Case Report, Clin Oncol Case Rep Vol: 5 Issue: 5
Endometrioid Cancer Transform to Carcinosarcoma after Hormonal Treatment: Epithelial-Mesenchymal Transition during Fertility Preserving Management in Two Women
Jie Yang1*, Huanwen Wu2, Yang Xiang1, Lingya Pan1 and Jiaxin Yang1
1Department of Obstetrics and Gynecology, Peking Union Medical College Hospital, National Clinical Research Center for Obstetric and Gynecologic Diseases, Beijing, China
2Department of Pathology, Peking Union Medical College Hospital, Beijing, China
*Corresponding Author: Jie Yang
Department of Obstetrics and Gynecology, Peking Union Medical College Hospital,
Chinese Academy of Medical Science, No. 1 Shuaifuyuan, Wangfujing, Dong Cheng District, Beijing 100730, China
E-mail: yangjie56@pumch.cn
Received: April 19, 2022; Manuscript No: COCR-22-61136;
Editor Assigned: April 22, 2022; PreQC Id: COCR-22-61136 (PQ);
Reviewed: May 07, 2022; QC No: COCR-22-61610 (Q);
Revised: May 09, 2022; Manuscript No: COCR-22-61610 (R);
Published: May 18, 2022; DOI: 10.4172/cocr.5(5).229
Citation: Yang J, Wu H, Xiang Y, Pan L, Yang J (2022) Endometrioid Cancer Transform to Carcinosarcoma after Hormonal Treatment: Epithelial-Mesenchymal Transition during Fertility Preserving Management in Two Women. Clin Oncol Case Rep 5:5
Abstract
Endometrial Cancer (EC) is the most common gynecologic malignancy. A significant number of patients are diagnosed during reproductive age in nowadays. The importance of fertility preservation for EC has become more evident than ever before in this patient population. Hormonal therapy for stage IA low-risk endometrial cancer is reported efficient and safe. However, the recurrence rate is high and the pregnancy outcomes are not satisfactory. We report two patients at reproductive age who were diagnosed of endometrioid EC and underwent fertility preservation treatment. Both of them achieved complete remission but later developed carcinosarcoma. This unusual disease progression may imply the underling mechanism of hormonal resistance. The epithelial-mesenchymal transition pathway may be involved in the EC cell evolution.
Keywords: Endometrial cancer; Fertility preservation; Epithelial-Mesenchymal transition; Tumor cell evolution; Hormonal resistance
Introduction
Endometrial Cancer (EC) is the most common gynecologic malignancy in developed countries and the incidence is continuously rising [1]. In the modern societies women are delaying childbearing and EC affects a significant proportion of reproductive age women who wish to preserve fertility [2]. Fertility sparing management by progestin for low-risk endometrial cancer were introduced since 1990s [3]. Till now the strategies for fertility preservation in EC are still limited to hormonal therapy. Large dose of Medroxyprogesterone Acetate (MPA) or Megestrol Acetate (MA) are the most frequently used progestin in fertility preserving and the remission rate is relatively high, which can achieve a 97% Complete Remission (CR) rate [4]. The long-term survival was reported no differences between patients who received progestin therapy and who received primary surgery [5]. Though hormonal therapy in EC showed a high remission rate, the pregnancy rate remains low with no more than 50% in most medical centers, and the recurrence rate is reported high as 40% [6]. Providing oncological safe options for fertility preservation in EC patients at reproductive age has become a crucial component of cancer survivorship care [2].
Here we describe two clinical cases of patients with endometrioid EC who underwent hormonal treatment for fertility preservation. Both of them achieved CR but developed carcinosarcoma later. The extreme phenomenon of Epithelial-Mesenchymal Transition (EMT) in these two patients’ cancer tissue was also supported by immunohistochemistry staining. We suspect EMT may be involved in EC progression and progestin resistance.
Case Report
Patient 1
A 22-year-old college girl with menstrual disorders of amenorrhea and periodic menorrhagia for five years presented at our outpatient clinic. Uterine ultrasonography showed thickening endometrium of 1.4 cm. A Dilation and Curettage (D&C) were performed and pathology diagnosis of focal endometrial cancer with atypical endometrial hyperplasia background. There were no other risk factors such as Lymph Vascular Space Invasion (LVSI), myometrial invasion or lymph node metastasis on pathology or imaging evaluation. Her BMI was 19.7. The patient had a strong will for fertility preservation. After in-depth discussion and fully consent, the patient and physician made a shared decision on fertility sparing management of her endometrial cancer. She was provided with oral MPA of 250 mg daily and hysteroscopy endometrial biopsy every three months. The endometrial evaluation showed mild to moderate atypical hyperplasia and endometrium decidual change at three, six and nine months. The patient achieved CR after one year’s treatment. She was prescribed oral contraceptives for maintenance and underwent ultrasonography every 6 months to monitor the endometrium thickness.
The patient had regular menstrual cycles and ultrasound showed endometrium thickness within 1 cm for 21 months. She presented at our outpatient clinic with spotting and menorrhagia two years after CR. The endometrium thickness on ultrasonography was 1.5 cm. Hysteroscopic endometrium curettage was performed with pathology reported carcinosarcoma of 30% grade 2 endometrioid cancer and 70% undifferentiated sarcoma. Immunohistochemistry (IHC) staining showed mutation-type p53 expression. Immunostaining for EMTrelated markers including E-Cadherin, Vimentin and β-catenin was also performed (Figure 1 left column). The expression of E-cadherin gradually decreased from epithelium to mesenchymal components, whereas nuclear expression of β-catenin gradually increased. The mesenchymal marker vimentin was abnormally overexpressed in the epithelium. The Positron Emission Tomography-Computed Tomography (PET/CT) showed no radioactive lesion intra-or extrauterus. After a multidisciplinary consultation with gynecologic oncologist, pathologist and radiologist, the patient decided to proceed with hysterectomy. A laparoscopic endometrial cancer staging surgery with hysterectomy, bilateral salpingectomy and pelvic lymphadenectomy was performed 38 days after diagnosis of uterine carcinosarcoma.
There was no residual tumor on specimens. No additional therapy was delivered to the patient. She was free of tumor two years after surgery.
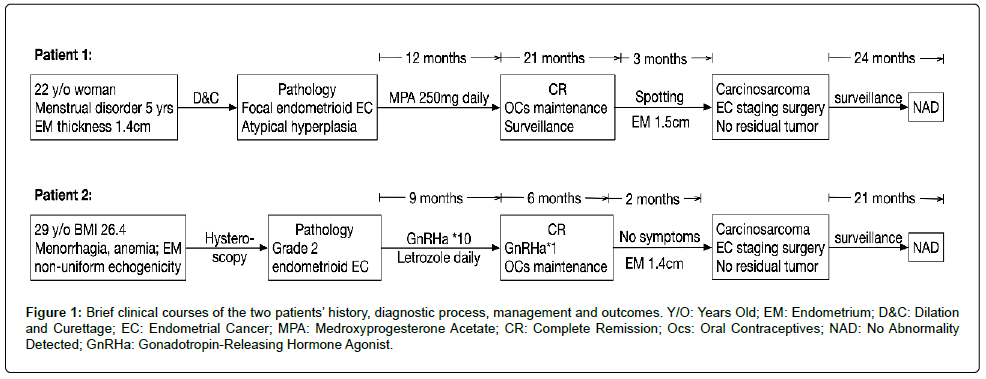
Figure 1: Brief clinical courses of the two patients’ history, diagnostic process, management and outcomes. Y/O: Years Old; EM: Endometrium; D&C: Dilation and Curettage; EC: Endometrial Cancer; MPA: Medroxyprogesterone Acetate; CR: Complete Remission; Ocs: Oral Contraceptives; NAD: No Abnormality Detected; GnRHa: Gonadotropin-Releasing Hormone Agonist.
Patient 2
A 29-year-old patient with Polycystic Ovary Syndrome (PCOS) presented with long period and menorrhagia caused severe anemia (hemoglobin 50 g/L). Ultrasonography showed the endometrium thickness of 0.8 cm with non-uniform echogenicity. Hysteroscopy and endometrial biopsy were performed with pathologic diagnosis of grade 2 endometrioid EC. Immunohistochemistry (IHC) staining showed mutation-type p53 expression. A PET/CT suggested intra uterus radioactive lesion and no signs of extra uterus dissemination. The patient desired fertility sparing treatment for her endometrial cancer. She was overweighted with BMI of 26.4. After consent, she was given Gonadotropin Releasing Hormone Agonist (GnRHa) every 28 days with daily letrozole for nine months then the patient achieved CR. She received one more dose of GnRHa for consolidation treatment.
Her menses resumed 6 months after last treatment and oral contraceptive pills were given periodically. However, she suffered from menstrual disorders and ultrasonography showed the endometrium thickness of 1.4 cm with rich blood flow signal 8 months after CR. Hysteroscopic endometrium curettage was performed with pathology reported carcinosarcoma of (20%) grade 2-3 endometrioid cancer and (80%) undifferentiated sarcoma. IHC analysis of EMT related markers suggested similar findings with patient 1. (Figure 2, right column) After comprehensively discussion with her gynecologic oncologist, the patient decided to take the radical surgery. She underwent the laparotomy hysterectomy, bilateral salpingo-oopherectomy and lymphadenectomy 28 days after diagnosis of sarcoma. Postoperative pathology did not identify any residual tumor or extra uterine metastasis. No additional treatment was given and she was free of disease 21 months after surgery.
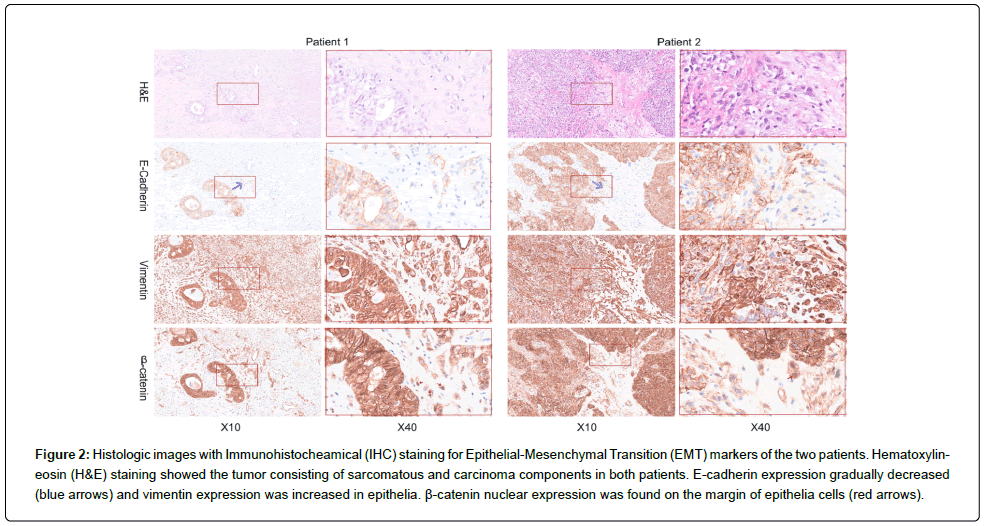
Figure 2: Histologic images with Immunohistocheamical (IHC) staining for Epithelial-Mesenchymal Transition (EMT) markers of the two patients. Hematoxylineosin (H&E) staining showed the tumor consisting of sarcomatous and carcinoma components in both patients. E-cadherin expression gradually decreased (blue arrows) and vimentin expression was increased in epithelia. β-catenin nuclear expression was found on the margin of epithelia cells (red arrows).
Discussion
Historically EC has been a disease mainly occurred in postmenopausal women. The standard treatment strategies for EC are surgical removal of uterus with or without gonadotoxic treatments such as adjuvant radiotherapy and chemotherapy. However, a significant number of patient encounter with a diagnosis of EC during reproductive age in nowadays. The percentage for patient under the age of 45 with newly diagnosed uterine cancer is 6.5%, based on Surveillance, Epidemiology and End Results (SEER) report [7]. Complicated with the sociodemographic transition toward pregnancy at advanced maternal age, the importance of fertility preservation for EC has become more evident than ever before in this patient population. Among the women with the diagnosis of EC, 65% are age <45 years who may wish to preserve fertility [2]. Therefore, providing oncological safe fertility preservation options has become the desire by both patients and gynecologic oncologists in modern cancer care.
Many retrospective and prospective studies have reported the successful achievement in fertility preserving management of EC [6]. The indications for fertility preserving treatment are mainly based on clinical and pathophysiological features. Conservative treatment can be proposed to women without any risk factors, which includes early stage, tumor localized in mucosa, without LVSI, well differentiated endometrioid cancer and no signs of extra-uterine metastasis. Though treatment for fertility preservation in EC patient was introduced since 1990’s, the strategies are still limited, including large dose of progestin, levonorgestrel system (LNG-IUS), GnRHa and hysteroscopic resection of tumor [8]. The hormonal treatment for early-stage EC is reported safe, with a CR rate from 55% to 87.5% and median time to CR of 4.5-12 months[9]. However, the recurrence rate is high up to 46.6% and the pregnancy outcomes are not satisfactory with the live birth rates of 18.1% to 52.6% [9]. Understanding the mechanism of varied responses to hormonal therapy in patients with similar clinical features is crucial to achieve higher remission and successful pregnancy outcomes. Basic mechanism study can help clinician with patient selection of whom are truly benefit from fertility-sparing treatment, making more accurate predictive models, as well as novel therapeutic strategy development.
In our report, both of the two patients had low-risk clinical characteristics: endometrioid histology, tumor limited in mucous, and no extra-uterine spreading on image studies. The only notable pathological feature of potential prognosticator was mutant p53 in IHC at diagnosis. It is difficult to diagnose high-grade endometrioid EC and the concordance among senior pathologists is low [10]. The Cancer Genome Atlas (TCGA) Research Network has performed an integrated genomic study of 373 EC and correlated the molecular profiles to the prognosis [11]. The result showed TP53 mutation is frequently presented in serous tumors and high-grade endometrioid EC. Furthermore, about 25% of high-grade endometrioid EC have a molecular phenotype similar to uterine serous carcinoma and the survival outcomes are also inferior than those without TP53 mutation [11]. After TCGA study, more researches on EC genomic profiling have shaped the nowadays post-surgical treatment paradigms, while there are scant studies in fertility preserving management of EC. In our opinion, the fertility preserving management of EC also warrant molecular studies to classify patients according to the predict hormonal therapeutic effect and develop the tailored treatment strategies that can improve successful delivery rate without detrimental to survival outcomes.
The two cases we reported here represented tumor evolution from carcinomatous elements to sarcomatous elements derivation after hormonal treatment. They are the best examples of EMT, presenting with trans-differentiations, expression of mesenchymal markers. The special clinical phenomenon gave us an idea of EMT pathway may be involved in the hormonal resistance during fertility preserving management of EC. EMT is a temporary and reversible physiological process in embryogenesis [12]. Recent studies reported EMT is also presented in many kinds of malignant tumors including EC [13]. Activation of EMT results in the loss of cell polarity, disruption of cellcell junctions, degradation of the underlying basement membrane and reorganization of the Extracellular Matrix (ECM) [12]. In the case of carcinoma cells, EMT status facilitates cell invasion and dissemination to distant tissues [14].
The EMT process includes transcriptional control by the zinc finger protein Snail and regulatory factors, such as SLUG, Zinc finger E-box Binding homeobox 1 (ZEB1) and ZEB2, Twist, and E12/E47, etc. [15]. It has been reported that EMT can be activated by different growth factors and cytokine signaling pathways, such as the transforming growth factor-β (TGF-β), Wnt, JAK/STAT, MAPK, and Notch pathways [16]. Expression of E-cadherin and certain cytokeratins is lost, while expression of markers associated with the mesenchymal state (N-cadherin, vimentin, fibronectin and β1 and β3 integrins) is activated [15]. β-catenin is involved in the process of WNT signaling pathway. In activation of WNT pathway, β-catenin translocate to the nucleus where it binds to the transcription factors to activate genes that drive EMT [12]. We performed IHC staining for E-cadherin, vimentin and β-catenin to evaluate EMT process. The IHC results showed ongoing process of EMT presenting gradually decreased expression of E-cadherin and high vimentin expression in epithelial cells, as well as nucleus staining of β-catenin.
It is reported that epithelial-like cancer cells can gradually acquire more-mesenchymal characteristics as tumor progression proceeds [12]. The resulting quasi-mesenchymal cells display elevated resistance to several therapeutic regimens [17]. In our report, the two patients achieved CR after hormonal treatment but developed carcinosarcoma during low dose hormonal maintenance therapy. We suspect that EMT was involved in the process of cancer cells acquire drug resistance in these two patients. The mechanisms of EMTmediated chemoresistance are well elucidated in other type of cancers [12]. EMT related factors SNAIL and SLUG promote chemoresistance by antagonizing p53-mediated apoptosis and by regulating genes involved in cell death and stem cell maintenance [18,19]. There is a lack of study in hormonal resistance and EMT process in endometrial cancer. The study on the role of progesterone in antiproliferative action on uterine epithelium suggested that Hand 2 is a critical regulator in suppressing the production of several Fibroblast Growth Factors (FGFs), which can be related with EMT [20]. Understanding the role of EMT process in hormonal treatment of EC may help us elucidate the mechanism of varied drug response and precise the stratified treatment for patient of reproductive age. Anti-EMT therapy may be a therapeutic strategy for fertility preserving management of EC.
Conclusion
In conclusion, we reported two patients who developed carcinosarcoma after fertility preserving management of low-risk EC. The molecular mechanism study is warranted to understand the drug resistance and improve the treatment outcomes.
References
- Lu KH, Broaddus RR (2020) Endometrial cancer. N Engl J Med 383: 2053-2064. [Google Scholar] [Cross Ref]
- Taylan E, Oktay L (2019) Fertility preservation in gynecologic cancers. Gynecol Oncol 155: 522-529. [Google Scholar] [Cross Ref]
- Ushijima K, (2019) Fertility sparing treatment for early stage endometrial cancer: Current situation and new strategy. J Gynecol Oncol 30: e117. [Google Scholar] [Cross Ref]
- Mitsuhashi A, Habu Y, Kobayashi T, Kawarai Y, Ishikawa H, et al. (2019) Long-term outcomes of progestin plus metformin as a fertility-sparing treatment for atypical endometrial hyperplasia and endometrial cancer patients. J Gynecol Oncol 30: 90. [Google Scholar] [Cross Ref]
- Greenwald ZR, Huang LN, Wissing MD, Franco EL, Gotlieb WH, et al. (2017) Does hormonal therapy for fertility preservation affect the survival of young women with early-stage endometrial cancer? Cancer 123: 1545-1554. [Google Scholar] [Cross Ref]
- Gallos ID, Yap J, Rajkhowa M, Luesley DM, Coomarasamy A, et al. (2012) Regression, relapse, and live birth rates with fertility-sparing therapy for endometrial cancer and atypical complex endometrial hyperplasia: A systematic review and metaanalysis. Am J Obstet Gynecol 207: e1-e12. [Google Scholar] [Cross Ref]
- Howlader NN, Noone AM, Krapcho M, Garshell J, Miller D, et al. (2014) SEER cancer statistics review 1975-2012. National Cancer Institute, 2014. [Google Scholar]
- Garzon S, Uccella S, Zorzato PC, Bosco M, Franchi M, et al. (2021) Fertility-sparing management for endometrial cancer: Review of the literature. Minerva Med 112: 55-69. [Google Scholar] [Cross Ref]
- Leone RMU, Khamisy FR, Bragazzi NL, Bogani G, Martinelli F, et al. (2021) Fertility-sparing treatment of patients with endometrial cancer: A review of the literature. J Clin Med 10(20). [Google Scholar] [Cross Ref]
- Gilks CB, Oliva E, Soslow RA (2013) Poor interobserver reproducibility in the diagnosis of high-grade endometrial carcinoma. Am J Surg Pathol 37: 874-881. [Google Scholar] [Cross Ref]
- Levine DA (2013) Integrated genomic characterization of endometrial carcinoma. Nature 497: 67-73.
[Google Scholar] [Cross Ref] - Dongre A, Weinberg RA (2018) New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol 20: 69-84. [Google Scholar] [Cross Ref]
- Zhou Q, Li W, Kong D, Liu Z, Shi Z, et al. (2019) DACH1 suppresses epithelial to mesenchymal transition (EMT) through Notch1 pathway and reverses progestin resistance in endometrial carcinoma. Cancer Med 8: 4380-4388. [Google Scholar] [Cross Ref]
- Lamouille S, Xu J, Derynck R et al. (2014) Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 15: 178-196. [Google Scholar] [Cross Ref]
- De Craene B, Berx G (2013) Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer 13: 97-110. [Google Scholar] [Cross Ref]
- Gonzalez DM, Medici D (2014) Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal 7: re8. [Google Scholar] [Cross Ref]
- Shibue T, Weinberg RA (2017) EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol 14: 611-629. [Google Scholar] [Cross Ref]
- Kurrey NK (2009) Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells 27: 2059-2068. [Google Scholar] [Cross Ref]
- Bharti R, Dey G, Mandal M (2016) Cancer development, chemoresistance, epithelial to mesenchymal transition and stem cells: A snapshot of IL-6 mediated involvement. Cancer Lett 375: 51-61. [Google Scholar] [Cross Ref]
- Li Q, Kannan A, DeMayo FJ, Lydon JP, Cooke PS, et al. (2011) The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science 331: 912-916. [Google Scholar] [Cross Ref]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 