Research Article, J Athl Enhanc Vol: 9 Issue: 1
Effects of 3D-Multiple Object Tracking on Reaction Time Performance in High-Performance Varsity Swimmers
*Corresponding Author:
Brian R Christie
Division of Medical Sciences
University of Victoria, Victoria, Canada
E-mail: brain64@uvic.ca
Received Date: January 03, 2020; Accepted Date: January 27, 2020 Published Date: February 03, 2020
Citation: Snowden TM, Hogan KC, Sparks TJ, Stein RG, Lysenko-Martin MR, et al. (2020) Effects of 3D-Multiple Object Tracking on Reaction Time Performance in High-Performance Varsity Swimmers. J Athl Enhanc 9:1. DOI: 10.37532/jae.2020.9(1).326
Abstract
Elite swimming is highly competitive; first place is often determined by only milliseconds. Improving off-the-block reaction time may serve as means to improve overall performance. The current study sought to determine whether repeated three-dimensional multiple object tracking training improves reaction times to auditory cues in high-performance varsity swimmers. Experimental participants (n=15; ages 18-25) were initially assessed for off-the block reaction times, as well as visual reaction times. Experimental participants then completed ten training sessions of three-dimensional multiple object tracking over a five-week period, after which off-the-block and visual reaction times were re-evaluated. Off-the-block reaction times improved in both the experimental and control groups (Ex: W=120, p=0.00072; Con: W=45, p=0.0039), however the improvement observed in the experimental group was significantly greater than that observed in the control group (W=10, p=0.00059). The experimental group also showed significant improvements in the ruler task, a measure of visual reaction time for both their right hand (W=111, p=0.0020) and left hand (W=113, p=0.0012). To our knowledge, this is the first evidence that training visual selective attention can positively affect auditory selective attention in nonvisually dominated sports.
Keywords: Reaction time; Athletic training; Multiple object tracking
Introduction
High performance swimming is an extremely competitive sport. For instance, at the 2018 National Collegiate Athletic Association (NCAA) Division 1 Championship men’s 50 m freestyle finals, when you look past the world record first place performance, a mere 0.53 seconds separated second through eighth place [1]. Further, according to Hoof (Riewald & Rodeo) [2] in the sprint events, a difference of just 0.1 seconds would have changed 65 Olympic medals winners between the 1972 and 2004 Olympic games, and more recently at the Rio 2016 Olympics, a difference of just 0.1 seconds would have changed 30 medal winners [3]. Thus, small reductions in the time to complete a race can have significant effects on a racers final standing.
Competitive pool swimming races can be broken down into four phases: the start, underwater segment, stroke swimming and turns. The starting phase of a race is considered to be the time it takes for the athlete’s feet to leave the block after hearing the signal, until the athlete’s head reaches 15 meters [4]. This phase can be further broken down into three: the on-block, flight and underwater phases [5]. In this study, we were interested in the on-block phase, and how to improve off-the-block reaction time. Off-the-block reaction time can be considered the initial stage of a race, and can be defined as the time it takes between the starting cue (auditory stimulus) and the athlete initiating movement and simultaneously beginning the flight phase (physical response). The auditory reaction time is one of the fastest processes in the human body, and averages between 140 milliseconds and 160 milliseconds [6], and in sprint running starts has been observed lower than 200 milliseconds. In the context of a swim start, the auditory reaction time is more complex. Auditory reaction time is dependent on multiple factors including: arrival of the sound to the ear, conversion by the ear to a neural signal, neural transmission and processing followed by muscular activation and soft tissue compliance [7].
Some studies have been conducted in order to determine the best physical practices of enhancing off-the-block reaction time including: plyometric training [8], repetitive dive practice [9], as well as plyometric training in conjunction with dive practice [10], all of which were found to successfully improve off-the-block reaction time. Although these techniques have been successful in improving off-the-block reaction time, a negative consequence of these practices is that they increase the physical workload on top of an already demanding training schedule. Additionally, repetitive dive practice use up pool time, which is often a limited resource for many teams, and time most coaches and athletes prefer to spend doing other types of in-water training. More recently, task-specific cognitive training (i.e. auditory reaction time training) was found to improve reaction times in adolescent swimmers, but not block times [11]. The effects of cognitive training in high-performance athletes remain unknown. Cognitive training shows promise in the context of off-the-block reaction time, and if found to provide similar improvements as physical training, it has the added benefit of enhancing performance without additional muscle exhaustion or taking away from limited pool time.
Three-dimensional multiple object tracking (3D-MOT) is a perception-based neurocognitive training task conducted in a virtual environment [12]. 3D-MOT training can be done using a software called NeuroTracker, a tool which concurrently activates multiple brain networks, requiring them to work together and integrate cognitive tasks such as working memory, complex motion integration, and distributed attention processing to track multiple objects in time and three-dimensional space [13]. This visual-tracking training tool has been making its way into the sport scene to enhance performance in visually dominated sports such as soccer [14], basketball [15], and volleyball [16], and in a recent review, NeuroTracker training had the most evidence for far-transfer effects and sport-related benefits than other commercially available cognitive training devices [17]. 3D-MOT most heavily recruits attentional resources, and results suggest that just 10 sessions of 3D-MOT training enhances multiple types of attention (i.e. sustained, selective, divided and inhibition) in both visual and auditory domains [12]. Attention dictates our senses and influences what we see, hear, feel, taste, and smell, and in regard to the swimming start, better attention to the starting signal may enhance off-the-block performance.
In this study, we sought to answer if 3D-MOT training could enhance off-the-block reaction time in high-performance varsity swimmers. We suggest that by participating in 3D-MOT training sessions, athletes may improve their auditory selective attention, allowing them to better focus on the starting signal and lead to subsequent improvements in off-the-block reaction time. Due to the extremely competitive nature of the sport, improving off-theblock reaction time could be used to better overall performance. We hypothesized those high-performance varsity swimmers who engage in 10 sessions of 3D-MOT over a period of 5 weeks will improve their off-the-block reaction times significantly more than controls.
Materials and Methods
Participants
Information about the study was disseminated to the swimmers early in the swimming season at the team’s annual welcome meeting. To be included, participants had to meet the initial criteria: a member of the University of Victoria Swim Team, not currently suffering from a concussion, the absence of color blindness, and the absence of any injuries that would prevent the athlete from diving. These criteria were needed to ensure that changes in off-the-block reaction time could not be attributed to external causes such as concussion or injury recovery.
The University Swim Team consisted of 31 members. Of these members, 15 (9 males and 6 females) athletes chose to participate in this research, and completed all 3D-MOT training sessions, citing personal improvement as their leading motive, while the remaining eligible recruits (7 males and 2 females) who cited time commitment as their main deterrent were used as a control group. The remaining 7 athletes did not meet inclusion criteria for the study. Table 1 show experimental and control group demographics.
Table 1: Experimental and control group demographics. (Experimental group, n:15; Control group, n:9)
Ethics
This research was done in accordance with ethics protocol 17-176 at the University of Victoria. Informed consent was obtained from all experimental and control group participants.
Apparatus and instruments
Ares-Omega timing system: To get an accurate off-the-block reaction time a starting system is required. This system is used to indicate the start of a race, and is operated by a starter, wherein their voice is used to signal instructions to the athletes, followed by a start gun generating the audible signal indicating to jump off the block. The starting system distributes audio signals to many loudspeakers, offsetting any disadvantage due to the speed of sound across the pool. Force plates within the starting blocks detect the initiation of movement after the starting gun has fired, and this is recorded as reaction time [18]. For this research, we used the starting equipment, starting blocks and control room to get accurate numbers for offthe- block reaction time (Figure 1 shows visual representations). The starting procedure was in accordance with Fédération Internationale De Natation (FINA) standards such that on the starter’s command “take your marks”, the athletes immediately took up their starting positions with at least one foot at the front of the starting block, and when all swimmers were still, the starter gave the starting signal [19]. The athletes were told to perform these dives as they would in a race (i.e. no anticipation of the starting signal) to reduce likelihood of a false start; however if a false start occurred (as determined by the start official), the offending athlete was asked to re-do the dive. Figure 2 represent visual representation of the swim start.
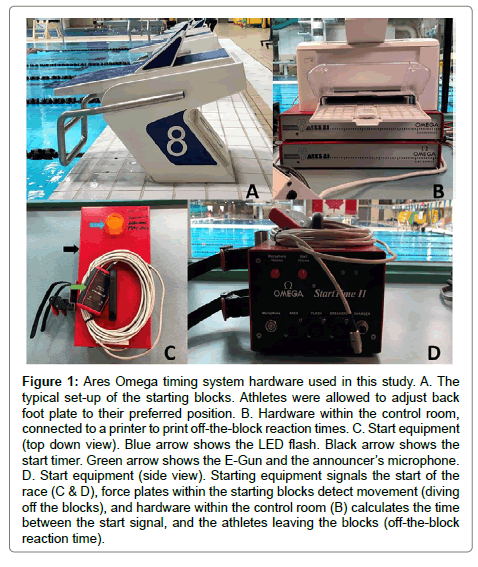
Figure 1: Ares Omega timing system hardware used in this study. A. The typical set-up of the starting blocks. Athletes were allowed to adjust back foot plate to their preferred position. B. Hardware within the control room, connected to a printer to print off-the-block reaction times. C. Start equipment (top down view). Blue arrow shows the LED flash. Black arrow shows the start timer. Green arrow shows the E-Gun and the announcer’s microphone. D. Start equipment (side view). Starting equipment signals the start of the race (C & D), force plates within the starting blocks detect movement (diving off the blocks), and hardware within the control room (B) calculates the time between the start signal, and the athletes leaving the blocks (off-the-block reaction time).
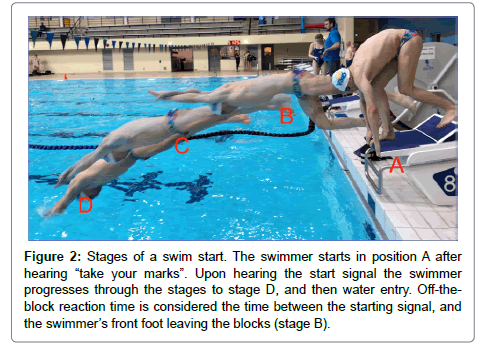
Figure 2: Stages of a swim start. The swimmer starts in position A after hearing “take your marks”. Upon hearing the start signal the swimmer progresses through the stages to stage D, and then water entry. Off-theblock reaction time is considered the time between the starting signal, and the swimmer’s front foot leaving the blocks (stage B).
Neuro tracker: A lab attendant guided all experimental participants through the experiment. The NeuroTracker was set to CORE mode during all training sessions. Each participant sat 150 centimeters away from their respective 52-inch TV screen, and wore Samsung 3D glasses. The participant identified their targets by pressing its respective key on a keyboard. The participant completed 3 subsequent CORE sessions per training session. Before each session, participants were briefed using a protocol developed in our laboratory that reads as follows:
“This is the NeuroTracker-a test of awareness and attention. Professional athletes, Special Forces soldiers, and executives use NeuroTracker to improve mental performance.
The next thing you will see is a cube with 8 yellow balls. Four of the balls will turn orange; these are your targets. The four that stay yellow are decoys. Your 4 targets will turn back to yellow and the balls will start moving around the cube. You need to pay attention to the 4 targets and track them for eight seconds. At the end of 8 seconds, you will have to identify them. If you get all 4 correct, you move up to a higher speed. If you miss any, you move to a lower speed. You will get 20 chances.” A breakdown of these phases is shown in Figure 3.
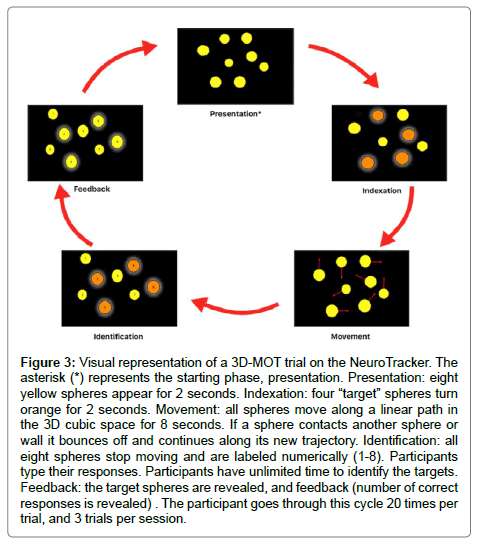
Figure 3: Visual representation of a 3D-MOT trial on the NeuroTracker. The asterisk (*) represents the starting phase, presentation. Presentation: eight yellow spheres appear for 2 seconds. Indexation: four “target” spheres turn orange for 2 seconds. Movement: all spheres move along a linear path in the 3D cubic space for 8 seconds. If a sphere contacts another sphere or wall it bounces off and continues along its new trajectory. Identification: all eight spheres stop moving and are labeled numerically (1-8). Participants type their responses. Participants have unlimited time to identify the targets. Feedback: the target spheres are revealed, and feedback (number of correct responses is revealed) . The participant goes through this cycle 20 times per trial, and 3 trials per session.
Ruler task: The experimental group partook in the ruler task, a measure of visual reaction time. The ruler task has been examined in children [20,21] and college students [22], and has been validated as a proper measure of reaction time [23]. Reaction time is considered the time between stimulus onset (seeing the ruler drop) and initiation of response (catching the ruler). This task is conducted by asking each participant to sit at a table with their forearm and hand extended over the edge. The participant holds their hand in an open position, as if holding a hockey puck between their thumb and index finger. The lab attendant line up a meter sticks between the thumb and index finger, at the same height as the digits, and then drops the meter stick. The participant catches the meter stick when they see it dropped. The researcher records the placement of the ruler (in cm) from the bottom of the participants’ hand. The participant repeats this task three times with each hand.
A separate control group (n=15) of similar age and athletic commitment was used to examine any learning effects in the ruler task. The control group completed the ruler task using the same methodology as the experimental group.
Procedure
We recruited participants from the University Swim Team. The first off-the-block reaction time set was collected after a regularly scheduled competition. A series of three off-the-block reaction times were collected and averaged for each eligible participant and control (N=24) using the Ares-Omega timing system. This session was conducted prior to any 3D-MOT training.
The ruler task and 3D-MOT sessions were conducted at the Concussion Laboratory located at the University campus. The experimental group (n=15) completed the ruler task at the beginning and end of the five-week period. Participants completed 3D-MOT training twice per week for 5 weeks (10 sessions in total), with the ruler task occurring immediately before their first training session and immediately after their last training session.
Following the 10 session training period all experimental and control group members partook in a second off-the-block reaction time data collection session, with data being collected in the same manner as the first data collection session. Figure 4 represents visual representation of the experimental procedure.
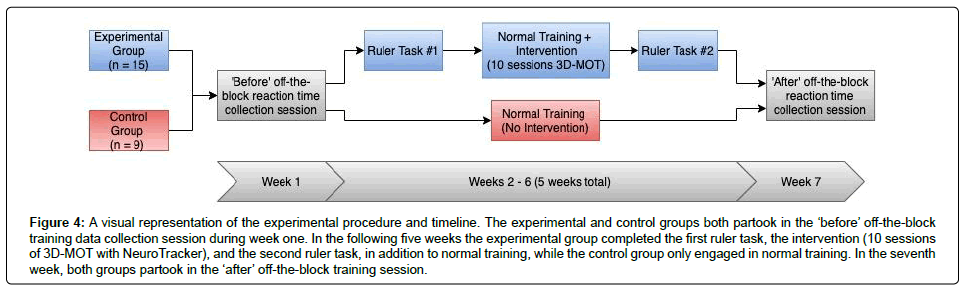
Figure 4: A visual representation of the experimental procedure and timeline. The experimental and control groups both partook in the ‘before’ off-the-block training data collection session during week one. In the following five weeks the experimental group completed the first ruler task, the intervention (10 sessions of 3D-MOT with NeuroTracker), and the second ruler task, in addition to normal training, while the control group only engaged in normal training. In the seventh week, both groups partook in the ‘after’ off-the-block training session.
Having each swimmer perform three subsequent dives in the ‘before’ and ‘after’ sessions allowed assessment of within-subject variability by assessing individual effect sizes, a necessary assessment given the small sample size used in this study. As described by Papic et al. [13], this additional data provided further support of real change due to the intervention (3D-MOT), and decreases the chance of making a Type I or Type II error.
For the remainder of this paper, the first off-the-block reaction time data collection session will be referred to as the ‘before’ session, and the second off-the-block reaction time data collection session will be referred to as the ‘after’ session, in relation to completion of 3D-MOT training.
Statistical analysis
We analyzed the off-the-block reaction times using single-factor within-group and between-group research designs. Data was analyzed using R [24]. Average differences between ‘before’ and ‘after’ off-theblock reaction times were calculated for both groups. A Shapiro-Wilk [25] test was conducted to assess normality of the data set. All data sets were non-normally distributed, thus non-parametric tests were performed. Paired Wilcoxon signed rank tests [26] were conducted to assess for changes in ‘before’ and ‘after’ off-the-block for both experimental and control groups. A Wilcoxon signed rank test was performed to identify any differences from ‘before’ to ‘after’ between the experimental and control groups. One off-the-block reaction time was recorded as >1.5 s, and was considered either a malfunction of the equipment, or a ‘non-race-like start’, and was excluded from analysis. An alpha level (α) of 0.05 was used to assess for significance. Because of the small sample size and non-normal distributions, we performed Vargha and Delaney’s A (using R package: effsize [27]) to assess the overall effect size between groups, as well as individual effect size to account for within-subject variability. Vargha and Delaney’s A runs on a scale of 0–1, with a value of 0.5 indicating no stochastic difference between groups. Effect size was determined as follows: large effect size; ≥ 0.71 or ≤ 0.29, medium effect size; 0.64 – 0.71 or 0.29 – 0.34, small effect size; 0.56 – 0.64 or 0.34 – 0.44 [28]. In the context of this study a value of A>0.5 is indicative that the before off-the-block reaction times were larger (slower) than the after offthe- block reaction times, and vice versa for a value of A<0.5. Paired Student’s T-Tests were used to examine differences in first and final 3D-MOT training sessions.
Results
Demographics
Age: Ages ranged from 18.2 to 25.4 years old (M=20.3, SD=2.25) in the experimental group, and 18.4 to 22.7 (M=19.4, SD=1.41) in the control group. There was no statistical difference in age between the control group and experimental group (t (21)=1.794, p=0.0867).
Change in off-the-block reaction times after 10 sessions of 3D-MOT
A paired Wilcoxon signed rank test demonstrated a significant improvement in off-the-block reaction time in the experimental group from the ‘before’ session to the ‘after’ session (M=0.0813 seconds, SD=0.0428, W=120, p=0.00072) (Figure 5). A large effect size (A ≥ 0.71) was observed in each experimental participant. An ANOVA table demonstrated no between group differences when accounting for years of varsity athletics (F4(10), p=0.149), as well as when accounting for type of swimmer (sprinter, middle distance, distance/open water) (F2(12), p=0.253). For a breakdown of off-theblock reaction times of experimental participants see Appendix A.
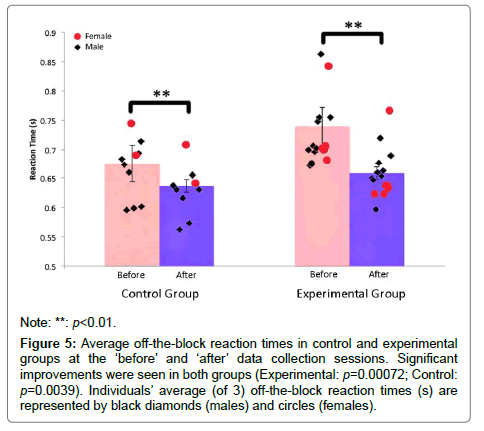
Figure 5: Average off-the-block reaction times in control and experimental groups at the ‘before’ and ‘after’ data collection sessions. Significant improvements were seen in both groups (Experimental: p=0.00072; Control: p=0.0039). Individuals’ average (of 3) off-the-block reaction times (s) are represented by black diamonds (males) and circles (females).
A second paired Wilcoxon signed rank test demonstrated a significant improvement in off-the-block reaction time in the control group (M=0.034 seconds, SD=0.0171, W=45, p=0.0039) (Figure 5). A large effect size was observed in a majority of control group participants. An ANOVA table demonstrated no between group differences when accounting for years of varsity athletics (F3(5), p=0.479), as well as when accounting for type of swimmer (sprinter, middle distance, distance/open water) (F2(6), p=0.0512).For a breakdown of off-the-block reaction times of the control group see Appendix A.
A Wilcoxon signed rank test demonstrated a significant difference between the change in the experimental group’s and control group’s off-the-block reaction times (M=0.0469 seconds, W=10, p=0.00059) (Figure 6). A Vargha and Delaney’s A test identified a large effect size (A=0.9259259) indicating that the differences in before and after off-the-block reaction time in the experimental group were larger than differences in the control group.
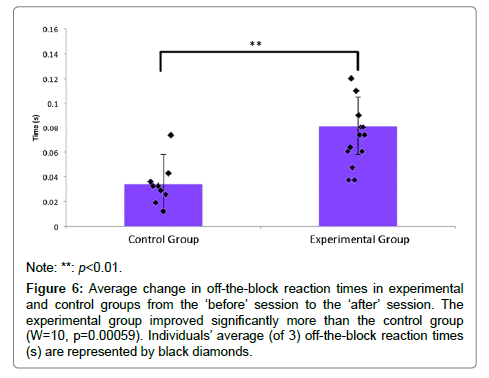
Figure 6: Average change in off-the-block reaction times in experimental and control groups from the ‘before’ session to the ‘after’ session. The experimental group improved significantly more than the control group (W=10, p=0.00059). Individuals’ average (of 3) off-the-block reaction times (s) are represented by black diamonds.
Change in visual reaction times
A paired Wilcoxon signed rank test demonstrated a significant difference between the ‘before’ and ‘after’ conditions in the right hand (W=113, p=0.0020), and in the left hand (W=113, p=0.0012) (Appendix B, Figure B1). In a separate control group, a paired Wilcoxin signed rank test established no significant difference between the ‘before’ and ‘after’ conditions in both the right and left hands (W=82, p > 0.05; W=102, p > 0.05).
Baseline group differences
A Wilcoxon signed rank test demonstrated that the control group’s initial off-the-block reaction times were significantly faster than the experimental group’s (W=22, p=0.0066), indicating baseline differences between the groups.
Improvements in 3D-MOT
Over the 10 sessions of 3D-MOT, experimental participants significantly improved their scores (t (14)=-8.496, p=6.753e-07) (Appendix C, Table C1). The average NeuroTracker scored increased logarithmically from 1.14 to 2.12 over 10 sessions (Appendix C, Figure C1).
Discussion
The purpose of this study was to examine if 10 sessions of 3D-MOT would improve off-the-block reaction time in highperformance varsity swimmers. We theorized that training visual selective attention using 3D-MOT would lead to improvement in auditory attention, as demonstrated by improvements in off-theblock reaction time. Thus we hypothesized that off-the-block reaction time would improve following ten sessions of 3D-MOT training. The experimental group significantly improved their 3D-MOT scores in 10 sessions, indicating improvements in attention, and we found that indeed, off-the-block reaction times significantly improved after 3D-MOT training, suggesting that auditory attention can be improved by training visual attention. We found that both the experimental and control groups significantly improved their offthe- block reaction times from the before session to the after session; however, improvements in both groups were expected. Previous research has established that practicing dive technique enhances dive performance [9,29], and the examined team practices diving technique regularly. Additionally, the ‘before’ session took place early in the season (second month of a seven month season); realistically every athlete should improve their off-the-block reaction times due to practice effects from daily training, as well as fitness development over the study period. However, more of interest was the finding that the experimental group improved their off-the-block reaction times significantly more than the control group. Further, larger individual effect sizes were observed in the experimental group compared to the control group.
The cognitive effects of 3D-MOT are widespread, and include enhancement of cognitive functions in aging populations [30], military populations [31], and younger, healthy populations [12], younger populations with neurodevelopmental conditions [32] as well as improvement in attention, visual information processing speed, and working memory in just ten sessions [12]. Researchers have also identified corresponding changes in brain waves related to attention and coordination in the frontal and occipital lobes after 3D-MOT by using quantitative electroencephalography (qEEG) [12]. The results of this study are some of the first to report audiovisual improvements via 3D-MOT, and to the best of our knowledge, the first results taking this finding into live sport. Parsons et al. [12] were the first to demonstrate improvements in auditory selective attention after 10 sessions of 3D-MOT training, despite this being a purely visual task; however, this was strictly in a controlled clinical setting using standardized neuropsychological assessments, and while interesting, this result did not provide an applicable realworld application. Within the sport of swimming, few studies have examined swimming start intervention, and even fewer have examined this in a university-level population. In the same starting style as this study (track start), with similar participant demographics, Blanksby et al. [9] found an improvement of 0.03 seconds in off-theblock reaction time after a repetitive dive start intervention. Our intervention found a greater improvement in off-the-block reaction time (0.08 seconds), suggesting that our 3D-MOT intervention may be of more benefit to high performance swimmers than repetitive dive practice. Interestingly, the improvement found by Blanksby et al. [9] was similar to the improvements seen in our control group (0.034 seconds), suggesting that the team’s typical weekly dive practice is sufficient enough to improve off-the-block reaction time, and that increasing time spent on dive practice may not provide any benefit. Although just hundredths of seconds, there is a lot to gain from improving off-the-block reaction time when the stakes are high, most notably in short distance races where the start component contributes most to overall time [33]. In both genders, a faster off-the-block reaction time was found to be significantly correlated with complete race performance for all but four short distance races at the 1999 Pan- Pacific Championships [34]. Having a faster reaction time not only means having a slight advantage over the field at the start of the race, but may also be indicative of overall performance in the sprinting events. In longer events off-the-block reaction time contributes less to the total racing time, but is still a means of improvement.
Processing visual information is linked to processing auditory information; however, more is understood about crossmodal plasticity following sensory deprivation than is understood about crossmodal plasticity during typical development [35]. In normal functioning adults, spatial and temporal processing is dominated by visual and auditory domains, respectively [36]. Our study complements the findings of McGovern et al. [35], in which enhancements of learned spatial tasks were found to transfer from the visual domain to the auditory domain, but not vice versa; however their study also examined task specific auditory to visual processing transfer, which our study did not. The authors further suggested that there may be one-way learning transfer from dominant to non-dominant sensory modalities [35]. While it is known that these crossmodal enhancements exist, the actual underlying mechanisms remain unclear. McGovern et al. [35], suggest that cross modal sensory calibration (i.e. the most accurate sense for a task calibrates another [37]) may help explain their findings. Another theory, the multiple resources theory [38], suggests that different attention-requiring modalities (e.g. touch, vision, audition) share, and are limited by a common attentional resource pool. Thus, training one area of attention (e.g. vision) may enhance the entire shared pool, leading to improved attention capacity in other modalities (e.g. audition).
The neural circuits underlying visual-auditory crossmodal transfer effects have not been explored in detail; however, there is evidence that sensory modalities are not separate, as once believed [39]. For example, using function magnetic resonance imaging (fMRI) the primary auditory cortex was found to be active when a talking face was viewed, even in the absence of sound [40]. More recently, using functional connectivity magnetic resonance imaging (fcMRI), it has been found that areas of the primary auditory cortex (namely, medial Heschl’s gyrus) are strongly coupled to the primary visual cortex via the anterior banks of the calcarine fissure [41]. Further, while these visual-auditory domain connections exist, many of these networks are strongly disrupted when engaging in a visual perception task; however, connections between the anterior visual cortex, an area important for peripheral vision, and the auditory cortex, are not disrupted [41]. Interestingly, 3D-MOT trains the use of peripheral visual search strategies (e.g. center-looking versus target looking), which may account for its translational improvements on the sports field [14]. One hypothesis we would like to propose is that because 3D-MOT training has been found to improve peripheral visual strategies [14], and neural pathways exist, and remain uninterrupted during multisensory processing, between the primary auditory cortex and peripheral vision processing areas [41], under Hebbian theory [42], 3D-MOT training, a visual task, may strengthen neural connections between the auditory and visual cortices, leading to enhancement of auditory processes (i.e. off-the-block reaction time) without direct sensory training.
While our research focused on one sensory domain influencing another, other research has examined the benefits of multisensory learning on unisensory performance [36]. Shams and Seitz [36] describe that multisensory processing occurs constantly in a real environment, and that unisensory training creates an unnatural learning environment that does not tap into multisensory mechanisms which humans evolved over time, and therefore may not produce translational results. One study examining multisensory learning facilitation of unisensory performance found that audio-visual perceptual training significantly improved learning time and overall performance in a visual task compared to a group that only partook in visual perceptual training [43]. Although the present study found significant translational effects of unisensory (visual) perceptual training on an auditory task (off-the-block reaction time), in a future study it would be interesting to include auditory stimuli in addition to 3D-MOT to identify any facilitative effects of multisensory perceptual training in the current context.
We found that 3D-MOT training improved visual reaction time, as measured by the ruler task. 3D-MOT using NeuroTracker software has been found to enhance visual processing speed [12], and since visual processing speed is a factor of visual reaction time [44], enhancement of visual processing speed via 3D-MOT training may explain the improvements seen in the ruler task. Originally the ruler task was added to this study as a secondary measure of reaction time, and this task was only conducted in the experimental group. However, to control for learning effects we examined a separate control group consisting of 15 athletes who completed the ruler task in the same manner as the experimental group, with approximately five-weeks between sessions. The ruler task is prone to practice effects [45], however, the most pronounced improvements were found to occur between the tenth and twentieth trial of the task, which occurred within 2 days of each other. The improvements demonstrated by the experimental group are unlikely due to practice effects, as each experimental participant completed only 3 trials on each hand per session, and the sessions were 5 weeks apart. Further, we did not find any significant improvements in the ruler task in either hand in the separate control group.
Additionally, we found the control group’s average ‘before’ off-the-block reaction time was significantly faster than the experimental group’s. A few factors must be considered here: reason for participation in the study, and group demographics. First, athletes who knew they had slower off-the-block reaction times were more likely to participate in the study in hopes of improving, thus potentially skewing the experimental groups’ ‘before’ off-the-block reaction times towards the slower end. Second, males and females have different reaction times, and thus group demographics should be considered. Average high-performance off-the-block reaction times can be calculated from recent NCAA Division 1 Swimming Championships’ sprint events; 0.64 seconds [1], and 0.70 seconds [46] for men and women, respectively. The experimental group included 9 males and 6 females (66.6% female), and in comparison the control group included 7 men and 2 females (28.5% female). It has been found that muscle contraction time is the same for males and females [47], but that males execute stronger motor responses than females [48], and this difference likely leads to different reaction times between the sexes, and aids in the explanation of the difference seen in average group reaction times in the before session. In this study, these demographics were not considered, as our focus was looking at improvements in off-the-block reaction time from the ‘before’ session to the ‘after’ session; however, this is something we believe should be investigated further.
We acknowledge that this study has limitations. The first is that the groups were not randomly selected. Due to the time commitment of this study (i.e. 1 hour per week for 5 weeks), and the demanding schedule of varsity athletes (i.e. 18–22+ hours a week of training, in addition to full course loads), we decided to let the athletes selfselect if they would like to participate in the full study, thus the groups were not sex-matched, nor equal size, and the experimental group likely had a motivation bias. Additionally, due to sample size constraints we did not include a positive control group, which could strengthen the evidence for 3D-MOT as an effective training tool, rather than placebo. However, although the mechanisms behind this enhancement remain speculative, in the context of high-performance sport the important take-away is that 3D-MOT training enhanced performance.
The finding of improved off-the-block reaction times following 3D-MOT training in varsity swimmers as demonstrated in this study may have important implications and suggest opportunities for future research. Future studies in the realm of swimming should determine whether the improvements in off-the-block reaction times are long lasting, by extending the study period over a competitive season, rather than just a 7-week period. Additionally, we believe the future studies with larger experimental groups should include sex and gender as factors, and conduct a randomized group trial while using this intervention. Further, future research should include a positive control group (e.g. playing a type of videogame, or auditory reaction time training) to eliminate the possibility of placebo effects. Similar to other studies using 3D-MOT as a training tool [12,49], the present study found that participants significantly improved their NeuroTracker scores in just 10 sessions. While 10 sessions elicited improvements in off-the-block reaction times, it remains unknown if this is the ideal number of training sessions. Future studies should investigate whether using different numbers of 3D-MOT affects results. While this research strictly looked at off-the-block reaction times in high-level varsity swimmers, future research should examine if 3D-MOT training can lead to enhancements in other non-visually dominated sports which also have an auditory starting signal (e.g. rowing, track and field, cycling). In a broader context, future studies should also aim to identify the neural pathways underlying cross modal perceptual training transfer.
Conclusion
This study demonstrated improvements in off-the-block reaction time after 10 sessions of 3D-MOT training. This finding lends itself to the field of sport’s science, as it suggests a new mechanism of training reaction time that had not previously been considered. Due to the small sample size, follow up studies should be conducted. The results of this study suggest that 3D-MOT may be an effective cognitive training tool in high-level athletes who play non-visually dominated sports.
Author Contribution
Conceptualization, T.S. and B.C.; methodology, T.M.S. and B.C.; software, T.M.S., B.C.; validation, T.M.S., K.H., T.J.S., R.S., M.L-M., B.C.; formal analysis, T.M.S.; investigation, T.M.S., K.H., .J.S., R.S.; resources, B.C.; data curation, T.M.S., K.H., T.J.S., R.S.; writingoriginal draft preparation, T.M.S.; writing-review and editing, T.M.S., B.C. and M.L-M.; visualization, T.M.S., M.L-M., B.C.; supervision, B.C.; project administration, T.M.S. and B.C.; funding acquisition, T.M.S and B.C.
Funding
This research was funded by CIHR, grant number 127050.
Acknowledgments
The researchers would like to thank the coaches and athletes of the university swim team for their enthusiastic participation in this research study, and their assistance in using the timing software.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- NCAA Mens (2018).
- Riewald S, Rodeo S (2015) The Science of Swimming Faster; Human Kinetics Publishers Inc.: Champagne.
- Rio (2016) Swimming.
- Cossor JM, Mason BR (2001) Swim Start Performances at The Sydney 2000 Olympic Games. In Proceedings of the International Symposium on Biomechanics in Sports.
- Tor E, Pease D, Ball K (2014) Characteristics of an elite swimming start.
- Thompson PD, Colebatch JG, Brown P, Rothwell JC, Day BL, et al. (1992) Voluntary stimulusâ€ÂÂsensitive jerks and jumps mimicking myoclonus or pathological startle syndromes. Mov Disord 7: 257-262.
- Pain MTG, Hibbs A (2007) Sprint starts and the minimum auditory reaction time. J Sports Sci 25: 79-86.
- Bishop DC, Smith RJ, Smith MF, Rigby HE (2009) Effect of Plyometric Training on Swimming Block Start Performance in Adolescents. J Strength Cond Res 23: 2137-2143.
- Blanksby B, Nicholson L, Elliott B (2002) Swimming: Biomechanical analysis of the grab, track and handle swimming starts: An intervention study. Sport Biomech 1: 11-24.
- Leprêtre P, Kazarine L, Puel F (2014) Does 4-weeks of simple reaction time training improve start performance in swimming ? 1-2.
- Papic C, Sinclair P, Fornusek C, Sanders R (2019) The effect of auditory stimulus training on swimming start reaction time. Sport Biomech 18: 378-389.
- Parsons B, Magill T, Boucher A, Zhang M, Zogbo K, et al. (2016) Enhancing Cognitive Function Using Perceptual-Cognitive Training. Clin EEG Neurosci 47:37-47.
- Cavanagh P, Alvarez GA (2005) Tracking multiple targets with multifocal attention. Trends Cogn Sci 9: 349-354.
- Romeas T, Guldner A, Faubert J (2016) 3D-Multiple Object Tracking training task improves passing decision-making accuracy in soccer players. Psychol Sport Exerc 22: 1-9.
- Mangine GT, Hoffman JR, Wells AJ, Gonzalez AM, Rogowski JP, et al. (2014) Visual tracking speed is related to basketball-specific measures of performance in NBA players. J Strength Cond Res 28: 2406-2414.
- Fleddermann MT, Heppe H, Zentgraf K (2019) Off-Court Generic Perceptual-Cognitive Training in Elite Volleyball Athletes: Task-Specific Effects and Levels of Transfer. Front Psychol 10.
- Harris DJ, Wilson MR, Vine SJ (2018) A systematic review of commercial cognitive training devices: Implications for use in sport. Front Psychol 9.
- Swiss Timing (2019).
- FINA Rules and Regulations (2018).
- Aranha VP, Sharma K, Samuel AJ, Joshi R, Kumar PS (2015) Reaction Time in Children by Ruler Drop Method: A Cross-Sectional Study Protocol. Pediatr Educ Res 3: 61-66.
- Aranha VP, Saxena S, Moitra M, Narkeesh K, Arumugam N, et al. (2017) Reaction time norms as measured by ruler drop method in school-going South Asian children: A cross-sectional study. HOMO J Comp Hum Biol 68: 63-68.
- Montare A (2009) The simplest chronoscope: Group and interindividual differences in visual reaction time. Percept Mot Skills 108: 161-172.
- Eckner JT, Whitacre RD, Kirsch NL, Richardson JK (2019) Evaluating a clinical measure of reaction time: An observational study. Percept Mot Skills 108: 717-720.
- RCoreTeam R: a language and environment for statistical computing.
- Royston P (1992) Approximating the Shapiro-Wilk W-test for non-normality. Stat Comput 2: 117-119.
- Rosner B, Glynn RJ, Lee MLT (2006) The Wilcoxon signed rank test for paired comparisons of clustered data. Biometrics 62: 185-192.
- Torchiano M (2019) Efficient Effect Size Computation. R package effsize version 0.7.6.
- R Handbook: Two-sample Mann-Whitney U Test.
- Lee CW, Huang C, Lin DC (2002) The strategy of muscular pre-tension during initial block phase in swimming grab start. Int Soc Biomech Sport 52-55.
- Legault I, Faubert J (2012) Perceptual-cognitive training improves biological motion perception: Evidence for transferability of training in healthy aging. Neuroreport 23: 469-473.
- Vartanian O, Coady L, Blackler K (2016) 3D Multiple Object Tracking Boosts Working Memory Span: Implications for Cognitive Training in Military Populations. Mil Psychol 28: 353-360.
- Tullo D, Guy J, Faubert J, Bertone A (2018) Training with a three-dimensional multiple object-tracking (3D-MOT) paradigm improves attention in students with a neurodevelopmental condition: a randomized controlled trial. Dev Sci 21: e12670.
- Argüelles J, Caynzos B (2014) XVth World Swimming Championships: Race phases’ contribution to the overall performance and the gender differences. In Proceedings of the IV NSCA International Conference 2014. Human Performance Development through Strength and Conditioning.; Murcia, Universidad Católica de Murcia.
- Mason B, Cossor J (2000) What can we learn from competition analysis at the 1999 pan pacific swimming championships? In proceedings of the international symposium on biomechanics in sports.
- McGovern DP, Astle AT, Clavin SL, Newell FN (2016) Task-specific transfer of perceptual learning across sensory modalities. Curr Biol 26: R20-R21.
- Shams L, Seitz AR (2008) Benefits of multisensory learning. Trends Cogn Sci 12: 411-417.
- Gori M, Del Viva M, Sandini G, Burr DC (2008) Young Children Do Not Integrate Visual and Haptic Form Information. Curr Biol 18: 694-698.
- Wickens CD (2008) Multiple resources and mental workload. Hum Factors 50: 449-455.
- Shimojo S, Shams L (2001) Sensory modalities are not separate modalities: Plasticity and interactions. Curr Opin Neurobiol 11: 505-509.
- Calvert GA, Bullmore ET, Brammer MJ, Campbell R, Williams SCR, et al. (1997) Activation of auditory cortex during silent lipreading. Science 276: 593-596.
- Eckert MA, Kamdar NV, Chang CE, Beckmann CF, Greicius MD, et al. (2008) cross-modal system linking primary auditory and visual cortices: Evidence from intrinsic fMRI connectivity analysis. Hum Brain Mapp 29: 848-857.
- Hebb DO (1949) The Organization of Behaviour. Wiley: New York, USA.
- Seitz AR, Kim R, Shams L (2006) Sound Facilitates Visual Learning. Curr Biol 16: 1422-1427.
- Potter MC, Faulconer BA (1975) Time to understand pictures and words. Nature 253: 437-438.
- Del Rossi G, Malaguti A, Del Rossi S (2014) Practice effects associated with repeated assessment of a clinical test of reaction time. J Athl Train 49: 356-359.
- NCAA Women (2016).
- Botwinick J, Thompson LW (1966) Components of Reaction Time in Relation to Age and Sex. J Genet Psychol 108: 175-183.
- Silverman IW (2006) Sex differences in simple visual reaction time: A historical meta-analysis. Sex Roles 54: 57-68.
- Faubert J (2013) Professional athletes have extraordinary skills for rapidly learning complex and neutral dynamic visual scenes. Sci Rep 3.
- Your Guide to NeuroTracker Scores, NeuroTracker (2018).
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi