Review Article, Clin Dermatol Res J Vol: 8 Issue: 4
Dermascopic Features of Androgenetic Alopecia: A Review Article
Zakaria Ali*, Jianjun Qiao and Hong Fang
Department of Dermatology, Zhejiang University School of Medicine, Hangzhou 310003, China
*Corresponding Author: Zakaria Ali
Department of Dermatology, Zhejiang
University School of Medicine, Hangzhou, 310003, China
Tel: 4591778556
E-mail: Ipeaceful@live.com
Received date: 24 January, 2023, Manuscript No. CDRJ-23-87860;
Editor assigned date: 27 January, 2023, PreQC No. CDRJ-23-87860 (PQ);
Reviewed date: 10 February, 2023, QC No. CDRJ-23-87860;
Revised date: 18 April, 2023, Manuscript No. CDRJ-23-87860 (R);
Published date: 25 April, 2023, DOI: 10.4172/2576-1439.1000209
Citation: Ali Z, Qiao J, Fang H (2023) Dermascopic Features of Androgenetic Alopecia: A Review Article. Clin Dermatol Res J 8:3.
Abstract
Background: Hair and scalp dermoscopy, also known as trichoscopy, is a useful, non-invasive method for diagnosing alopecia and other scalp and hair conditions. It is gaining popularity, but there are still few review reports on the dermascopic findings of AGA. Objective: The aim of this study is to review the clinical and trichoscopic features of AGA patients.
Methods: A comprehensive search was conducted with the PubMed, Cochrane Library, Embase, and Scopus databases. We identified eligible published articles up to February 2022.
Results: Included were 18 articles yielding 3202 patients with androgenic alopecia. Most patients with androgenic alopecia displayed yellow dots, black dots, white dots, honeycomb pigmentation, vellus hairs, tapered hair, peripilar sign, Hair thickness heterogeneity, and perifollicular hyperpigmentation under the dermoscope.
Conclusion: This study has shown the significances of trichoscopy with androgenic alopecia patients, which can prove important to monitor early disease activity.
Keywords: Androgenic alopecia, Trichoscopy, Dermoscopy, Hai rloss, Pattern hair loss
Introduction
Androgenetic alopecia is a genetic disorder characterized by progressive transformation of terminal hairs into indeterminate and eventually vellus hairs. It is an extremely common disease that affects men and women [1]. Men with this condition, called male pattern baldness, can start losing hair from their teens through their twenties. It is characterized by a receding hairline and progressive hair loss from the crown and frontal scalp [2]. Women with a condition called female pattern baldness do not see significant thinning until they are 40 or older. Women experience a general thinning over the entire scalp, with the most extensive hair loss at the crown [3]. Standard methods used to diagnose hair diseases include clinical examination, hair loss pattern, traction test, trichogram, biopsy and screening blood tests. Their different in sensitivity, reproducibility and invasiveness. Trichoscopy is very useful for in vivo diagnosis of scalp and hair conditions and can greatly improve clinical management. Both handheld dermatoscope and video dermatoscope can be utilized [4]. The basic principle of dermatoscopy is trans-illumination of a lesion and studying it with high magnification to visualize subtle features. Structures visualized by trichoscopy include hair shafts, hair follicle openings, perifollicular epidermis, and dermal micro vessels. Trichoscopy allows analyzing acquired and congenital hair diseases [5]. Recent studies have gathered evidence that the use of trichoscopy in the clinical evaluation of hair disorders improves diagnostic options beyond the simple clinical examination.
Literature Review
Search strategy
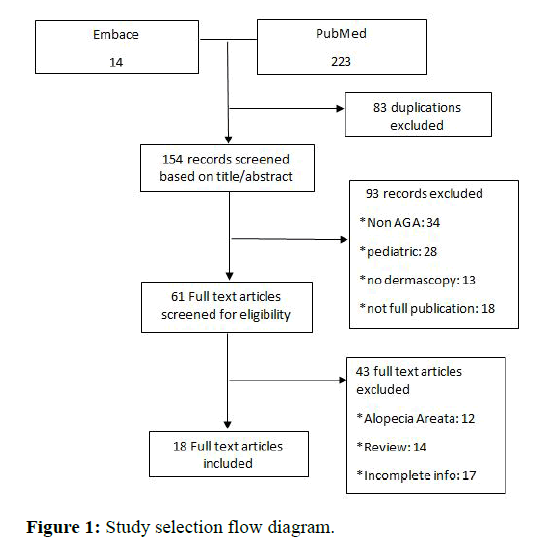
A systematic search of three databases-PubMed, cochrane library, embase, and scopus was performed in February 2021 (Figure 1). A total of 18 observational study articles were included into the review and analysis [6-9]. Articles concerning data on androgenic alopecia were included. The frequency of trichoscopic features was reported in reference to the total number of included patients with AGA. The search terms were ‘‘alopecia’’ and ‘‘androgenic alopecia,’’ ‘‘trichoscopy’’ and ‘‘dermascopy,’’ ‘‘(review)’’ and ‘‘pattern hair loss. Articles written in English were included. There were no limitations on article type. After the selection process, the references of all included articles were assessed for missing publications [10].
Study eligibility, selection criteria, and screening
Independent assessment of the titles and abstracts was performed out independently. All articles reporting cases of AGA and dermascopic screening were used in this study. Both males and females with AGA was included in the article [11]. The diagnosis of AGA was based on Hamilton-Norwood scale for male patients, and Ludwig classification for females patients. The following criteria were used to exclude articles. Pediatric patients (<18 years), patients with any scalp disorders such as irreversible alopecia, trichotillomania, alopecia area ta and scalp psoriasis were excluded from the study [12-14]. Articles that failed to mention the necessary trichoscopic results were also excluded.
Data extraction and statistical analysis
The following variables where gathered as available: Nationality of patients, sample size, age, gender, hair loss severity, areas of scalp observed, examination tool, and dermascopic findings [15].
Results and Discussion
Systemic search results
We included 18 articles yielding 2302 patients with androgenic alopecia that was examined with dermascopy. The majority of articles comprised of observational studies (71%) followed by case control studies (25%). The mean age at presentation was 48.2 years [16]. With a male to female ratio at 3:6:1.
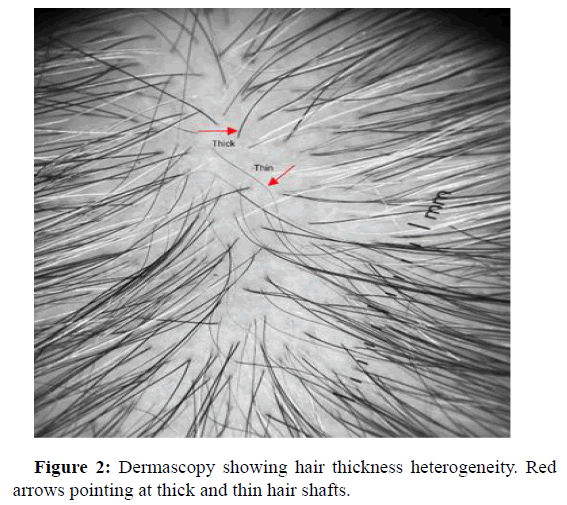
Hair thickness heterogeneity
The heterogeneity of hair thickness is characterized by the simultaneous presence of hair of different thicknesses. Vellus, thin, intermediate and thick. In the case of androgenic alopecia, a change in the diameter of more than 20% of the hair in the androgen dependent region is considered an important diagnostic criterion for androgenic alopecia (Figure 2). Kasumagic, et al., and Adriana, et al., both had most common trichoscopic finding as hair thickness heterogeneity. Kasumagic, et al., showed that all of the participants (104/104) displayed HTH with different thicknesses: Vellus, thin inter mediate and thick hairs [17]. Adriana Rakowska, et al. showed that mean percentage of thin hairs in androgenic alopecia was significantly higher than in healthy controls [18].
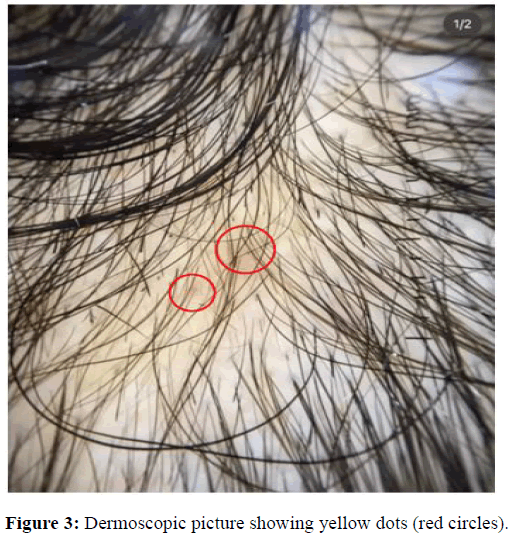
Yellow dots
Trichoscopic yellow dots correspond to an enlarged follicular infundibulum filled with keratotic material and/or sebum [19]. They appear as round or polycyclic structures of various sizes, yellowish pink to tan in color and usually hairless. This difference in results can be explained by the difference in the ethnic groups included in the studies, which means a difference in the activity of the sebaceous glands, as well as the degree of pigmentation of the scalp in advanced stages [20]. Yellow dots were found in 70.88% of patients during examinations in both the early and late stages of AGA. On the other hand, Shruthi, et al., emphasized yellow dots being higher in late AGA in their study. In nearly all studies, yellow dots were observed. Emina, et al.; and Minu, et al. showed 100 % presence of yellow dots in their study (Figure 3).
Black dots
Black dots indicate broken hair follicles on the surface of the skin. They are located in the follicular ostia and are considered a specific trichoscopic sign of alopecia. Shruthi Madhavi, et al., screened the hair shaft and root, as well as tests for hair anchorage and fragility on 100 cases. The mean age of the study group was 26 ± 14.8 years. She found that the common trichoscopic follicular features noted were black hair at 48%. Another study done by Melike, et al., shows a study where black dots sized 1 mm-2 mm were determined in alopecia, and were different from the regular black dots or HCPP seen in alopecia previously (Figure 4). A biopsy from one of these areas in a patient showed no perifollicular, epidermal or dermal infiltration but revealed intense demodex colonisation in follicular ostia.
White dots
It usually represents dead follicles being replaced by fibrous ducts. They can sometimes be confused with the eccrine duct openings which also appear pale, but they can be distinguished because they have a well-defined, rounded structure, are regularly spaced, and can be seen on normal and diseased scalps. In the study conducted by Melike Kibar, et al., white dots were related to severe androgenic alopecia, and that cumulative sun damage makes these dots more visible with honeycomb pigmentation. Soha S. Tawfik et al., reported white dots in 20.3% (16/79) of patients, and according to her study it was related to findings in advanced androgenetic alopecia.
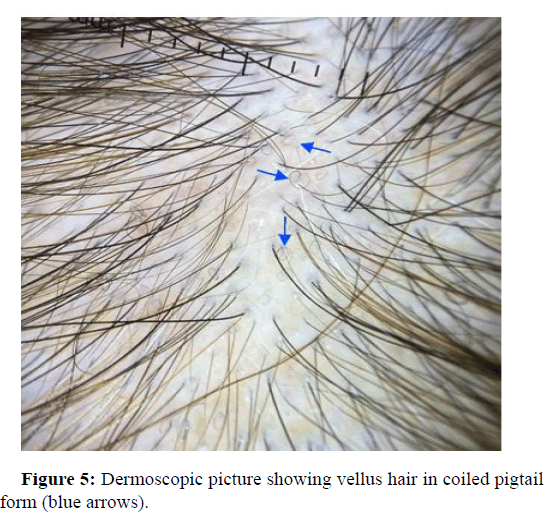
Vellus hairs
Vellus hairs are fine hairs less than 0.04 mm in diameter. Vellus hairs are a sensitive indicator of hair growth. These regrowth hairs can be curled as a pigtail and can be seen in alopecia cases. In AGA patients, the occurrence of vellus hair has been reported in the articles with a frequency of 65%-84%. Jin Park, et al., reports trichoscopic results of three hundred and twenty seven participants, with different alopecia types. Vellus hairs were the most common findings in AGA patients at 55.3%. On the other hand, Adriana Rakowska, et al., calculated dermoscopic image in 20-fold magnification which only showed 2 short hairs in frontal and occipital area and 3 in temporal area in some patients (Figure 5).
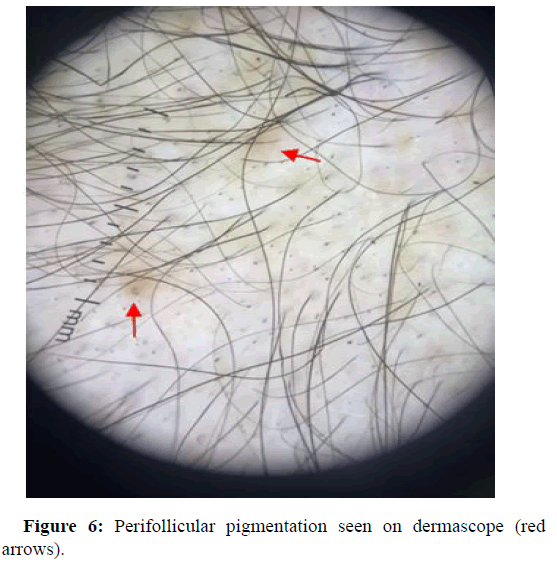
Perifollicular pigmentation
The dermascopic findings of perifollicular pigmentations are thought to be the result of dermal infiltrates in androgenic alopecia patients. Isha Verma, et al., reports of a >3:1 ratio of hair with perifollicular discoloration at the frontal and occipital area in their patients, which were observed in the later stages of alopecia. In other case Emina Kasumagic, et al., showed us that perifollicular pigmentations was seen in 40.38% of patients in the study group (Figure 6). In some repots it states that perifollicular inflammation is allegedly due to the effects of cosmetics, chemicals, ultraviolet light, deposits of mucus and melanocytes, however, the pathogenesis remains not know.
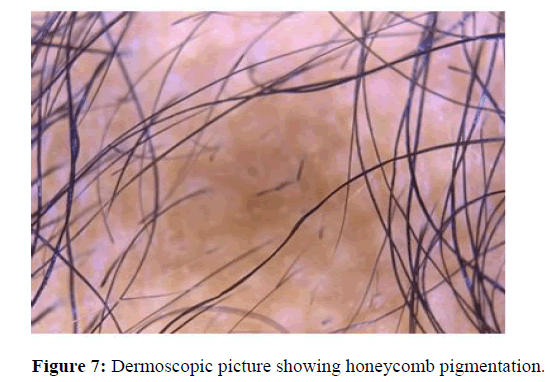
Honeycomb pigmentation
The pigment network consists of a network of intersecting pigment lines that form a honeycomb pattern. The anatomical basis of the pigment network is the melanin of keratinocytes or melanocytes. This pattern was observed in most of the studies found. Jin Park, et al., observed honeycomb pigment network in 19.7% of his patients with the age range of the patient group being 2∼81 years. In another study Soha S Tawfik, et al., showed us honeycomb scalp pigmentation in 17.7%. In this study it is also suggested that it is seen more often in ethnic groups of darker skin with alopecia. In the study conducted by Amudha Ummiti, et al., with patients at the age group between 18 and 70 years shows that honeycomb pigmentation was found in 87.9% of patients, which is statistically significant, and was most commonly seen in all stages of alopecia (Figure 7).
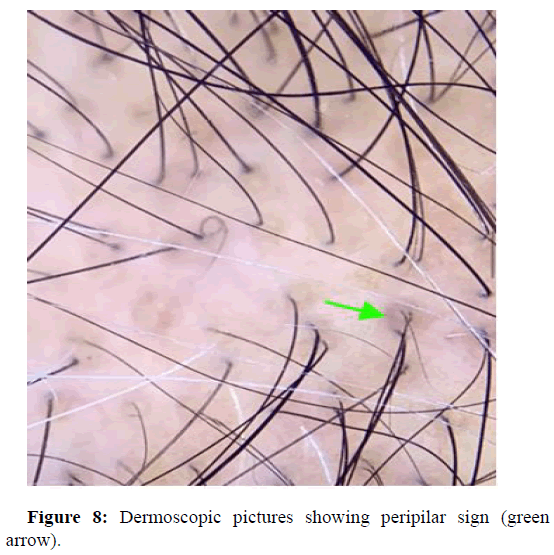
Peripilar sign
Is usually seen as a subtle brown halo and is a specific finding seen in early stages of alopecia. It reflects perifollicular inflammation. Soha S Tawfik, et al., reported of a study that observed androgenic alopecia in seventy nine dark skinned patients with alopecia. Clinical and trichoscopic examination revealed, peripilar brown halo in 32.9% of patients, and also peripilar white halo in 10.1%. The study concluded that peripilar signs are characteristic signs of androgenetic alopecia in ethnic groups of darker skin types (Figure 8). Shoba Mani, et al., showed a study where 47 patients presenting with alopecia were subjected to dermoscopy with a video dermoscope of magnification 50X and 200X. Out of the 47 atients 27 displayed peripilar signs. Marwa Said, et al., reports of a study performed on a total of 200 patients. Among the high statistically significant trichoscopic findings were peripilar sign at 61%. Najam U Saqib, et al., showed a hospital based observational study of 200 alopecia patients, with a median age of onset of 21 years. Peripilar halo among these patients was at 88.7%. Jin Park, et al., observed three hundred and twenty-seven patients with alopecia, where he found 36.1 percent of the study group with peripilar sign (Table 1).
| Article (year): Study design | Country | Sample Size | Age, years | Female | Hair loss severity | Scalp regions observed | Examination toll | Trichoscopic findings |
|---|---|---|---|---|---|---|---|---|
| Ruiming Hu et al. Case control study | China | 950 patients | 18-60 years | 200/950 | Grade II-VII (H-N) scale, grade F1-F3 Ludwig scale | 3-4 images were taken of vertex, frontal and temporal hair line | 10-fold magnification dermascope | HSTH of more than 20%, BPPS, WPPS, yellow dots, focal atrichia and scalp honeycomb pigmentation (P<0.001), and less com mon trichoscopic features were pinpoint white dots (P<0.05). |
| Emina Kasumagic et al. Case-control study | Bosnia | 104 patients | 22-69 years | 44/104 | Grade II-VII (H-N) scale, F2-F3 Ludwig scale | Frontal, occipital and both temporal areas of the scalp | MoleMax II videodermatoscope or handheld dermatoskope DermLite II pro | HTHS=104 (100%), yellow dots=55 (52.88%), vellus hairs=51 (49.03%), Perifollicular hyperpigmented=42 (40.38%). |
| Amudha Ummiti et al. Observational study | India | 91 patients | 18-70 years | 66/91 | Grade II-VII (H-N) scale, grade F1-F3 Ludwig scale | Crown, midscalp, frontal region. | eScope Oitez Digital Microscope, optical magnification 10-40×, 200× | BPPS was seen in early grades of AGA P <0.01, WPPS and focal atrichia were seen in later grades of AGA P <0.01. honeycomb pigmentation was commonly seen in all stages. |
| Shruthi Madhavi et al. Observational study | India | 100 patients | Mean age of 26 ± 14.8 years. | 57/100 | Grade III-IV (H-N) scale, grade F1-F3 Ludwig scale | Frontal, occipital and both temporal areas of the scalp | Dermoscope Dermlite DL3N with ×10 magnification | Broken hair (48%), black dots (48%), single hair follicle unit (45%), short vellus hair (94.1%), upright hair (41%), and yellow dots (40%), honey comb pigment pattern (64.7%) |
| Jin Park et al. Case-control study | South Korea | 327 patients | 20=81 years | 178/327 | Grade II-VII (H-N) scale, grade F1-F3 Ludwig scale | Frontal, vertex and occiput | Polarized-light handheld dermoscope Dermlite DL 3; 3Gen 3 or 4-fold optical zoom canon camera | Hair diversity 71.2%, short vellus hairs (55.4%), peripilar sign 31.7%, single hairs 25.2%, uniform thinning 15.7%, honeycomb pigment 19.6% |
| Najam U Saqib et al. Observational study | India | 200 patients | 19-70 years | 200/200 | Grade F1-F3 Ludwig scale | USB-connected video dermoscopeat magnifications ranging from 20× to 220× | Hair diversity >20%, with a singular hair coming out of follicular openings and thin hair 100%. Other findings were vellus hair 98.3%, peripilar sign 88.7%, yellow dots 28.7%, and focal atrichia 16.5%. | |
| Shigeki Inui et al. Case-control study | Japan | 60 patients | 18-65 years | 10/50 | (H-N) scale, of the 50 AGA patients, 10 had type III AGA, 16 type III vertex, 21 type IV and three type Va. According to Ludwig classification, all 10 AGA patients had type II hair loss. | Central scalp, frontal, and vertex | DermLite II Pro dermoscope (3Gen, San Juan Capistrano, CA, USA), Nikon | Peripilar signs were seen in 66%, Yellow dots was 63,7%, Vellus hairs 51% (49.03) Coolpix 4500 digital camera. |
| Adriana Rakowska et al. Observational study | Poland | 59 patients | 18=56 years | 28/59 | Grade F3 Ludwig scale | Vertal and frontal scalp | Trichoscopy with 20-fold magnification. | Increased number of yellow dots 46% and thin hairs 53%, as well as decreased average hair thickness in frontal area. |
| Melike Kibar et al. Observational study | Turkey | 206 patients | 18-75 years | 143/206 | Grade I-VII (H-N) scale, grade F1-F3 Ludwig scale | Parietal, frontal, occipital, and lesional areas. | ×100 magnification dermascopy | Diversity (HDD), Structure less Red Areas (SRA), Brown Dots (BD), and Perifollicular White Scales (PWS) (p<0.001). |
| Krishnendra Varma et al. Observational study | India | 269 patients | 18-50 years | 96/269 | Grade Il-VII (H-N) scale, grade F1-F3 Ludwig scale | vertex, frontal and temporal hair line | Dermascopy with camera magnifications ranging from 20X to 220X. | Peripilar sign (77%) followed by short vellus hair (75%), honey comb pigmentation (46%), single follicular units (40%) and yellow dots (37%). |
| Melike Kibar et al. Observational study | Turkey | 201 patients | 25-60 years | 138/201 | Grade Il-VII (H-N) scale, grade F1-F3 Ludwig scale | Vertex, frontal and temporal hair line | Molemax 3 videodermoscopy. | Trichoscopic findings of a honeycomb hyperpigmentation pattern, cumulus like clustered white dots, white dots and black dotted pigmentation. |
| Minu Jose Chirame et al. Observational study | India | 22 patients | 18-60 years | 9/22 | Grade Il-VII (H-N) scale, grade F1-F3 Ludwig scale | Bilateral fronto-temporal, bilateral parieto-temporal | Non polarized Heine delta 20 mini dermoscope (10× magnification) | Yellow dots (100%), diameter diversity>20% (95.1%), thin hair (90.9%), vellus hair (40.9%), honeycomb pigment network (40.9%), peripilar sign (9%) |
| Isha Verma et al. Case-control study | India | 50 patients | 18-69 years | 50/50 | Grade 3-4 Sinclair scale | Frontal, occipital, and left temporal after parting of hairs with comb | Magnified images and provided results in millimeters) at 58 × (covers an area of 9 mm2) | >4 Yellow dots in the frontal scalp zone, >2-1 ratio of single hair units (frontal: occiput) and >3-1 ratio of hair with perifollicular discoloration (frontal: occiput) are commonly noted in late stages of FAGA i.e. grade 4/ 5 and >1.5-1 ratio of vellus hairs (frontal/occiput) in early stages i.e. grade 2/3 while lower mean hair thickness in frontal area and >10%. |
| Marwa Said et al. Observational study | Egypt | 200 patients | 18-65 years | 200/200 | Grade F1-F3 Ludwig scale | Vertex, frontal and temporal hair line | Trichoscopy ×100 magnification | Yellow dots, 45%, peripilar sign, 61%, hair diameter diversity, 100%, and single hair pilosebaceous unit was, 96%. |
| Tugba Rezan Ekmekci et al. Observational study | Turkey | 60 patients | Mean age: (32.21 8.37) | 60/60 | Grade F2 Ludwig scale | Midscalp and on the occiput | TG with digital camera attached to a dermoscope | On the midscalp, increase of percentage of thin hair in consistent with clinical stages in the AGA was noted. |
| Shoba Mani, et al. Observational study | India | 65 patients | 20-65 years | 28/65 | Grade l-VII (H-N) scale, grade F1-F3 Ludwig scale | Frontal, vertex and occiput | Dermoscopy with videodermoscope of magnification 50X and 200X. | Peripilar pigmentation (27), honeycomb pigmentation (16), Yellow dots (7), Pilosebaceous single hair unit (27). |
| Adriana Rakowska et al. Observational study (2) | Poland | 59 patients | 19-58 years | 59/59 | Grade F2-F3 Ludwig scale | Frontal, occipital and both temporal areas of the scalp | 20-fold and 70-fold magnification | Yellow dots noted 1.59 6 2.0 mm the mean hair thickness was 0.061 6 0.008 mm. in the frontal area vs. 0.058 6 0.007 mm in the occipital area (P, 0.001). |
| Soha S Tawfik et al. Observational study | India | 79 patients | 18-54 years. | 79/79 | Grade F1-F3 Ludwig scale | Frontal scalp, vertex, area, righ and left temporal scalp, occiput | Dermlite DLIII dermoscope with 10-fold magnification. | 97.4% (77/79), peripilar brown halo seen in 32.9%, peripilar white halo in 10.1%, and honeycomb like scalp pigmentation in 17.7% of patients, yellow dots in 15.2%, white dots in 20.3%, and hidden hair in 7.6%. |
Table 1: Baseline characteristics.
Conclusion
Trichoscopy is a useful non-invasive diagnostic tool which can aid in diagnosing of androgenic alopecia. Several characteristic findings suggestive AGA can be identified by trichoscope. Yellow dots and short vellus hairs were the most common, and thus most sensitive trichoscopic findings. Followed by, black dots, white dots, honeycomb pigmentation, vellus hairs, tapered hair, peripilar sign, hair diversity, and Perifollicular hyperpigmentation. These early onset findings may prove to be very important to monitor early disease activity of androgenic alopecia. Further studies are needed to substantiate this finding and its relevance to the severity of androgenic alopecia.
Data availability statement
All data included in this review are available in the articles listed in references. Images are available from the corresponding author, APC, upon reasonable request.
References
- Khunkhet S, Chanprapaph K, Rutnin S, Suchonwanit P (2021) Histopathological evidence of occipital involvement in male androgenetic alopecia. Front Med 2353.
[Crossref] [Google Scholar] [PubMed]
- Mesinkovska NA, Bergfeld WF (2013) Hair: What is new in diagnosis and management: Female pattern hair loss update: Diagnosis and treatment. Dermatol Clin 31:119-127.
[Crossref] [Google Scholar] [PubMed]
- Mathur M, Acharya P (2020) Trichoscopy of primary cicatricial alopecias: An updated review. J Eur Acad Dermatol Venereol 34:473-484.
[Crossref] [Google Scholar] [PubMed]
- Widaty S, Pusponegoro EH, Rahmayunita G, Astriningrum R, Akhmad AM, et al. (2019) Applicability of trichoscopy in scalp seborrheic dermatitis. Int J Trichology 11:43.
[Crossref] [Google Scholar] [PubMed]
- Kasumagic-Halilovic E (2021) Trichoscopic findings in androgenetic alopecia. Med Arch 75:109.
[Crossref] [Google Scholar] [PubMed]
- Hu R, Xu F, Han Y, Sheng Y, Qi S, et al. (2015) Trichoscopic findings of androgenetic alopecia and their association with disease severity. J Dermatol 42:602-607.
[Crossref] [Google Scholar] [PubMed]
- Ummiti A, Priya PS, Chandravathi PL, Kumar CS (2019) Correlation of trichoscopic findings in androgenetic alopecia and the disease severity. Int J Trichology 11:118.
[Crossref] [Google Scholar] [PubMed]
- Rakowska A, Slowinska M, Kowalska-Oledzka E, Olszewska M, Rudnicka L (2008) Trichoscopy criteria for diagnosing female androgenic alopecia. Nat Prec 1.
- Kibar M, Aktan S, Lebe B, Bilgin M (2015) Trichoscopic findings in alopecia areata and their relation to disease activity, severity and clinical subtype in Turkish patients. Australas J Dermatol 56:e1-6.
[Crossref] [Google Scholar] [PubMed]
- Chiramel MJ, Sharma VK, Khandpur S, Sreenivas V (2016) Relevance of trichoscopy in the differential diagnosis of alopecia: A cross-sectional study from North India. Indian J Dermatol Venereol Leprol 82:651.
[Crossref] [Google Scholar] [PubMed]
- Verma I, Madke B, Singh AL, Choudhary S (2021) A clinico-trichological study of female androgenetic alopecia. Int J Trichology 13:9.
[Crossref] [Google Scholar] [PubMed]
- Said M, El‐Sayed SK, Elkhouly ND (2020) Trichoscopic evaluation of frontal hairline recession in Egyptian female patients. J Cosmet Dermatol 19:2706-2716.
[Crossref] [Google Scholar] [PubMed]
- Rezan Ekmekci T, Koslu A (2006) Phototrichogram findings in women with androgenetic alopecia. Skin Res Technol 12:309-312.
[Crossref] [Google Scholar] [PubMed]
- Mani S, Manickam N, Gopalan K (2018) Role of dermoscopy in the diagnosis of alopecia. J Pak Assoc Dermatol 28:320-328.
- Rakowska A, Slowinska M, Kowalska-Oledzka E, Olszewska M, Rudnicka L (2009) Dermoscopy in female androgenic alopecia: Method standardization and diagnostic criteria. Int J Trichology 1:123.
[Crossref] [Google Scholar] [PubMed]
- Tawfik SS, Sorour OA, Alariny AF, Elmorsy EH, Moneib H (2018) White and yellow dots as new trichoscopic signs of severe female androgenetic alopecia in dark skin phototypes. Int J Dermatol 57:1221-1228.
[Crossref] [Google Scholar] [PubMed]
- Rudnicka L, Rakowska A, Olszewska M (2013) Trichoscopy: How it may help the clinician. Dermatol Clin 31:29-41.
[Crossref] [Google Scholar] [PubMed]
- Jain N, Doshi B, Khopkar U (2013) Trichoscopy in alopecias: Diagnosis simplified. Int J Trichology 5:170.
[Crossref] [Google Scholar] [PubMed]
- Kibar M, Aktan S, Bilgin M (2014) Scalp dermatoscopic findings in androgenetic alopecia and their relations with disease severity. Ann Dermatol 26:478-484.
[Crossref] [Google Scholar] [PubMed]
- Lima CD, Lemes LR, Melo DF (2017) Yellow dots in trichoscopy: Relevance, clinical significance and peculiarities. An Bras Dermatol 92:724-726.
[Crossref] [Google Scholar] [PubMed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi