the use of convalescent plasma. Worldwide there are over 66 clinical trials, as reported by Clinical Trials.gov, actively recruiting COVID-19 patients to study the effect of convalescent plasma. In many studies plasma from noninfected patients is used as the placebo arm of the trial, to ensure that any benefits identified are indeed specific for SARS-CoV-2 antibodies.
To better understand the potential role of CP in Covid-19 treatment, it’s important to highlight the following:
• CP is a “scarce resource” and thus should be reserved to critically ill patients,
• CP isn ’ t a “ single active principle ” product, but contains a multiplicity of active ingredients,
• Human plasma is the defined first-line therapy in diseases like TTP, presenting with clinical and pathological features similar to Covid-19,
• Based on the objects of the trials, the timing, the quantity, the frequency, the haematological and clinical parameters monitoring and the side effects need to be evaluated and defined,
• Human non-convalescent plasma must be used as placebo in the control arm.
This paper supports the hypothesis that the worsening of Covid-19 clinical symptoms is due to the catastrophic “ unfriendly fire ” of recruited neutrophils, with over helming and imbalancing of serine proteolytic cascades activator proteases over inhibitors, serious endotheliopathy, NETosis, ULVWF release, hypercoagulability and diffuse micro/macrothrombi formation.
This condition in the paper has been described as “proteolytic storm”, which advances and sustains/is sustained by the well-known “cytokine storm” as shown in Figure 3.
Following this hypothesis, CP doesn’t supply only NAbs, but also supplies:
Other antibodies able to mediate/neutralize pathways such as complement activation, antibody-dependent cellular cytotoxicity and/or phagocytosis 29,30, limiting immune complexes formation and cytokine release such as IL-1β and TNFα,
SERPIN family serine proteases inhibitors of the four inter connected proteolytic cascades (clotting, complement, fibrinolysis and kallikrein),
ADAMTS13 metallo protease, which cleaves ULVWF, reducing hypercoagulability and thrombogenicity,
SERPINA1 inhibiting neutrophil elastase deleterious effects, mainly at alveolar capillary level,
SERPING1 counter acting platelet activation action on clotting and complement cascade35 and P-selectin/HGMB1 expression [6,18],
Other inhibitors like the generic Alfa2 Macroglobulin and Thrombomodulin,
CP Nabs boost a much stronger immune response of newly dendritic cells inf-cDC236.
Conclusion
CP can be considered a very effective “ fire extinguisher ” for neutrophils “unfriendly fire ” and its consequences of “proteolytic storm”, hypercoagulability, thrombosis and sepsis with MOF, but the absence of clinical trials, with human plasma as placebo in control arm, doesn’t allow to exclude that human non convalescent plasma can obtain at least part of the same expected clinical outcomes.
References
- Wu Z, McGoogan JM (2020) Characteristics of and important lessons from the corona virus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 323: 1239-1242.
- Wu C, Chen X, Cai Y, Xia J, Zhou X, et al. (2020) Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA.
- Hoffmann M, Kleine-Weber H, Schroeder S, Kru¨ger N, Herrler T, et al. (2020) SARS-CoV-2 Cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181: 271–280.
- Muus C, Luecken MD, Eraslan G, Waghray A, Heimberg G, et al. (2020) Integrated analyses of single-cell atlases reveal age, gender, and smoking status associations with cell type-specific expression of mediators of SARS-CoV-2 viral entry and highlights inflammatory programs in putative target cells. Bio Rxiv preprint.
- Blanco-Melo D, Nilsson-Payant BE, Liu WC, Uhl S, Hoagland D, et al. (2020) Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 181: 1–10.
- Mason RJ (2020) Pathogenesis of COVID-19 from a cell biologic perspective. Eur Respir J.
- Magro C, MulveyJJ, Berlin D, Nuovo G, Salvatore S, et at. (2020) Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Translational Research.
- Zhou H, Deng M, Liu Y, Yang C, Hoffman R, et al. (2018) Platelet HMGB1 is required for efficient bacterial clearance in intra-abdominal bacterial sepsis in mice. Blood Adv 2: 638-648.
- Conti P, Ronconi G, Caraffa A, Gallenga CE, Ross R, etal. (2020) Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents 34.
- Zhang C, Wu Z, Li JW, Zhao H, Wang GQ (2020) The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents 55.
- Delgado-Rizo V, Martínez-Guzmán MA, Iñiguez-Gutierrez L, García-Orozco A, Alvarado-Navarro A et al. (2017) NeutrophilExtracellularTraps and Its implications in inflammation: An Overview. Front Immunol 8.
- Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, et al. (2020) Neutrophil extracellular traps in COVID-19. JCI Insight.
- Barnes BJ, Adrover JM, Baxter-Stoltzfus A, Borczuk A, Cools-Lartigue J, et al. (2020) Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med 217: e20200652.
- Von Brühl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M,et al. (2012) Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med 209: 819-835.
- Varatharajah N, Rajah S (2020) Microthrombotic Complications of COVID-19 Are Likely Due to Embolism of Circulating Endothelial Derived Ultra large Von Wille brand Factor (eULVWF) Decorated-Platelet Strings. Fed Pract 37.
- Garland KS, Reitsma SE, Shirai T, Zilberman-Rudenko J, Tucker EI, et al. (2017) Removal of the C-Terminal Domains of ADAMTS13 by Activated Coagulation Factor XI induces Platelet Adhesion on Endothelial Cells under Flow Conditions. Front Med 4: 232.
- Massberg S, Grahl L, von Bruehl ML, Manukyan D, Pfeiler S, et al. (2010) Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med 16: 887–96.
- Lehman HK, Segal BH (2020) The role of neutrophils in host defense and disease. J Allergy Clin Immunol.
- Bernardo A, Ball C, Nolasco L, Choi H, Moake JL, et al. (2005) Platelets adhered to endothelial cell-bound ultra-large von Wille brand factor strings support leukocyte tethering and rolling under high shear stress. J Thromb Haemost 3: 562‐570.
- Chang JC (2018) TTP-like syndrome: novel concept and molecular pathogenesis of endotheliopathy-associated vascular microthrombotic disease. Thrombosis Journal 16.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi