Case Report, Clin Oncol Case Rep Vol: 5 Issue: 3
Durable Response with Trabectedin in A Patient with High-Grade Myxoid Liposarcoma and Orbital Metastases
Nadia Hindi1,2,3, Irene Carrasco García3,4, and Javier Martin Broto1,2,3*
1Department of Oncology, Fundación Jimenez Díaz University Hospital and Villalba General Hospital, Madrid
2Fundación Instituto de Investigación Sanitaria Fundación Jimenez Díaz (FIIS-FJD), Madrid
3Advanced Therapies and Biomarkers in sarcoma (ATBsarc group), CITIUS III Seville, Spain
4Medical Oncology Department, Virgen del Rocio University Hospital, Seville, Spain
*Corresponding Author:
Javier Martin Broto
Oncology Department,
Fundación Jiménez Díaz University Hospital,
Av. Reyes Catolicos 2, Madrid, Spain
E-mail: jmartin@atbsarc.org
Received: January 19, 2022, Manuscript No: COCR-22-53136;
Editor assigned: January 21, 2022, PreQC No: P-53136;
Reviewed: March 01, 2022; QC No: Q-53136;
Revised: March 04, 2022, Manuscript No: R-53136;
Published: March 14, 2022, DOI: 10.4172/cocr.5(3).219
Citation: Hindi N, García IC, Broto JM (2022) Durable Response with Trabectedin in A Patient with High-Grade Myxoid Liposarcoma and Orbital Metastases. Clin Oncol Case Rep 5:3
Abstract
Background: High-grade Myxoid Liposarcoma (MLPS) has a high metastatic risk. Besides pulmonary metastasis, as in other soft-tissue sarcoma, MLPS shows a characteristic capacity of developing distant metastasis in other body sites, such as abdominal cavity/retroperitoneum, soft part, and bone. Orbital metastases are exceedingly rare in MLPS. In patients with advanced MLPS not amenable with local therapies, systemic chemotherapy remains the backbone treatment. Trabectedin has become an interesting therapeutic option for the treatment of adult patients with advanced soft tissue sarcoma, after failure of anthracyclines and ifosfamide, or who are unsuited to receive these agents. Given the exceptional nature of orbital Liposarcoma (LPS) secondary to MLPS, the use of trabectedin in these patients has not been yet elucidated.
Case Presentations: Here, we report the case of a patient with high-grade MLPS and orbital metastases who was successfully treated with trabectedin. The patient achieved very prolonged stable disease, during 77 cycles of trabectedin therapy and with a manageable safety profile with no severe adverse events detected.
Conclusion: Our results additionally confirm that trabectedin enable a long-term therapy conferring relevant disease control with a manageable safety profile and without cumulative toxicities.
Keywords: Myxoid liposarcoma; High-grade myxoid liposarcoma; Metastasis; Orbital; Trabectedin; Durable response
Introduction
Liposarcomas (LPS) are malignant tumors of mesodermal origin probably arising from multipotent mesenchymal stem cells [1]. LPS are the most common subtype of all Soft-Tissue Sarcomas (STS) as account for 15%-20% of all. The LPS are classified into four subgroups: myxoid/high-grade, well-differentiated/dedifferentiated, pleomorphic and myxoid pleomorphic [2]. The myxoid/high-grade subtype represents around one-third (32.9%) of all diagnosed LPS [3]. The underlying oncogenic-driver mechanism is the translocation of FUS and DDIT3 (also known as CHOP) genes, present in almost 95% of cases [4]. This translates into an earlier onset of disease, developing approximately 10 years before other subtypes [5]. The high-grade myxoid LPS (MLPS) requires diagnostic confirmation through a core biopsy, showing a mix of non-lipogenic mesenchymal cells and small lipoblasts that forms a myxoid stroma with a distinctive vasculature [4,6]. It is usually localized in lower extremities [5], and it is prone to metastasize to non-pulmonary tissues, especially retroperitoneum, bone, and contralateral limb. Metastases into fat rich areas (such as the orbit and the epidural region) are very rare [7]. Since this subtype is radiosensitive, radiotherapy is frequently employed in front-line of therapy of localized disease, in addition to surgery [8]. Early reports with doxorubicin-based therapy showed response rates over 40% in advanced high-grade MLPS [9,10].
Trabectedin (Yondelis®) is a semisynthetic antineoplastic drug originally isolated from the sea squirt Ecteinascidia turbinata. It is recommended for the treatment of adults with advanced STS, after failure of anthracyclines and ifosfamide, or who are unsuited to receive these agents. Efficacy data of trabectedin are based mainly on LPS and leiomyosarcoma patients. In particular, it has demonstrated superior efficacy in the treatment of advanced LPS and leiomyosarcoma, compared to conventional dacarbazine [11]. Trabectedin toxicity is tolerable and manageable; thus, it has become an interesting therapeutic option for long-lasting treatment of metastatic STS [12-16]. Given the exceptional event of orbital metastatic myxoid liposarcoma, there are hardly few case reports in the literature [7,17]. Here, we report the case of a patient with metastatic high-grade MLPS who received treatment with trabectedin for more than four years.
Case Presentation
A 40-year-old male patient with no relevant medical history was diagnosed with a myxoid LPS in June 2003, following a non-oncological intralesional resection of a mass in his right distal thigh. Surgery without biopsy was the first diagnostic maneuver. A local progression was observed in a short period of time after initial therapy. Given the evidence of a locally advanced relapse, together with the intralesional initial resection and several local wound complications, a radical surgery with transfemoral amputation was eventually performed in November 2003. Afterward, the patient was followed up with no administration of any adjuvant therapy. In April 2005, a metastatic inguinal lymph node was identified and subsequently removed. The histopathological study of samples obtained during the surgical procedure confirmed the diagnosis of high-grade MLPS. Subsequently, the patient received four cycles of postoperative ifosfamide followed by radiotherapy and restarted clinical follow-up. In October 2011, the patient complained of visual impairment and orbital pain. The ophthalmologic study revealed the presence of a soft-tissue mass in the left orbit (Figure 1). Once a restaging study ruled out other disease spread, and given the time interval from the last resection (six years), a surgery was planned, which consisted of the complete resection of the mass, with osteotomy of the ascending process malar bone, and an osteosynthesis. The pathological analysis of the surgical specimen from the orbital mass, confirmed the diagnosis of metastatic high-grade MLPS, resected with affected margins. Molecular confirmation of the diagnosis was obtained by Fluorescence In Situ Hybridization (FISH), which demonstrated the rearrangement in C/EBP Homologous Protein (CHOP) gene. The imaging study after surgery showed the presence of residual disease, and in early 2012 the patient initiated systemic therapy with trabectedin 1.5 mg/m2 given as a 24-h infusion every 3 weeks. After approximately seven months of therapy, in which the patient received only five cycles of trabectedin due to hematological toxicity, the patient experienced clinical (diplopia) and radiological progression in the orbit, and the treatment was withdrawn. By the end of 2012, the patient received radiotherapy at the dose of 50 Gy for local control purposes (Figure 2). Again, follow-up was re-started, and at the beginning of 2014, new orbital progression was evidenced, both clinically (diplopia) and radiologically. In March 2014, the patient underwent a palliative debulking (R2) surgery. Eventually, the orbital exenteration was indicated and it was performed in August 2014. Afterwards, the patient was referred to the sarcoma unit. In October 2014, liver and soft-part metastases in the diaphragm were detected by postoperative imaging reassessment. The patient was then enrolled in a clinical trial and started therapy with pazopanib 800mg orally, in a daily basis. After three cycles of pazopanib, the dose was reduced to 600 mg per day due to grade 3 neutropenia. In May 2015 (with a progression-free survival of seven months), the patient experienced a rapid, symptomatic clinical progression (i.e. pain); however, his general condition was very good, with an Eastern Cooperative Oncology Group (ECOG) performance status of 0. Given the histologic subtype, and the previously suboptimal therapy with trabectedin, a rechallenge with trabectedin (1.5 mg/m2) was started. The patient was also pretreated with dexamethasone, given orally at a dose of 4 mg, 24h and 12 h before trabectedin, and intravenously with 20 mg of dexamethasone just before the start of trabectedin infusion. After one cycle of treatment, the patient experienced a clear clinical response with both pain relief and volumetrically reduction. A complete radiological response was subsequently evident. After 16 cycles, trabectedin administration schedule was changed to every four weeks in order to improve tolerance since the patient complained of grade 2 fatigue and presented myositis with elevation of creatinine phosphokinase. In March 2017, an orbital reconstruction was performed due to the absence of detectable disease. In October 2019 (after four years and 62 cycles of trabectedin with stable disease and a complete response in both the abdominal wall and the orbit), the patient experienced trabectedin extravasation, with a necrotic cutaneous lesion. Thus, the central venous catheter was removed, and the treatment was withdrawn. After eight months of follow-up without therapy, in June 2020 the patient reported orbital disturbances and a mass effect on the orbit. The magnetic resonance imaging and thoracoabdominal computerized tomography revealed local orbital progression together with local invasion of the cavernous sinus, and bone metastasis in humerus and femur. Trabectedin treatment was restarted again at a dose of 1.5 mg/m2 every 28 days, in combination with granulocyte colony stimulating factor support. After the first cycle of rechallenge, the patient showed again a clear clinical benefit, with disappearance of orbital pain and tumor volume reduction. At the moment of the drafting of this paper, in October 2021, the patient had received, since 2012, a total of 77 cycles (15 cycles since the last rechallenge), and with very long-lasting disease stabilization (Figure 3).
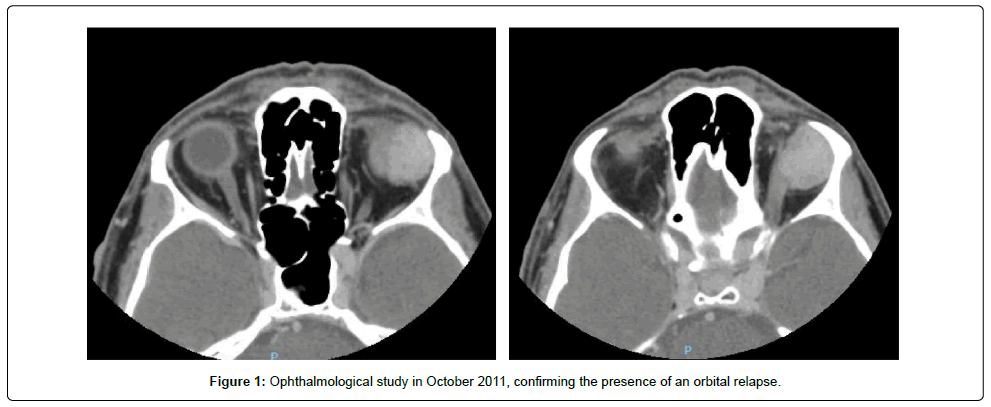
Figure 1: Ophthalmological study in October 2011, confirming the presence of an orbital relapse.
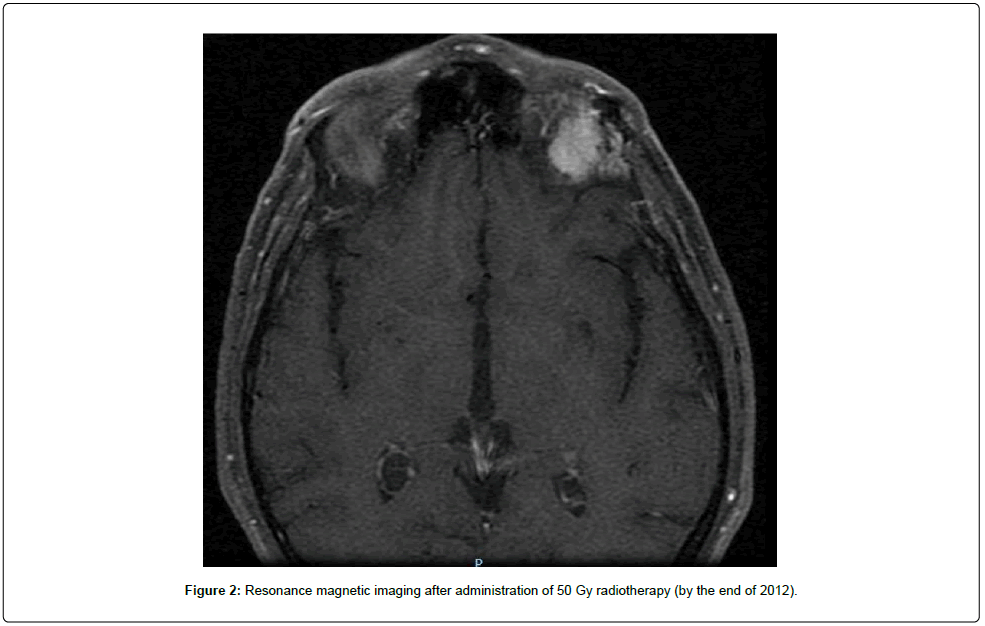
Figure 2: Resonance magnetic imaging after administration of 50 Gy radiotherapy (by the end of 2012).
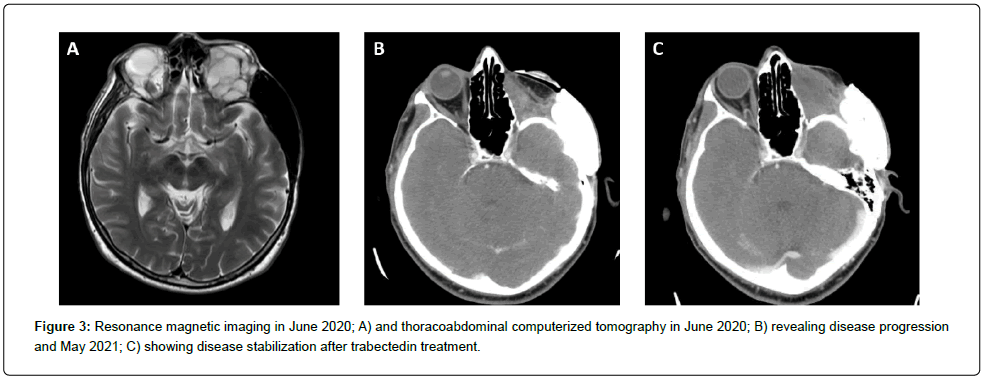
Figure 3: Resonance magnetic imaging in June 2020; A) and thoracoabdominal computerized tomography in June 2020; B) revealing disease progression and May 2021; C) showing disease stabilization after trabectedin treatment.
Discussion
Available data about treatment in patients with orbital myxoid LPS or orbital metastases is quite scarce [7,18-21]. Wide local excision combined with postoperative radiotherapy is the upfront treatment for localized myxoid LPS. Despite treatments, cases of orbital relapses after the successful excision of the primary tumor have been described in the literature [7,18-21]. Additionally, approximately 12.5% of primary orbital LPS ultimately result in the death of the patient [22]. To our knowledge, none of the published reports have used trabectedin. Therefore, this is the first ever reported case in which a patient diagnosed with high-grade MLPS metastatic to the orbit has been treated with trabectedin.
The efficacy of trabectedin in STS has been well established in both clinical trials and real-world evidence [23-28]. Regarding clinical trials, trabectedin has exhibited an improved PFS compared with dacarbazine (4.2 vs. 1.5 months) and best supportive care (3.1 vs. 1.5 months) in patients with advanced STS. Moreover, PFS (5.6 vs. 0.9 months) and overall survival (OS: not reached vs 8.0 months) were higher than best supportive care in patients with translocation-related sarcomas [25]. The maintenance of trabectedin therapy is also crucial in the management of STS [23]. In the randomized phase II T-DIS trial, patients that continued with trabectedin administration until progression or unacceptable toxicity showed a significant higher PFS at 6 months (51.9%) that those who stopped the treatment (23.1%) after six initial cycles. Real-world evidence has corroborated the clinical benefits of trabectedin found in trials, as trabectedin has demonstrated durable disease control, with a median PFS ranging from 3.6 to 5.9 months and an adequate safety profile, representative of a suitable drug for long-term treatment [27]. In addition, greater efficacy of trabectedin has been observed when administered at earlier lines. In RetrospectYon database, patients who received trabectedin at second-line showed a significant greater median PFS and OS (4.8 and 12.9 months, respectively) than at fourth-line (3.8 and 9.5 months) [24]. Accordingly, discontinuation of trabectedin (after the sixth cycle) resulted in a rapid progression of the disease, with PFS and OS rates decreasing from 11.7 and 24.9 months respectively (in patients that continued the treatment), to 7.6 and 16.9 months (in those who stopped the treatment). Regarding high-grade MLPS, data from the French compassionate-use program showed a higher OS (33.4 vs. 13.9 months), PFS (10.5 vs. 2.8 months), and treatment response (21% vs. 8%) than other STS subtypes [29]. The especial sensitivity of high-grade MLPS to trabectedin was reflected in a retrospective series in 2007, where 51% of patients showed at least a partial response [30]. Additionally, it has been established that trabectedin induces a detachment of the fusion transcript FUS-CHOP from the target promoters. As a result, the adipocytic differentiation is reactivated [31].
In our case the long-term treatment with trabectedin was well tolerated, with durable response, and stabilized disease of the orbital metastasis. In general, the safety of trabectedin was lined up with prior experience and reports reflecting the well-characterized safety profile [11,23-29]. After 77 cycles, Adverse Events (AEs) were mild, i.e. asymptomatic grade 3 neutropenia, grade 2 fatigue, one episode of non-complicated trabectedin extravasation, and three vertebrae osteoporotic fractures (probably related with the intermittent use of steroids for trabectedin premedication). The hematological toxicity that led to trabectedin poor dose intensity in 2012 was probably related to a deficient premedication. Fatigue improved by increasing the cycle duration (i.e. from 21 to 28 days).
Conclusion
We report the case of a patient with high-grade MLPS with prolonged response, who was successfully treated with trabectedin. The long-term treatment with trabectedin was safe and resulted in durable disease control.
Disclaimer
Acknowledgment: Authors also express gratitude to Meisys (Madrid, Spain) for writing assistance.
Conflict of interest statement: Javier Martin-Broto reports research grants from PharmaMar, Eisai, Immix BioPharma and Novartis outside the submitted work; honoraria for advisory board participation and expert testimony from PharmaMar, Eli Lilly and Company, Bayer and Eisai; and research funding for clinical studies (institutional) from PharmaMar, Eli Lilly and Company, Adaptimmune Therapeutics, AROG, Bayer, Eisai, Lixte, Karyopharm, Deciphera, GSK, Novartis, Blueprint, Nektar, Forma, Amgen and Daiichi Sankyo.
Irene Carrasco García reports honoraria for advisory board participation and expert testimony from PharmaMar and research funding for clinical studies (institutional) from PharmaMar, Eli Lilly and Company, Adaptimmune Therapeutics, Karyopharm, Deciphera, GSK, Novartis, Blueprint, Nektar, Forma, Amgen and Daiichi Sankyo.
Nadia Hindi reports research grants from PharmaMar, Eisai, Immix BioPharma and Novartis outside the submitted work; honoraria for advisory board participation and expert testimony from PharmaMar; and research funding for clinical studies (institutional) from PharmaMar, Eli Lilly and Company, Adaptimmune Therapeutics, AROG, Bayer, Eisai, Lixte, Karyopharm, Deciphera, GSK, Novartis, Blueprint, Nektar, Forma, Amgen and Daiichi Sankyo.
References
- Rodriguez R, Rubio R, Menendez P (2012) Modeling sarcomagenesis using multipotent mesenchymal stem cells. Cell Res 22: 62-77.
Google Scholar Cross Ref - Fletcher CDM, Hogendoorn PCW, Mertens F, Hogendoorn PCW, Mertens F (2013) WHO classification of tumours of soft tissue and bone. Washington, DC, IARC Press.
Google Scholar - Toro JR, Travis LB, Wu HJ, Zhu K, Fletcher CD, et al. (2006) Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978-2001: An analysis of 26,758 cases. Int J Cancer 119: 2922-2930.
Google Scholar Cross Ref - Crago AM, Dickson MA (2016) Liposarcoma: Multimodality management and future targeted therapies. Surg Oncol Clin N Am 25: 761-773.
Google Scholar Cross Ref - Dürr HR, Rauh J, Baur-Melnyk A, Knösel T, Lindner L, et al. (2018) Myxoid liposarcoma: Local relapse and metastatic pattern in 43 patients. BMC Cancer 18: 304.
Google Scholar Cross Ref - Moreau LC, Turcotte R, Ferguson P, Wunder J, Clarkson P, et al. (2012) Myxoid\round cell liposarcoma (MRCLS) revisited: an analysis of 418 primarily managed cases. Ann Surg Oncol 19: 1081-1088.
Google Scholar Cross Ref - Lin S, Gan Z, Han K, Yao Y, Min D (2015) Metastasis of myxoid liposarcoma to fat-bearing areas: A case report of unusual metastatic sites and a hypothesis. Oncol Lett 10: 2543-2546.
Google Scholar Cross Ref - Chowdhry V, Goldberg S, DeLaney TF, Cote GM, Chebib I, et al. (2018) Myxoid liposarcoma: Treatment outcomes from chemotherapy and radiation therapy. Sarcoma 2018: 8029157.
Google Scholar Cross Ref - Patel SR, Burgess MA, Plager C, Papadopoulos NE, Linke KA, et al. (1994) Myxoid liposarcoma. Experience with chemotherapy. Cancer 74: 1265-1269.
Google Scholar Cross Ref - Katz D, Boonsirikamchai P, Choi H, Lazar AJ, Wang WL, et al. (2012) Efficacy of first-line doxorubicin and ifosfamide in myxoid liposarcoma. Clin Sarcoma Res 2: 2.
Google Scholar Cross Ref - Demetri GD, von Mehren M, Jones RL, Hensley ML, Schuetze SM, et al. (2016) Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: Results of a phase III randomized multicenter clinical trial. J Clin Oncol 34: 786-793.
Google Scholar Cross Ref - Grosso F, Sanfilippo R, Virdis E, Piovesan C, Collini P, et al. (2009) Trabectedin in myxoid liposarcomas (MLS): a long-term analysis of a single-institution series. Ann Oncol 20: 1439-1444.
Google Scholar Cross Ref - De Sanctis R, Marrari A, Santoro A (2016) Trabectedin for the treatment of soft tissue sarcomas. Expert Opin Pharmacother 17: 1569-1577.
Google Scholar Cross Ref - Haslbauer F (2018) Long-term progression-free survival in a patient with metastatic leiomyosarcoma of the inguinal region treated with trabectedin. Case Rep Oncol 11: 246-251.
Google Scholar Cross Ref - Cordeiro M, Casanova JM, Rodrigues J, Freitas J, Fonseca R, et al. (2020) Long-term response after 94 cycles of trabectedin in a patient with metastatic leiomyosarcoma of the lower extremity. Case Rep Oncol 13: 113-119.
Google Scholar Cross Ref - Matsuda S, Tanaka K, Kawano M, Iwasaki T, Itonaga I, et al. (2020) Long-term disease control by trabectedin in a patient with dedifferentiated liposarcoma: A case report. Medicine (Baltimore) 99: e18689.
Google Scholar Cross Ref - Hannachi Sassi S, Braham E, Bhouri L, Mrad K, Abbes I, et al. (2007) Métastase orbitaire d'un liposarcome Orbital metastasis of liposarcoma. J Fr Ophtalmol 30: e28.
Google Scholar Cross Ref - Fezza J, Sinard J (1997) Metastatic liposarcoma to the orbit. Am J Ophthalmol 123: 271-272.
Google Scholar Cross Ref - Tehrani AH, Heegaard S, Prause JU, Fledelius HC, Daugaard S (2003) Liposarcoma metastatic to the orbit. Eur J Ophthalmol 13: 108-112.
Google Scholar Cross Ref - Fabi A, Salesi N, Vidiri A, Mirri A, Ferraresi V, et al. (2005) Retroperitoneal liposarcoma with metastasis to both orbits: an unusual metastatic site. Anticancer Res 25: 4769-4771.
Google Scholar - Kim SE, Kim JH, Yang SW (2020) Dedifferentiated transformation in metastatic liposarcoma of orbit and brain. Orbit 39: 437-440.
Google Scholar Cross Ref - Borbolla-Pertierra AM, Morales-Baños DR, Martínez-Nava LR, Garrido-Sánchez GA, López-Hernández CM, et al. (2017) Orbital liposarcoma. Arch Soc Esp Oftalmol 92: 86-92.
Google Scholar Cross Ref - Le Cesne A, Blay JY, Domont J, Tresch-Bruneel E, Chevreau C, et al. (2015) Interruption versus continuation of trabectedin in patients with soft-tissue sarcoma (T-DIS): A randomised phase 2 trial. Lancet Oncol 16: 312-319.
Google Scholar Cross Ref - Le Cesne A, Ray-Coquard I, Duffaud F, Chevreau C, Penel N, et al. (2015) Trabectedin in patients with advanced soft tissue sarcoma: a retrospective national analysis of the French Sarcoma Group. Eur J Cancer 51: 742-750.
Google Scholar Cross Ref - Kawai A, Araki N, Sugiura H, Ueda T, Yonemoto T, et al. (2015) Trabectedin monotherapy after standard chemotherapy versus best supportive care in patients with advanced, translocation-related sarcoma: a randomised, open-label, phase 2 study. Lancet Oncol 16: 406-416.
Google Scholar Cross Ref - Le Cesne A, Blay J, Cupissol D, Italiano A, Delcambre C, et al. (2018) Results of a prospective randomized phase III T-SAR trial comparing trabectedin (T) vs best supportive care (BSC) in patients with pretreated advanced soft tissue sarcoma (ASTS): A French Sarcoma Group (FSG) trial. J Clin Oncol 36: 11508.
Google Scholar Cross Ref - de Sande González LM, Martin-Broto J, Kasper B, Blay JY, Le Cesne A (2020) Real-world evidence of the efficacy and tolerability of trabectedin in patients with advanced soft-tissue sarcoma. Expert Rev Anticancer Ther 20: 957-963.
Google Scholar Cross Ref - Le Cesne A, Blay JY, Cupissol D, Italiano A, Delcambre C, et al. (2021) A randomized phase III trial comparing trabectedin to best supportive care in patients with pre-treated soft tissue sarcoma: T-SAR, a French Sarcoma Group trial. Ann Oncol 32: 1034-1044.
Google Scholar Cross Ref - Blay JY, Italiano A, Ray-Coquard I, Le Cesne A, Duffaud F, et al. (2013) Long-term outcome and effect of maintenance therapy in patients with advanced sarcoma treated with trabectedin: an analysis of 181 patients of the French ATU compassionate use program. BMC Cancer 13: 64.
Google Scholar Cross Ref - Grosso F, Jones RL, Demetri GD, Judson IR, Blay JY, et al. (2007) Efficacy of trabectedin (ecteinascidin-743) in advanced pretreated myxoid liposarcomas: a retrospective study. Lancet Oncol 8: 595-602.
Google Scholar Cross Ref - Di Giandomenico S, Frapolli R, Bello E, Uboldi S, Licandro SA, et al. (2014) Mode of action of trabectedin in myxoid liposarcomas. Oncogene 33: 5201-5210.
Google Scholar Cross Ref
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 