Case Report, Clin Oncol Case Rep Vol: 5 Issue: 4
Cutaneous Squamous Cell Carcinoma and Epidermolysis Bullosa: An Unholy Alliance-Case Report and Review of The Literature
Ethan Kallick1, Maya Garg2, Jeffrey Uchin3, and Howard Edington1*
1Surgery, Cancer Institute of Allegheny Health Network, Pittsburgh, Pennsylvania
2Carnegie Mellon University Biomedical Engineering, Pittsburgh, Pennsylvania
3Dermatopathology Pathology Institute of Allegheny Health Network, Pittsburgh, Pennsylvania
*Corresponding Author: Howard Edington
Surgery, Cancer Institute of Allegheny Health Network, Pittsburgh, Pennsylvania, USA
E-mail: Howard.EDINGTON@ahn.org
Received: March 30, 2022; Manuscript No: COCR-22-58955;
Editor Assigned: April 02, 2022; PreQC Id: COCR-22-58955 (PQ);
Reviewed: April 19, 2022; QC No: COCR-22-58955 (Q);
Revised: April 23, 2022; Manuscript No: COCR-22-58955 (R);
Published: April 30, 2022; DOI: 10.4172/cocr.5(4).225
Citation: Kallick E, Garg M, Uchin J, Edington H (2022) Cutaneous Squamous Cell Carcinoma and Epidermolysis Bullosa: An Unholy Alliance-Case Report and Review of The Literature. Clin Oncol Case Rep 5:4
Abstract
A female patient with a clinical history of Epidermolysis Bullosa (EB) presented with a large, necrotic mass on the posterior aspect of the right leg. The patient had previously undergone a biopsy of the mass at an outside institution that was non-diagnostic for malignancy; the mass had been presumed to be a venous stasis ulcer, and was treated as such. Upon presentation to our clinic, clinical suspicion was that this mass was a wound-derived Squamous Cell Carcinoma (SCC). Repeat biopsy was obtained, with careful consideration to biopsy tumor as opposed to necrotic and granulation tissue within the mass. Pathology confirmed the clinical suspicion and imaging showed that the disease was metastatic. The patient underwent surgery to remove the mass, followed by systemic therapy. This case highlights the phenomenon of skin cancer arising in chronically inflamed or abnormal skin, including scars and/or wounds. This case also elucidates the importance of managing this unique patient population at increased risk of cutaneous malignancy, as well as the salient issues that naturally ensue with obtaining proper diagnosis, including the necessity of repeat biopsy if the initial biopsy results run counter to what clinical suspicion engenders.
Keywords: Epidermolysis bullosa (EB); Squamous cell carcinoma (SCC); Biopsy; Skin cancer; Wound-derived squamous cell carcinoma; Marjolina’s ulcer
Introduction
Nonmelanoma Skin Cancer (NMSC) is the most diagnosed malignancy in the United States [1-3]. The number of cases per annum is generally accepted to exceed all other malignancies put together [4]. Basal Cell Carcinoma (BCC) and Squamous Cell Carcinoma (SCC) account for the vast majority of NMSC (>95%) [4].
The overwhelming majority of NMSK patients are managed in the office and do well. The incidence of metastasis and mortality are generally low (see discussion). However, there are recognized patient populations at increased risk for accelerated and aggressive tumor transformation and progression. Early identification of patients at elevated risk in concert with timely initial diagnosis and aggressive treatment is likely to impact the ultimate course of the disease and prognosis. Once metastasis has occurred, survival is significantly decreased [5]. The pathogenesis of cutaneous squamous cell carcinoma is understood to be multifactorial, including both intrinsic and extrinsic factors and their interplay. Extrinsic factors include UV and other ionizing radiation exposure, various chemical carcinogens and induced immunosuppression (organ transplant) [4-6]. Viral etiologies, including HPV, have been associated [7]. Intrinsic factors include chronic scarring and inflammation8. Various genodermatoses are linked to elevated risk and accelerated tumor progression [8,9]. Perhaps the best studied malady is xeroderma pigmentosum, in which an inherited defect in DNA repair prevents the normal host response to UV radiation exposure [10,11].
Epidermolysis Bullosa (EB), likely due to its propensity to cause chronic skin inflammation, is one such example of a disorder that is associated with SCC oncogenesis. EB encompasses a heterogeneous group of genetic skin fragility/blistering disorders, arising from mutations in genes encoding dermal-epidermal adhesion proteins that are crucial for physiologic function of the basement membrane [12-14].
Case Report
An 80-year-old Caucasian female with a clinical history of EB presented with a large, fungating, and necrotic tumor of the posterior aspect of the right leg measuring 13 cm × 15 cm (Figure 1). She had a long history of intermittent skin blistering and desquamation and although her disease was never subtyped, she did show the clinical changes consistent with the broad diagnosis (Figure 2). She, in addition, had a multiple year history of a chronic and progressive wound of the posterior aspect of the right leg. The wound was presumed to be a venous stasis ulcer and was treated with compression therapy including Unna boot applications, without resolution. The patient had previously undergone a skin shave biopsy of the mass, which was interpreted at an outside institution as necroinflammatory changes, non-diagnostic for SCC (Figure 3). Initial physical exam confirmed the presence of a foul smelling, fungating tumor of the posterior right leg, superficial to the Achilles tendon. She had an ipsilateral enlarged inguinal lymph node consistent with metastatic disease. Subsequent internal review of that initial outside biopsy and a subsequent biopsy would confirm the clinical impression of invasive squamous cell carcinoma.
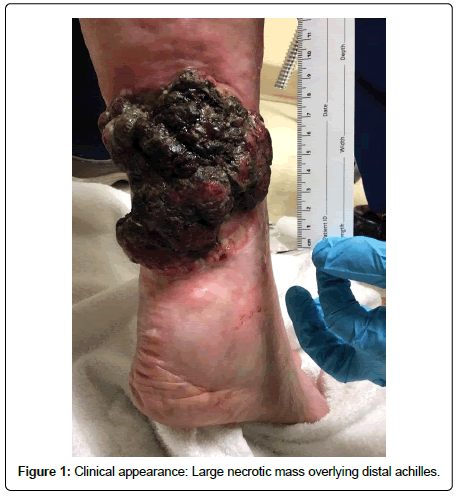
Figure 1: Clinical appearance: Large necrotic mass overlying distal achilles.
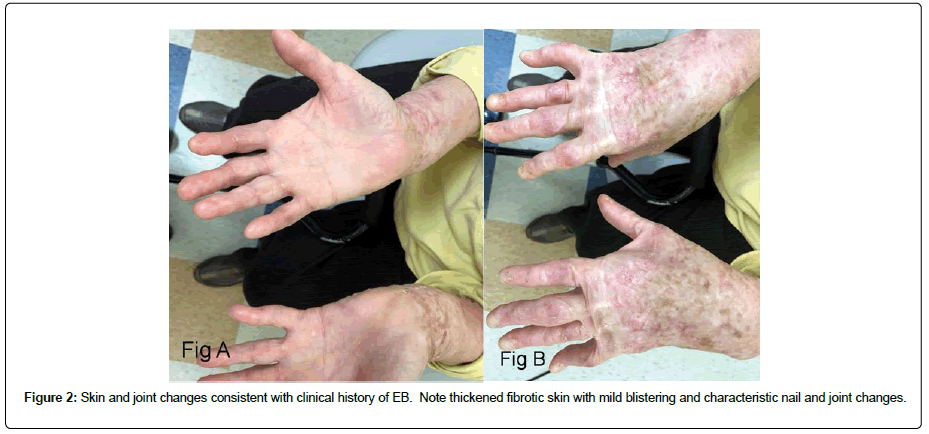
Figure 2: Skin and joint changes consistent with clinical history of EB. Note thickened fibrotic skin with mild blistering and characteristic nail and joint changes.
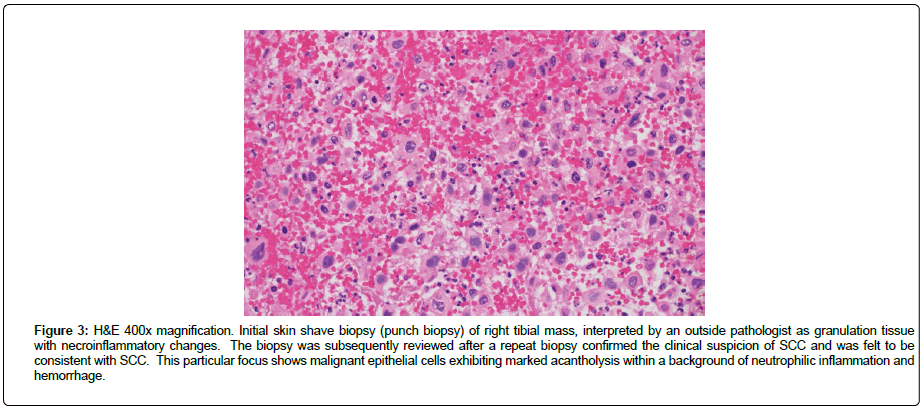
Figure 3: H&E 400x magnification. Initial skin shave biopsy (punch biopsy) of right tibial mass, interpreted by an outside pathologist as granulation tissue with necroinflammatory changes. The biopsy was subsequently reviewed after a repeat biopsy confirmed the clinical suspicion of SCC and was felt to be consistent with SCC. This particular focus shows malignant epithelial cells exhibiting marked acantholysis within a background of neutrophilic inflammation and hemorrhage.
Staging imaging was scheduled and an expedited palliative surgical resection was performed after maggot infestation was reported. The tumor was resected with free gross margins. The resection site was left open pending final pathology and completion of staging imaging.
Histologic sections of that resection specimen showed a poorly differentiated squamous cell carcinoma, composed of large atypical cells with epithelioid morphology, large nuclei, and prominent nucleoli exhibiting marked acantholysis accompanied by foci of tumoral necrosis. There were rare foci in which the poorly differentiated tumor cells could be seen arising from a focus of more well-differentiated squamous cell carcinoma (Figures 4, Figure 5). Epithelial lineage of the tumor cells was immunohistochemically confirmed with positive staining for pan-cytokeratin and p40.
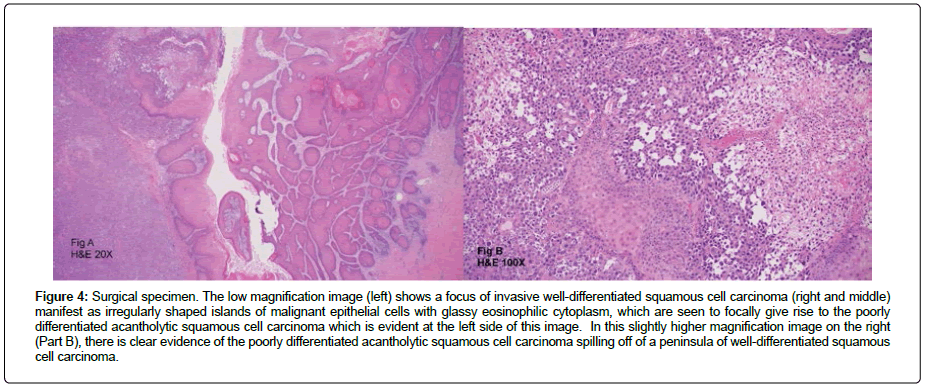
Figure 4: Surgical specimen. The low magnification image (left) shows a focus of invasive well-differentiated squamous cell carcinoma (right and middle) manifest as irregularly shaped islands of malignant epithelial cells with glassy eosinophilic cytoplasm, which are seen to focally give rise to the poorly differentiated acantholytic squamous cell carcinoma which is evident at the left side of this image. In this slightly higher magnification image on the right (Part B), there is clear evidence of the poorly differentiated acantholytic squamous cell carcinoma spilling off of a peninsula of well-differentiated squamous cell carcinoma.
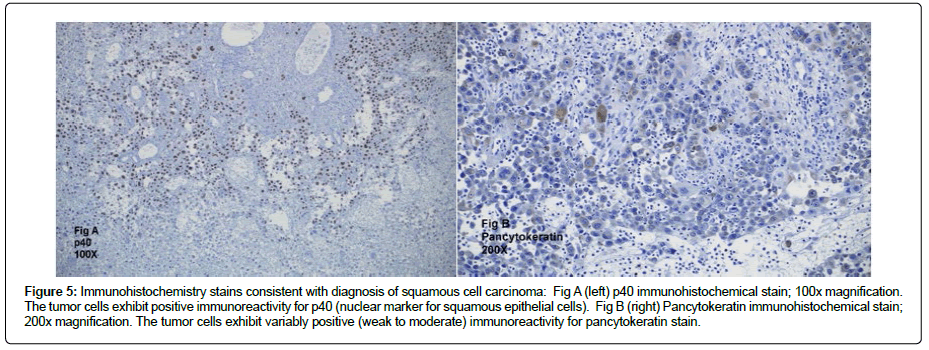
Figure 5: Immunohistochemistry stains consistent with diagnosis of squamous cell carcinoma: Fig A (left) p40 immunohistochemical stain; 100x magnification. The tumor cells exhibit positive immunoreactivity for p40 (nuclear marker for squamous epithelial cells). Fig B (right) Pancytokeratin immunohistochemical stain; 200x magnification. The tumor cells exhibit variably positive (weak to moderate) immunoreactivity for pancytokeratin stain.
The postoperative course was uneventful and the open wound was easily managed with dressing changes. Imaging studies were completed and showed extensive pulmonary and ilioinguinal metastases (Figures 6, Figure 7).
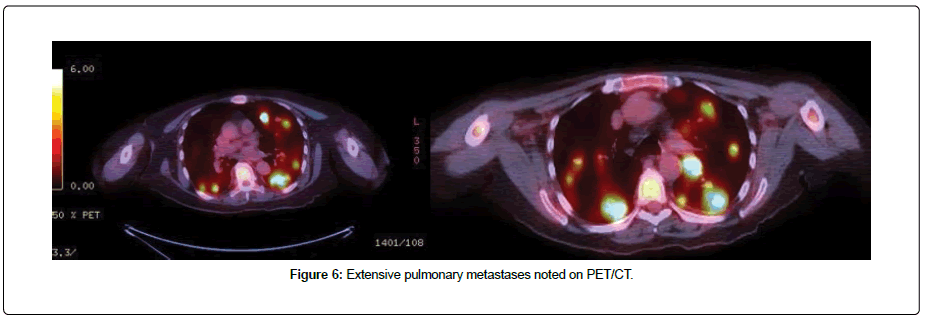
Figure 6: Extensive pulmonary metastases noted on PET/CT.
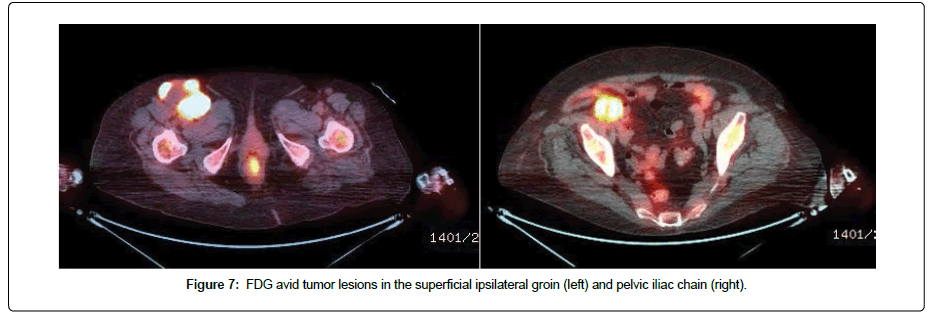
Figure 7: FDG avid tumor lesions in the superficial ipsilateral groin (left) and pelvic iliac chain (right).
Systemic treatment was initiated with Cemiplimab, an anti-PD1 drug with demonstrated response rates in advanced or metastatic SCC [15].
Discussion
Incidence of NMSK
It is generally accepted that the incidence of NMSC exceeds all the other malignancies put together [4] with an annual cost of $8.1 billion [16]. However, the actual number of NMSC cases is unknown because such cases are not reported to cancer registries, so incidence data is derived from medical claims data, survey data, and special studies [17-19]. It should be noted that melanoma statistics are in fact reported [1], which underscores the fact that clinically, NMSC tends to be underemphasized. The most recent study of NMSC occurrence estimated that in 2012, 5.4 million cases were diagnosed among 3.3 million people [1].
The mortality risk associated with NMSC is low: A study that examined deaths due to NMSC in Rhode Island between 1988-2000 showed mortality rates of 0.29 and 0.08 per 100,000 people per year for nongenital SCC and BCC, respectively [20]. Similarly, NMSC metastasis risk is relatively low; metastasis is thought to occur in <0. 1% of BCC patients [21,22]. However, metastasis in SCC is higher and is commonly reported as occurring in approximately 3%-9% of patients [23,24]; in this SCC cohort with metastatic progression, metastasis occurs most frequently to the regional lymph nodes and then systemically (often to the lungs, bone, and brain). Factors that may increase the risk of SCC metastasis include tumor diameter >2 cm, depth of invasion >4 cm, poor differentiation, scar carcinoma, perineural invasion of nerves >0.1 mm in diameter, immunosuppression, and history of previous treatments [4,5]. The 5-year survival rate for patients with metastatic cutaneous SCC remains guarded, at 34% [5].
Pathophysiology
There is a clear and definitive link between Ultraviolet (UV) skin exposure and SCC; this causative relationship is highlighted by a correlation between SCC incidence and geographic latitude, with SCC incidence doubling with each 8 to 10-degree decline in geographic latitude towards the equator [25,26]. Ultraviolet-B light (290 nm to 320 nm) is the most carcinogenic [27] and thus, is most implicated in SCC carcinogenesis. While UV-exposure is responsible for approximately 90% of NMSC cases [28-30], there is a subset of SCC patients in which SCC originates in abnormal skin, as described previously, with pathology arising from varied clinical entities as burns, varicose ulcers, Human Papilloma Virus (HPV) infection, chronic inflammation, carcinogenic chemicals, chronic immunosuppression, chronic wounds, and several genetic syndromes. Perhaps most relevant to this report is Marjolin’s ulcer, a SCC that develops in old scars and/or chronic wounds, with latency periods of up to 30 years [31,32]. Marjolin’s ulcer represents a more aggressive clinical course with metastasis occurring between 18% to 38% of the time [33] and mortality rates as high as 32.6% [34].
Intrinsic and extrinsic risk factors
Numerous risk factors have been identified which increase the likelihood of developing SCC, and portend a poor prognosis due to early and aggressive metastatic behavior. Whether multiple risk factors have an additive or synergistic impact is not known; however, clinical experience suggests the latter.
In a subset of patients, SCC is associated with long-standing wounds, irritation, or inflammation, which is clinically pertinent given that long-standing wounds have a 2% risk of harboring a SCC [35]. SCC associated with long-standing wounds was first described in the setting of burn wounds (Marjolin’s ulcer), however, malignant degeneration may occur in any chronic wound with features that include venous stasis ulcers, decubitus ulcers, hidradenitis, and chronic osteomyelitis. The possibility of malignancy should be considered in any chronic wound, and a biopsy should be performed, with particular attention paid to ensure biopsy of the tumor, as opposed to surrounding fibrotic or granulation tissue. Timely diagnosis is crucial, given that wound or scar-associated SCC tends to be more aggressive than its UV-induced counterpart, with metastases occurring more frequently (from 20% to 30%) [36,37], and overall prognosis for patients with metastatic disease being relatively poor, as previously described [38].
Indeed, Marjolin’s ulcer can arise in a chronic blistering wound as result of EB. EB is a broad diagnosis and includes a number of entities which have a common clinical presentation- skin fragility manifest as blistering in a setting of minimal to insignificant trauma (e.g rubbing); histologically this appears as subepidermal pauciinflammatory blistering. It is suggested that EB patients should be considered a population at elevated risk for SCC. This proposition has been confirmed for some of the EB subtypes. Particularly, severe recessive dystrophic EB (RDEB-S), is associated with increased risk of aggressive mucocutaneous SCC [39].
Four types of EB are recognized clinically, characterized by the level of skin cleavage/blistering: EB simplex (EBS), junctional EB (JEB), dystrophic EB (DEB), and Kindler EB (KEB) [14]. SCC in the EB patient arises in tissue with phenotypic features of chronic skin blistering, wounds, and fibrotic scarring [40]. Furthermore, in EB-associated malignant degeneration, multiple primary SCC’s often develop and have a propensity towards progression and metastasis [14]. As previously mentioned, a subtype of DEB termed severe recessive DEB (RDEB-S) has been described, in which SCC oncogenesis occurs almost invariably in all patients and is associated with a very aggressive biological behavior and attendant high morbidity and mortality [40]. According to the USA National EB Registry, the risk of developing at least one SCC for RDEB-S patients is 67.8% by age 35 and approaches 90.1% by age 55 [39]. Furthermore, EB-associated SCC’s are the leading cause of death in individuals with recessive DEB (RDEB-S) [39]. A study from Robertson et al. highlights the risk of SCC in EB (and particularly RDEB-S), ultimately showing that among 44 EB patients with SCC, 70% had RDEB-S and among RDEB-S patients, SCC tended to present earlier as compared to all other collective EB subtypes (median age 29. 5 years compared to 47.1 years) [14]. Furthermore, RDEB-S SCC patients tended to have multiple tumors (mean 5.8) as well as high rate of mortality (64.5%), with median survival after first SCC of 2.4 years [14].
Despite the clear link between EB (and especially RDEB-S) and SCC, further investigation is needed to understand the molecular mechanisms responsible for the development of SCC in EB patients [40]. Recent evidence suggests that mutations in the COL7A1 gene represent an important antecedent of SCC tumorigenesis in RDEB-S patients, with COL7A1 knockout in fibroblasts, keratinocytes, and extracutaneous tissue preceding a complex series of pro-tumorigenic molecular processes that include inflammation, angiogenesis, and tumor cell invasion [40]. Moreover, chronic inflammation plays a role in EB pathogenesis, which is supported by cytokine imbalance, and especially excessive plasma levels of the pro-inflammatory cytokine Interleukin-6 (IL-6) [41,42] in both EB animal models and patients; this evidence suggests that inflammation contributes to pathological perturbation of the dermal microenvironment while simultaneously leading to exacerbation of systemic disease manifestations [40]. These pathophysiological mechanisms are not mutually exclusive; instead, they can, and do, occur in concert: this is supported by a COL7A1 knockout mice model, which shows excessive inflammation in the upper dermis [43] as well as increased serum concentrations of IL-6 [40,41].
While more needs to be learned about the unique pathophysiological mechanisms that underlie SCC development in the EB patient, it is clear that this patient population remains a unique challenge to treat and ultimately necessitates a multidisciplinary care approach from surgical oncology, medical oncology, radiation oncology, and dermatology alike. This patient also demonstrates the difficulty in diagnosing SCC when present in tandem with chronic wounds, as the initial biopsy did not render a diagnosis of a carcinoma. This indicates that SCCs arisen from chronic wounds and/ or EB may require multiple biopsies to confirm either the presence or absence of a carcinoma. While surgical excision is curative for localized disease in up to 95% of patients [44], systemic treatment is necessary if SCC has metastasized. In the SCC patient with metastatic disease, a common combination chemotherapy regimen initiated post-surgery is cisplatin, 5-fluorouracil, paclitaxel, and methotrexate [45]. Second-line options of epidermal growth factor receptor (EGFR) inhibitors such as cetuximab or erlotinib may be employed in patients with advanced, unresectable SCC if polychemotherapy fails [46,47]. Importantly, the first anti-PD1 drug cemiplimab was recently approved by the US Food and Drug Administration in September 2018 and is an effective therapy with demonstrated responses in roughly 50% of SCC patients with advanced or metastatic disease [15]. This may be an appropriate choice for patients unable to tolerate the toxicity of front line therapy. Response statistics underscore the need for better systemic therapy perhaps guided by relevant biomarkers.
Conclusion
Ultimately, for the vast majority of patients, SCC is a highly curable disease with surgical excision providing durable cure. However, there are a subset of patients who may be identified as being at elevated risk for the development of an aggressive form of SCC and for whom metastatic potential and mortality rate are significantly elevated. These high risk group patients can be identified according to both extrinsic and intrinsic factors including EB as described. Patients at elevated risk should benefit from increased surveillance perhaps in a multidisciplinary skin cancer clinic. Importantly, a high index of clinical suspicion should be present in this group- particularly in patients having multiple risk factors as the case described. Clinicians should be aware of the possibility of sampling bias in any tumor, especially a large necrotic superficially sampled one. Additional sampling is warranted when a superficial subtotal biopsy read is not in keeping with the clinical impression of malignancy.
References
- American Cancer Society (2022) Cancer Facts and Figures 2022. Am Cancer Soc.
- Miller D, Weinstock M (1994) Nonmelanoma skin cancer in the United States: Incidence. J Am Acad Dermatol 30: 774-778.
Google Scholar Cross Ref - Parker SL, Tong T, Bolden S, Wingo PA (1997) Cancer statistics, 1997. CA Cancer J Clin 47: 5-27.
Google Scholar - Acarturk T, Edington H (2005) Nonmelanoma skin cancer. Clin Plast Surg 32: 237-248.
Google Scholar Cross Ref - Rowe DE, Carroll RJ, Day Jr CL (1992) Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. J Am Acad Dermatol 26: 976-990.
Google Scholar Cross Ref - Tessari G, Girolomoni G (2012) Nonmelanoma skin cancer in solid organ transplant recipients: Update on epidemiology, risk factors, and management. Dermatol Surg 38: 1622-1630.
Google Scholar Cross Ref - Wang J, Aldabagh B, Yu J, Arron ST (2014) Role of human papillomavirus in cutaneous squamous cell carcinoma: A meta-analysis. J Am Acad Dermatol 70: 621-629.
Google Scholar Cross Ref - BazaliÅ?ski D, Przybek-Mita J, BaraÅ?ska B, WiÄ?ch P (2017) Marjolin’s ulcer in chronic wounds-Review of available literature. Contemp Oncol (Pozn) 21: 197-202.
Google Scholar Cross Ref - Schierbeck J, Vestergaard T, Bygum A (2019) Skin cancer associated genodermatoses: A literature review. Acta Dermato-Venereologica 99: 360-369.
Google Scholar - Baykal C, Atçi T, Yilmaz Z, Büyükbabani N (2021) Skin tumors in xeroderma pigmentosum: Evaluation of a large series and a literature review. J Cutan Pathol 48: 884-895.
Google Scholar - Cancer ASCO (2020) Xeroderma Pigmentosum. Cancer net.
- Bardhan A, Bruckner-Tuderman L, Chapple I, Fine JD, Harper N, et al. (2020) Epidermolysis bullosa. Nat Rev Dis Primers 24: 78.
Google Scholar Cross Ref - Sawamura D, Nakano H, Matsuzaki Y (2010) Overview of epidermolysis bullosa. J Dermatol 37: 214-219.
Google Scholar Cross Ref - Robertson SJ, Orrin E, Lakhan MK, O’Sullivan G, Felton J, et al. (2021) Cutaneous squamous cell carcinoma in epidermolysis bullosa: A 28-year retrospective study. Acta Dermato-Venereologica 101: 1-8.
Google Scholar Cross Ref - Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, et al. (2018) PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. New Eng J Med 379: 341-351.
Google Scholar Cross Ref - Guy Jr GP, Machlin SR, Ekwueme DU, Yabroff R (2015) Prevalence and costs of skin cancer treatment in the US, 2002-2006 and 2007-2011. Am J Prev Med 48:183-187.
Google Scholar Cross Ref - Rogers HW, Weinstock MA, Harris AR, Hinckley MR, Feldman SR, et al. (2010) Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Derm 146: 283-287.
Google Scholar Cross Ref - Office of the General Surgeon (2014) The surgeon general’s call to action to prevent skin cancer. Exec Summary.
Google Scholar - Karagas M, Weinstock M, Nelson H (2006) Keratinocyte carcinomas (basal and squamous cell carcinomas of the skin). In: cancer epidemiology and prevention (3rd Edition). Oxford University Press 1230-1250.
Google Scholar - Lewis KG, Weinstock MA (2004) Nonmelanoma skin cancer mortality (1988-2000): The Rhode Island follow-back study. Arch Dermatol 140: 837-842.
Google Scholar Cross Ref - Von Domarus H, Stevens PJ (1984) Metastatic basal cell carcinoma. Report of five cases and review of 170 cases in the literature. J Am Acad Dermatol 10: 1043-1060.
Google Scholar Cross Ref - Wermuth BM, Fajardo LF (1970) Metastatic basal cell carcinoma. A review. Arch Pathol 90: 458-462.
Google Scholar - Shreve C, Shropshire C, Cotter DG (2020) Metastatic squamous cell carcinoma: A cautionary tale. Cureus 12: 1-5.
Google Scholar Cross Ref - Weinberg AS, Ogle CA, Shim EK (2007) Metastatic cutaneous squamous cell carcinoma: An update. Dermatol Surg 33: 885-899.
Google Scholar Cross Ref - Johnson TM, Rowe DE, Nelson BR, Swanson NA (1992) Squamous cell carcinoma of the skin (excluding lip and oral mucosa). J Am Acad Dermatol 26: 467-484.
Google Scholar Cross Ref - Rudolph R, Zelac DE (2004) Squamous cell carcinoma of the skin. Plast Reconstr Surg 114: 82e-94e.
Google Scholar Cross Ref - De Gruijl FR (1996) Photobiology of photocarcinogenesis. Photochem Photobiol 63: 372-375.
Google Scholar Cross Ref - Teng Y, Yu Y, Li S, Huang Y, Xu D, et al. (2021) Ultraviolet Radiation and Basal Cell Carcinoma: An Environmental Perspective. Front Public Health 9: 1-12.
Google Scholar Cross Ref - Marks R (1995) The epidemiology of non-melanoma skin cancer: who, why and what can we do about it. J Dermatol 22: 853-857.
Google Scholar Cross Ref - Pleasance ED, Cheetham RK, Stephens PJ, McBride DJ, Humphray SJ, et al. (2010) A comprehensive catalogue of somatic mutations from a human cancer genome. Nature 463: 191-196.
Google Scholar Cross Ref - Yu N, Long X, Lujan-Hernandez JR, Hassan KZ, Bai M, et al. (2013) Marjolin’s ulcer: A preventable malignancy arising from scars. World J Surg Oncol 11: 313.
Google Scholar Cross Ref - Kowal-Vern A, Criswell BK (2005) Burn scar neoplasms: A literature review and statistical analysis. Burns 31: 403-413.
Google Scholar Cross Ref - Goldman GD (1998) Squamous cell cancer: a practical approach. Semin Cutan Med Surg 17: 80-95.
Google Scholar - Novick M, Gard DA, Hardy SB, Spira M (1977) Burn scar carcinoma: A review and analysis of 46 cases. J Trauma 17: 809-817.
Google Scholar - Arons MS, Lynch JB, Lewis SR, Blocker TG (1965) Scar tissue carcinoma. i. a clinical study with special reference to burn scar carcinoma. Ann Surg 161: 170-188.
Google Scholar Cross Ref - Fleming ID, Amonette R, Monaghan T, Fleming MD (1995) Principles of management of basal and squamous cell carcinoma of the skin. Cancer 75(2 Suppl):699-704.
Google Scholar - Preston DS, Stern RS (1992) Nonmelanoma cancers of the skin. N Engl J Med 327: 1649-1662.
Google Scholar Cross Ref - Akoz T, Erdogan B, Gorgu M, Aslan G (1997) The necessity for aggressive treatment with Marjolin’s ulcers of the scalp. Plast Reconstr Surg 100: 805-806.
Google Scholar - Fine JD, Johnson LB, Weiner M, Li KP, Suchindran C (2009) Epidermolysis bullosa and the risk of life-threatening cancers: the National EB Registry experience, 1986-2006. J Am Acad Dermatol 60: 203-211.
Google Scholar Cross Ref - Condorelli AG, Dellambra E, Logli E, Zambruno G, Castiglia D (2019) Epidermolysis bullosa-associated squamous cell carcinoma: From pathogenesis to therapeutic perspectives. Int J Mol Sci 20: 5707.
Google Scholar Cross Ref - Liao Y, Ivanova L, Zhu H, Plumer T, Hamby C, et al. (2018) Cord blood-derived stem cells suppress fibrosis and may prevent malignant progression in recessive dystrophic epidermolysis bullosa. Stem Cells. 36: 1839-1850.
Google Scholar Cross Ref - Esposito S, Guez S, Orenti A, Tadini G, Scuvera G, et al. (2016) Autoimmunity and cytokine imbalance in inherited epidermolysis bullosa. Int J Molecular Sci 17: 1625.
Google Scholar Cross Ref - Fritsch A, Loeckermann S, Kern JS, Braun A, Bosl MR, et al. (2008) A hypomorphic mouse model of dystrophic epidermolysis bullosa reveals mechanisms of disease and response to fibroblast therapy. J Clin Invest 118: 1669-1679.
Google Scholar Cross Ref - Breuninger H, Eigentler T, Bootz F, Hauschild A, Kortmann RD, et al. (2013) Brief S2k guidelines-Cutaneous squamous cell carcinoma. J Dtsch Dermatol Ges Suppl 3:37-45.
Google Scholar Cross Ref - Ricci F, Paradisi A, Fossati B, Mancini M, Curatolo P, et al. (2015) Giant neglected squamous cell carcinoma of the skin. Dermatologic Ther 28: 230-234.
Google Scholar Cross Ref - Misiakos EP, Damaskou V, Koumarianou A, Goulomi AR, Patapis P, et al. (2017) A giant squamous cell carcinoma of the skin of the thoracic wall: A case report and review of the literature. Journal of Medical Case Reports 11: 136.
Google Scholar Cross Ref - Stratigos A, Garbe C, Lebbe C, Malvehy J, del Marmol V, et al. (2015) Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur J Cancer 51: 1989-2007.
Google Scholar Cross Ref
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 