Case Report, Clin Oncol Case Rep Vol: 5 Issue: 5
Everolimus and Lenvatinib in a Kidney Metastatic Epithelioid Angiomyolipoma
Flora Brouillard-Saby1, Chahrazed Boukhatmi1, Pierre Laurent- Puig2,3,4, Laetitia Marisa4, Nicolas Derive4, Virginie Verkarre5 and Stephane Oudard1*
1Institut du cancer Paris CARPEM, APHP, Department of Oncology, Hôpital Européen Georges-Pompidou, Paris, France
2Institut du cancer Paris CARPEM, APHP, Department of Biology, Hôpital Européen Georges-Pompidou, Paris, France
3Centre de recherche des Cordeliers, Inserm, Sorbonne University, Paris Cité University, Paris, France
4Laboratoire de Biologie Médicale multisites SeqOIA, Paris, France
5Institut du cancer Paris CARPEM, APHP, Department of Pathology, Hôpital Européen Georges-Pompidou, Université Paris-Cité, Paris, France
*Corresponding Author: Stephane Oudard
Institut du cancer Paris
CARPEM, APHP, Department of Oncology, Hôpital Européen Georges-
Pompidou, Paris, 20 rue Leblanc, 75015 Paris 01.56.10.21.59, France
E-mail: stephane.oudard@aphp.fr
Received: April 23, 2022; Manuscript No: COCR-22-61610;
Editor Assigned: April 25, 2022; PreQC Id: COCR-22-61610 (PQ);
Reviewed: May 11, 2022; QC
No: COCR-22-61610 (Q);
Revised: May 13, 2022; Manuscript No: COCR-22-
61610 (R);
Published: May 23, 2022; DOI: 10.4172/cocr.5(5).230
Citation: Saby FB, Boukhatmi C, Puig P, Marisa L, Derive N (2022) Everolimus and Lenvatinib in a Kidney Metastatic Epithelioid Angiomyolipoma. Clin Oncol Case Rep 5:5
Abstract
Objective: Report the efficacy and safety of a treatment with everolimus and lenvatinib in a patient with metastatic E-AML of the kidney.
Methods: We report one case of E-AML treated at European Georges Pompidou hospital with everolimus and lenvatinib.
Results: The patient had an E-AML with pulmonary and hepatic metastases associated with somatic inactivation of TSC2. After 12 months of treatment, she demonstrated a prolonged partial response with 62% tumor shrinkage. Tolerability was manageable (grade 1 hypertension and renal failure, grade 2 diarrhea).
Conclusion: Combination of everolimus plus lenvatinib was highly effective in this case of metastatic E-AML with TSC2 inactivation. Prospective evaluation of this combination is needed.
Keywords: Angiomyolipoma, Computed tomograph,
Introduction
Recognized by the World Health Organization (WHO) since 2004, renal Angiomyolipoma (AML) and Epithelioid Angiomyolipoma (E-AML) are two distinct mesenchymal renal tumors that arise from epithelioid perivascular cells [1]. Whereas AML is composed of an admixture of abnormal blood vessels, smooth muscle cells and mature adipose tissue, E-AML is characterized by at least 80% of epithelioid cells. In contrast to AML, which represent 1% of all renal tumors and is considered a benign tumor, E-AML is rare, accounting for 4.6% of all AML, but with malignant potential occurring in one-third of cases with metastasis leading to death [2]. E-AML mainly affects women (78% of cases) with a median age between 38 and 50 years. Most renal E-AML are sporadic, but some are associated with tuberous sclerosis complex (TSC) caused by germline mutation of TSC1 or TSC2 genes [3].
E-AML are associated with low fat on Computed Tomography (CT) and can be confused with Renal Cell Carcinoma (RCC) [4], with challenging microscopical diagnosis requiring immunohistochemistry. They are characterized by proliferation of epithelioid cells, with positive expression of melanocytic markers such as HMB-45, Melan-A without cytokeratin and paired-box gene 8 (PAX8) expression.
Standard treatment for early-stage tumors is surgical resection [5]. Metastatic E-AML has been treated with systemic chemotherapy with no success [6]. The oncogenesis of AML and E-AML is mostly driven by loss of function of TSC1 or TSC2 leading to activation of Mammalian Target of Rapamycin (mTOR) [7]. In line with medical treatment of AML using mTOR inhibitors [8-10], several cases have reported successful treatment of E-AML with targeted therapies such as mTOR and multikinase inhibitors [11-14]. However, data are lacking regarding the efficacy of combination therapy with these two treatments in patients with metastatic E-AML.
Here, we report a case of aggressive E-AML treated at European Georges Pompidou hospital with everolimus plus lenvatinib.
Case Report
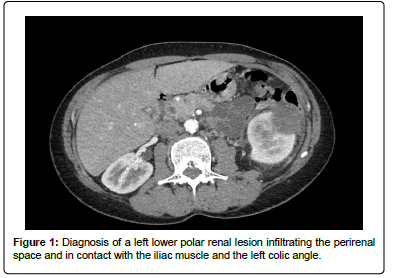
A 54-year-old woman with no notable medical history except for a total conservative hysterectomy, surgery for bilateral hallus valgus, and lipoma removal was referred to our institution in November 2020 for the exploration of nausea, vomiting and hyperleukocytosis. Abdominal CT scan identified a left lower polar renal lesion measuring (52 × 52 × 57) mm, infiltrating the perirenal space and in contact with the iliac muscle and the left colic angle (Figure 1).
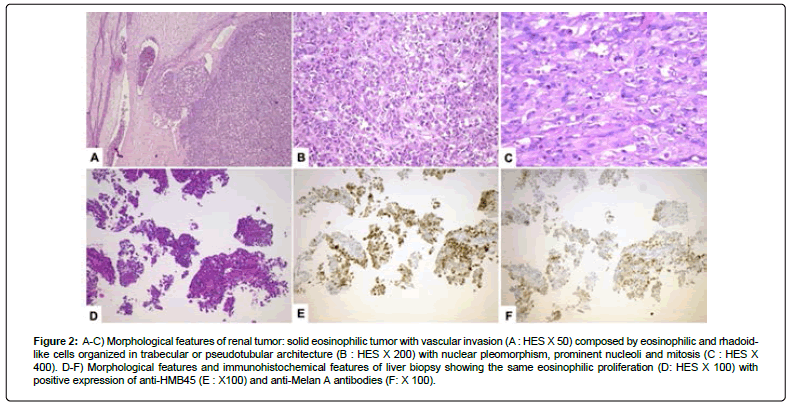
Reviewed by two experts in uropathology tumors, a biopsy showed an epithelioid angiomyolipoma with adverse features, with positive melanocytic markers and negative cytokeratin and PAX8. The extension assessment (chest and bone scans) was normal. Left enlarged nephrectomy with left colectomy was performed using laparotomy in January 2021. On pathological report, the E-AML measured 70 mm and was partially necrotic, with extension into the peri renal fat and without infiltration of the adherent left colonic segment. Numerous tumoral emboli were identified (Figure 2A). Tumoral cells proliferation were composed of eosinophilic or rhabdoid-like cells mimicking RCC organized in trabecular or tubular like pattern (Figure 2B), with frequent mitosis (Figure 2C). Melan-A and HMB45 were expressed weakly and heterogeneously without expression of PAX8 Ki 67 was 60%.
Figure 2: A-C) Morphological features of renal tumor: solid eosinophilic tumor with vascular invasion (A : HES X 50) composed by eosinophilic and rhadoidlike cells organized in trabecular or pseudotubular architecture (B : HES X 200) with nuclear pleomorphism, prominent nucleoli and mitosis (C : HES X 400). D-F) Morphological features and immunohistochemical features of liver biopsy showing the same eosinophilic proliferation (D: HES X 100) with positive expression of anti-HMB45 (E : X100) and anti-Melan A antibodies (F: X 100).
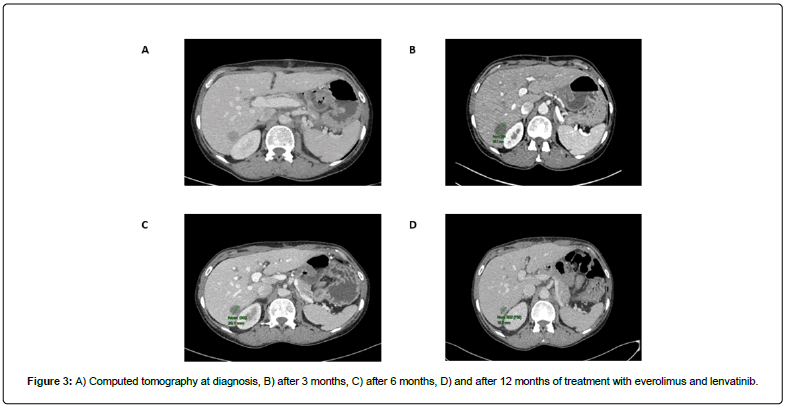
No additional postoperative treatment was received. One month later, in February 2021, a new CT scan revealed lymph nodes, and pulmonary and hepatic metastases (Figure 3A).
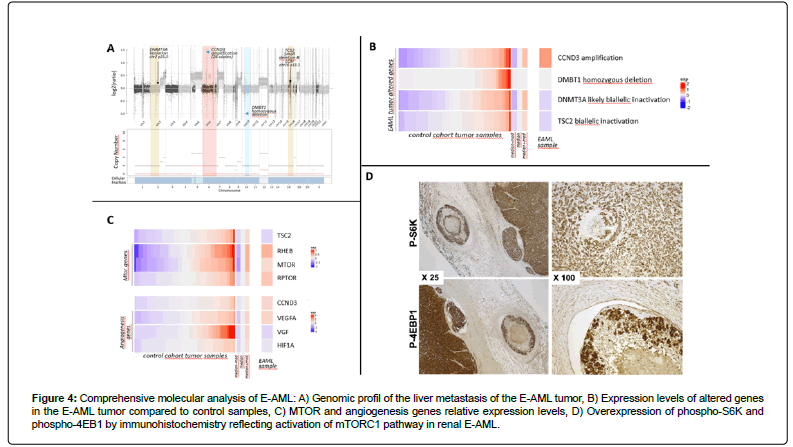
A liver biopsy confirmed hepatic localization of the known E-AML (Figure 2D) with positivity of HMB45 (Figure 2E) and Melan-A (Figure 2F) and negativity of PAX8. Whole exome sequencing and RNA sequencing was performed on the liver biopsy. The genomic profile of the tumor revealed a quasi-complete loss of heterozygosity by isodisomy of the genome (Figure 4A). Furthermore, a region on chromosome 6 including the CCND3 gene coding the Cyclin D3 protein was amplified at 26 copies, and a region on chromosome 10 showed a homozygous deletion including the DMBT1 gene. The tumor mutational load was 0.95 mutations/Mb. No microsatellite instability was observed. Only two mutations were considered to be likely oncogenic. The first of these led to inactivation of TSC2 via a small deletion (NM_000548.5 (TSC2):c.5252_5259+19del; p.(Arg1751Hisfs*21)) and loss of heterozygosity of the other allele.
Figure 4: Comprehensive molecular analysis of E-AML: A) Genomic profil of the liver metastasis of the E-AML tumor, B) Expression levels of altered genes in the E-AML tumor compared to control samples, C) MTOR and angiogenesis genes relative expression levels, D) Overexpression of phospho-S6K and phospho-4EB1 by immunohistochemistry reflecting activation of mTORC1 pathway in renal E-AML.
The second oncogenic mutation was a missense mutation of DNMT3A (NM_022552.5(DNMT3A):c.1915C>T; p.(Leu639Phe)) coding a methyltransferase gene. This mutation was associated with loss of heterozygosity of the other allele. All of these gene alterations were confirmed at the RNA expression level with upregulation of CCND3 and downregulation of TSC2 and DNMT3A transcripts, and with the absence of DMNB1 expression (Figure 4B). Since inactivation of TSC2 suggested susceptibility to mTOR inhibitors, key genes of the mTOR and angiogenesis pathways were also investigated at the expression level. Overexpression of mTOR and RPTOR, direct targets of the TSC2 gene, was observed. Also, increased angiogenesis was observed through the up-regulation of vascular endothelial growth factor A (VEGFA) but not VEGF or hypoxia inducible factor 1A (HIF1A) (Figure 4C), suggesting that amplification of CCND3 might contribute to the upregulation of this pathway [15]. Finally, we identified an overexpression of both Phospho-S6 Ribosomal Protein (Ser240/244) antibody (Cell Signaling, D68F8, 1/300) (phospho-S6K) and Phospho-4EBP1 (Thr37/46) antibody (Cell Signaling, 236B4, 1/100) (phospho-4EBP1) on tumoral cells highlighting activation of mTORC1 pathway (Figure 4D). These alterations were in favor of a combination of a mTOR inhibitor and an angiogenesis inhibitor.
The patient initiated treatment with everolimus 5 mg/day plus lenvatinib 18 mg/day, a treatment option for RCC based on results of the phase III CLEAR study [16-18]. A CT scan, performed every 3 months, showed an objective response with 43% tumor shrinkage at 3 months, 53% at 6 months, and 62% at 9 and 12 months according to Response Evaluation Criteria in Solid Tumors 1.1 (Figure 3B, 3C and 3D respectively). Tolerability was manageable, with grade 1 hypertension and grade 1 left ventricular dysfunction requiring optimization of treatment, grade 1 renal failure requiring a reduction of the lenvatinib dose to 14 mg/day, as well as grade 1 nausea, anorexia, asthenia, and hand foot syndrome, and grade 2 diarrhea. We continue to monitor the patient every 3 months, with her next CT scan planned for May 2022.
Discussion
In 2004, the WHO classification of renal cancer defines E-AML as a potentially malignant mesenchymal cancer, characterized by the proliferation of epithelioid cells with metastatic potential [1]. Malignant E-AML occurs in one-third of cases leading to an aggressive tumor with metastasis and can lead to death. Risk factors for malignant transformation are tumor size, tumor necrosis, presence of venous invasion and high mitotic activity [19].
Most AMLs are sporadic but more than half of patients with Tuberous Sclerosis develop AML. TSC1 and TSC2 genes are tumor suppressor genes, encoding for hamartin and tuberin, respectively, which are involved in the Rheb/mTOR/p70S6 kinase signaling pathway. Mutation in these genes results in an mTORC1-dependent activation of the Hypoxia Inducible Factor (HIF) which upregulates the expression of VEGF, increasing angiogenesis and tumor growth. The relationship between TSC1/TSC2 and mTORC1 explains the use of mTOR inhibitors in TSC associated renal E-AMLs [10,20]. The efficacy of the combination of the mTOR inhibitor everolimus with the anti-angiogenic TKI lenvatinib in kidney cancer is known based on the results of the phase III CLEAR study [17]. This study compared a treatment with everolimus + lenvatinib versus pembrolizumab + lenvatinib versus sunitinib as first-line therapy in patients with metastatic clear cell RCC. Compared with sunitinib, everolimus + lenvatinib was associated with improved progression-free survival (hazard ratio [HR] 0.65, p<0.001) and an objective response rate of 53.3% (including 10% complete responses), but no improvement in overall survival (HR 1.15, p=0.3). These results may suggest that the combination of everolimus plus lenvatinib may be applicable for metastatic E-AMLs.
Conclusion
The combination of everolimus plus lenvatinib showed considerable anti-tumor activity with manageable tolerability, and can be proposed as a useful treatment option in patients with metastatic E-AML with a constitutional or somatic mutation of TSC1 or TSC2. Prospective evaluation of the combination would be useful.
Acknowledgement
We thank Dr Hélène Bonte, Compiègne, France for providing us sample of the initial nephrectomy.
References
- He W, Cheville JC, Sadow PM, Gopalan A, Fine SW, et al. (2013) Epithelioid angiomyolipoma of the kidney: Pathological features and clinical outcome in a series of consecutively resected tumors. Modern Pathol 26: 1355-1364. [Google Scholar] [Cross Ref]
- Delgado R, Bojorge BDL, Albores‐Saavedra J (1998) Atypical angiomyolipoma of the kidney: a distinct morphologic variant that is easily confused with a variety of malignant neoplasms. Cancer: Interdis Internat J Am Cancer Soc 83: 1581-1592. [Google Scholar] [Cross Ref]
- Tsai CC, Wu WJ, Li CC, Wang CJ, Huang CH, et al. (2009) Epithelioid angiomyolipoma of the kidney mimicking renal cell carcinoma: a clinicopathologic analysis of cases and literature review. Kaohsiung J Med Sci 25: 133-140. [Google Scholar] [Cross Ref]
- Cong X, Zhang J, Xu X, Zhang M, Chen Y. (2018) Renal epithelioid angiomyolipoma: magnetic resonance imaging characteristics. Abdominal Radiol 43: 2756-2763. [Google Scholar] [Cross Ref]
- Luo J, Liu B, Wang Y, Li J, Wang P, et al. (2014) Comprehensive clinical and pathological analysis of aggressive renal epithelioid angiomyolipoma: report of three cases. Oncotargets Therapy 7: 823. [Google Scholar] [Cross Ref]
- Cibas ES, Goss GA, Kulke MH, Demetri GD, Fletcher CD. (2001) Malignant epithelioid angiomyolipoma (sarcoma ex angiomyolipoma') of the kidney: a case report and review of the literature. Am J Surg Pathol 25:121-126. [Google Scholar]
- Kenerson H, Folpe AL, Takayama TK, Yeung RS. (2007) Activation of the mTOR pathway in sporadic angiomyolipomas and other perivascular epithelioid cell neoplasms. Hum Pathol 38: 1361-1371. [Google Scholar] [Cross Ref]
- Kingswood JC, Belousova E, Benedik MP, Carter T, Cottin V, et al. (2019) Renal angiomyolipoma in patients with tuberous sclerosis complex: findings from the TuberOus SClerosis registry to increase disease Awareness. Nephrol Dialysis Transplant 34: 502-508. [Google Scholar] [Cross Ref]
- Bissler JJ, Christopher Kingswood J. (2018) Renal manifestation of tuberous sclerosis complex. In Am J Med Genetics Part C: Seminars in Med Genetics 178: 338-347. [Google Scholar] [Cross Ref]
- Bissler JJ, Kingswood JC, Radzikowska E, Zonnenberg BA, Frost M, et al. (2013) Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 381: 817-824. [Google Scholar] [Cross Ref]
- Shitara K, Yatabe Y, Mizota A, Sano T, Nimura Y, et al. (2011) Dramatic tumor response to everolimus for malignant epithelioid angiomyolipoma. Jpn J Clin Oncol 41: 814-816. [Google Scholar] [Cross Ref]
- Wolff N, Kabbani W, Bradley T, Raj G, Watumull L, et al. (2010) Sirolimus and temsirolimus for epithelioid angiomyolipoma. J Clin Oncol: Off J Am Soc Clin Oncol, 28(5), 65-68. [Google Scholar] [Cross Ref]
- Saoud R, Kristof TW, Judge C, Chumbalkar V, Antic T, et al. (2021) Clinical and pathological features of renal epithelioid angiomyolipoma (PEComa): A single institution series. In Urol Oncol: Seminars Orig Investig Elsevier. [Google Scholar] [Cross Ref]
- Citak EC, Yilmaz EB, Yaman E, Kaya S, Taskinlar H, et al. (2015) Malignant epitheloid angiomyolipoma of the kidney in a child treated with sunitinib, everolimus and axitinib. Canadian Urolog Asso J 9: 542. [Google Scholar] [Cross Ref]
- Demin DE, Bogolyubova AV, Zlenko DV, Uvarova AN, Deikin AV, et al. (2018) The novel short isoform of securin stimulates the expression of cyclin D3 and angiogenesis factors VEGFA and FGF2, but does not affect the expression of MYC transcription factor. Mol Bio 52: 436-445. [Google Scholar] [Cross Ref]
- Jacob A, Shook J, Hutson T. (2021) The implementation of lenvatinib/everolimus or lenvatinib/pembrolizumab combinations in the treatment of metastatic renal cell carcinoma. Expert Rev Antican Therapy 21: 365-372. [Google Scholar] [Cross Ref]
- Perego G, Barzaghi P, Vavassori I, Petrelli F. (2020) Treating metastatic clear-cell renal cell carcinoma: beyond immunotherapy. Medical Oncol 37: 1-5. [Google Scholar] [Cross Ref]
- Motzer R, Alekseev B, Rha SY, Porta C, Eto M, et al. (2021) Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. New Eng J Med 384: 1289-1300. [Google Scholar] [Cross Ref]
- Brimo F, Robinson B, Guo C, Zhou M, Latour M, et al. (2010) Renal epithelioid angiomyolipoma with atypia: a series of 40 cases with emphasis on clinicopathologic prognostic indicators of malignancy. Am J Surgical Pathol 34: 715-722. [Google Scholar] [Cross Ref]
- Espinosa M, Roldán-Romero JM, Duran I, de Álava E, Apellaniz-Ruiz M, et al. (2018) Advanced sporadic renal epithelioid angiomyolipoma: Case report of an extraordinary response to sirolimus linked to TSC2 mutation. BMC Cancer 18: 1-5. [Google Scholar]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi