Case Report, Clin Oncol Case Rep Vol: 3 Issue: 5
Extreme Response to Immunotherapy in an Estrogen Receptor Positive Breast Cancer: A Case Report
Rebecca Fitzpatrick* and Melody Cobleigh
Department of Internal Medicine, Division of Hematology, Oncology and Cell Therapy, Rush University Medical Center, Chicago, USA
*Corresponding Author : Rebecca Fitzpatrick, Department of Internal Medicine
Division of Hematology, Oncology and Cell Therapy, Rush University Medical
Center
1620 W Harrison St. Chicago, IL 60612, USA
E-mail: Rebecca_a_
fitzpatrick@rush.edu
Received: August 04, 2020 Accepted: September 28, 2020 Published: October 08, 2020
Citation: Fitzpatrick R, Cobleigh M (2020) Extreme Response to Immunotherapy in an Estrogen Receptor Positive Breast Cancer: A Case Report. Clin Oncol Case Rep 3:5. DOI: 10.37532/cocr.2020.3(5).145
Abstract
Introduction: Currently, immune checkpoint inhibitors are not approved by the FDA for HR-positive breast cancer, although an extreme response was seen in the case below.
Case: The patient is a 72 year old, woman who was diagnosed in 1996, at age 48, with stage T2N2aM0, ER-positive infiltrating ductal cancer in her left breast. She completed 4 cycles of doxorubicin and cyclophosphamide and she received adjuvant tamoxifen, until 2004, then was switched to letrozole, which was completed in April, 2011, thus completing 15 years of endocrine treatment. She recurred in 2017 with pleomorphic invasive lobular carcinoma, ER 5%, PR 0%, and HER2 not amplified by FISH. She eventually progressed through 5 lines of treatment. The 2/2019 biopsy specimen was sent for next generation sequencing. The tumor was found to have a high Mutational Burden (MB) (21 m/MB, 96th percentile for breast cancer). Pembrolizumab was provided on compassionate plea from the manufacturer in 3/2019. She had a complete response with resolution of severe brachial plexopathy pain.
Discussion: Pembrolizumab is associated with good outcomes in cancer other than breast cancer. In these cancers, a higher mutation burden is associated with response. The patient in this case had a high tumor MB, which prompted treatment with pembrolizumab. This case shows the importance of next generation sequencing and PD-L1 staining, enabling the use of immune checkpoint inhibitors as a possible treatment option for HR-positive breast cancer if the tumor has a high tumor MB and/or expression of PD-L1 in TILs.
Keywords: Breast cancer; Immune system; Lobular carcinoma; Pembrolizumab
Introduction
Breast cancer is common worldwide. Hormone-Receptor (HR)- positive breast cancer is the most common subcategory [1,2]. A large percentage of women will either present with metastatic disease or with metastatic reoccurrence [3]. In one study, it was found that only 95% of women with metastatic breast cancer will not survive 9 years after diagnosis [4]. This is all in spite of the fact there are many different treatment options for this type of breast cancer, including chemotherapy, endocrine therapy, cyclin-dependent kinase inhibitors and PI3 kinase inhibitors [5]. Immune checkpoint inhibitors are approved by the US FDA for treatment of triple-negative breast cancer, but not for HR-positive breast cancer [6]. We observed an extreme response to an immune checkpoint inhibitor in a patient with HRpositive, HER2-negative breast cancer.
Case Report
The patient is a 72 year old woman who was diagnosed in 1996, at age 48, with infiltrating ductal cancer in her left breast. The cancer was 4 cm, Grade 2, with 5/10 involved nodes. The cancer was ER positive and HER2 was not tested at that time. She completed 4 cycles of adriamycin/cytoxan. She then received adjuvant tamoxifen, which she took until 2004. She was then switched to letrozole, which was completed in April, 2011, completing 15 years of endocrine treatment.
In 2017, she noticed a fullness in her left axilla. Biopsy of left chest infraclavicular nodes revealed pleomorphic invasive lobular carcinoma, ER 5%, PR 0%, and HER2 not amplified by FISH. The mass was unresectable. Germline testing revealed no mutations or VUS's were found in the ATM, BARD1, BRCA1, BRCA2, BRIP1, CDH1, CHEK2, EPCAM (deletion/duplication analysis only), MRE11A, MSH2, MSH6, MUTYH, NBN, NF1, MLH1, PALB2, PMS2, PTEN, RAD50, RAD51C, RAD51D, SMARCA4, STK11, and TP53 genes.
Paclitaxel was started in March 2017. Re-biopsy of her left Rotter’s node showed ER 30%, PR 0, Ki-67>20%, HER-2/neu equivocal. A PET scan in 6/2017 showed a response to paclitaxel. Maintenance palociclib and fluvestrant were started. Disease progression occurred in 12/2017. Nab-paclitaxel was started but she had disease progression by 4/2018. Capecitabine was started but again her disease progressed. Metronomic methotrexate and cytoxan were started in 1/2019 with progression in 10/19. A biopsy on 2/2019 was sent for next generation sequencing. The tumor’s had a high mutational burden (21 m/MB, 96th percentile for breast cancer).
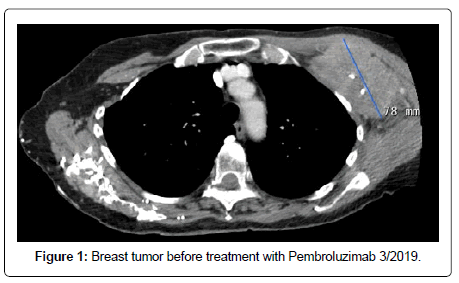
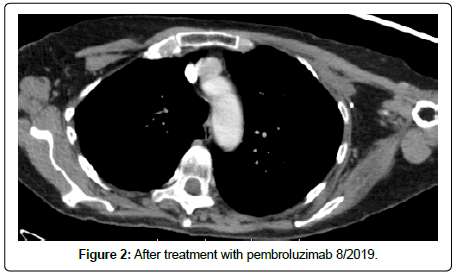
Pembrolizumab was provided on compassionate plea from the manufacturer in 3/2019. By this time the patient was highly symptomatic from brachial plexopathy and lymphedema of her left arm. The L arm was unusable. Manual lymphatic drainage was ineffective and she was talking narcotics for pain relief. CT scan of the tumor before starting immunotherapy is below in Figure 1. She experience partial relief of symptoms after one dose. A follow up CT scan is shown below on 8/2019 in Figure 2, shows resolution of lymphadenopathy. Her CEA fell from 905 U/ml to normal and CA 15.3 fell from 733 U/ml to normal after starting pembrolizumab. Subsequent analysis of her biopsy revealed PD-L1 (SP142): =1% tumor area occupied by immune cells. She continues responding, with complete resolution of pain and off of narcotics through August of 2020.
Discussion
One of the most common cancers in the world is breast cancer [7]. In women, the majority of breast cancers are hormone-receptor positive tumors [8]. In hormone-receptor positive breast cancer, one of the main treatment options is adjuvant endocrine therapy after surgery or chemotherapy. There is a high risk of late reoccurrence with hormone-receptor positive breast cancer, which is why it is recommended to complete 10 years of treatment with endocrine therapy [9]. The patient in the case above completed 15 years, and yet she still had a recurrence. Many women do have disease progression on endocrine therapy even with the combination of CDK 4/6 inhibitors [10]. CDK 4/6 inhibitors work with endocrine therapy, like tamoxifen, by making cells more sensitive to endocrine therapy by inhibiting cell growth [11]. Our patient received these therapies as well as chemotherapy for her reoccurrence but her disease progressed, until she tried an immune checkpoint inhibitor, pembrolizumab.
Immune checkpoint inhibitors are FDA-approved for many cancers, including lung, bladder, and melanoma [12-14]. Breast cancer historically has not been as sensitive to immune checkpoint inhibitors [12]. Hormonal receptor-positive breast cancers have lower expression of tumor-infiltrating lymphocytes, which would make the cells more sensitive to an immune checkpoint inhibitor [3]. The patient in the case had a high tumor mutational burden, which predicts response to immune checkpoint inhibitors in other types of cancer [15]. Pembrolizumab is an immune checkpoint inhibitor that was used in the patient in the case above.
Pembrolizumab binds PD-1 to inhibit T-cells suppression in cancer by blocking PD-L1 and PD-L2, facilitating immune response against tumor cells [10]. PD-L1 is expressed in>1% of tumor-infiltrating lymphocytes in about half of triple-negative metastatic breast cancers [16]. One trial with pembrolizumab as a monotherapy in hormonereceptor positive, HER2 negative breast cancers showed a median response duration of 12 months [12]. There was an overall response rate in these breast cancers treated with pembrolizumab of 12% [17]. In a recent study, pembrolizumab combined with chemotherapy did not show a statistically significant improvement in progression free survival, but some patients did have improved control [17].
Conclusion
Hormone-receptor positive breast cancers are extremely common. Currently, there are many different modalities to treat hormone-receptor positive breast cancer. Despite all of these options, progression on treatment is common. This case shows the importance of immune checkpoint inhibitors as a possible treatment option for hormone-receptor positive breast cancer if the tumor has a high tumor mutational burden and/or high tumor-infiltrating lymphocyte percentage.
Disclosure
Declarations of interest
None.
References
- Cazzaniga ME, Verusio C, Ciccarese M, Fumagalli A, Sartori D, et al. (2020) Is there still a role for endocrine therapy alone in HR+/HER2-advanced breast cancer patients? Results from the analysis of two data sets of patients treated with high-dose fulvestrant as first-line therapy in the real-world setting: The EVA and GIM-13 AMBRA studies. Breast Care 15: 30-37.
- Ditsch N, Schmidt M (2019) Treatment of advanced hormone receptor-positive (HR) HER2-negative breast cancer. Geburtshilfe Frauenheilkunde 79: 1328-1335.
- Barroso-Sousa R, Krop IE, Trippa L, Tan-Wasielweski Z, Li T, et al. (2019) A phase II study of pembrolizumab in combination with palliative radiotherapy (RT) for hormone receptor-positive (HR+) metastatic breast cancer (MBC). J Clin Oncol 37: 1047.
- Güth U, Elfgen C, Montagna G, Schmid S (2019) Long-term survival and cure in distant metastatic breast cancer. Oncol 97: 82-93.
- Giuliano M, Schettini F, Rognoni C, Milani M, Jersalem G, et al. (2019) Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: A systematic review and network meta-analysis. Lancet Oncol 20: 1360-1369.
- Cortes J, Andre F, Gonçalves A, Kummel S, Martin M, et al. (2019) IMpassion132 Phase III trial: Atezolizumab and chemotherapy in early relapsing metastatic triple-negative breast cancer. Future Oncol 15: 1951-1961.
- Harbeck N, Gnant M (2017) Breast Cancer. Lancet 389: 1134-1150
- Lin WZ, Xu QN, Wang HB, Li XY (2017) Fulvestrant plus targeted agents versus fulvestrant alone for treatment of hormone-receptor positive advanced breast cancer progressed on previous endocrine therapy: A meta-analysis of randomized controlled trials. Breast Cancer 24: 345-352.
- Burstein HJ, Lacchetti C, Griggs JJ (2018) Adjuvant endocrine therapy for women with hormone receptor–positive breast cancer: ASCO clinical practice guideline focused update. J Oncol Pract 15: 106-107.
- Rugo HS, Delord JP, Im SA, Ott P, Piha-Paul S, et al. (2018) Safety and antitumor activity of pembrolizumab in patients with estrogen receptor–positive/human epidermal growth factor receptor 2-negative advanced breast cancer. Clin Cancer Res 24: 2804-2811.
- Sobhani N, D’Angelo A, Pittacolo M, Roviello G, Miccoli A, et al. (2019) Updates on the cdk4/6 inhibitory strategy and combinations in breast cancer. Cells 8: 321.
- Force J, Leal JHS, Mcarthur HL (2019) Checkpoint blockade strategies in the treatment of breast cancer: Where we are and where we are heading. Curr Treat Options Oncol 20: 35.
- Reck M, Rodriguez-Abreu D, Robinson AG, Hiu R Csoszi T, et al. (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375:1823-1833.
- Robert C, Schachter J, Long GV, Arance A, Grob JJ, et al. (2015) Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 372: 2521-2532.
- Thomas A, Routh ED, Pullikuth A, Jin G, Su J, et al. (2018) Tumor mutational burden is a determinant of immune-mediated survival in breast cancer. OncoImmunology 7: e1490854.
- Adams S, Loi S, Toppmeyer D, Cescon DW, Laurentiis M, et al. (2019) Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: Cohort B of the phase II KEYNOTE-086 study. Ann Oncol 30: 405-411.
- Shah AN, Flaum L, Helenowski I, Santa-Maria C, Jain S, et al. (2020) Phase II study of pembrolizumab and capecitabine for triple negative and hormone receptor-positive, HER2-negative endocrine-refractory metastatic breast cancer. J Immunother Cancer 8: e000173.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi