Case Report, Clin Dermatol Res J Vol: 8 Issue: 3
Fatal Dermatological Squeal Following Depot Medroxyprogesterone Acetate (DMPA) Administration: A Catastrophic Hypersensitivity Case Report
Aisha Siddiqui1*, Farina Fatima Siddiqui1, Muhmmad Hamza Gul2, Yugam Rajeshkumar Oza3 and Miruthu Varshini Jayaprakash4
1Department of Internal Medicine, Liaquat University of Medical Health and Science, Jamshoro, Pakistan
2Department of Medicine, Bacha Khan Medical College Mardan, Kpk, Pakistan
3Department of Internal Medicine, Grodno State Medical University, Grodno, Belarus
4Department of Medicine, Rajah Muthiah Medical College, Annamalai University, Tamil Nadu, India
*Corresponding Author: Aisha Siddiqui,
Department of Internal Medicine,
Liaquat University of Medical Health and Science, Jamshoro, Pakistan
E-mail: 254dr.rehman@gmail.com
Received date: 23 August, 2023, Manuscript No. CDRJ-23-111105;
Editor assigned date: 25 August, 2023, Pre QC No. CDRJ-23-111105 (PQ);
Reviewed date: 08 September, 2023, QC No. CDRJ-23-111105;
Revised date: 15 September, 2023, Manuscript No. CDRJ-23-111105 (R);
Published date: 22 September, 2023, DOI: 10. 4172/2576-1439.1000209
Citation: Siddiqui A, Siddiqui FF, Gul MH, Oza YR, Jayaprakash MV (2023) Fatal Dermatological Squeal Following Depot Medroxyprogesterone Acetate (DMPA) Administration: A Catastrophic Hypersensitivity Case Report. Clin Dermatol Res J 8:3.
Abstract
The use of depot progesterone as a contraceptive every three months by a considerable number of women has raised concerns regarding the potential for hormones and chemicals to trigger fatal immune responses resulting in skin reactions. The focus of our case report is a 25-year-old woman who suffered a fatal dermatological condition after using this contraceptive. It is noteworthy that this specific condition has not been documented in medical literature before.
Keywords: Depot progesterone; Fatal dermatology lesion;
Acantholysis; Hypersensitivity response
Introduction
Dermatological emergencies are a serious matter that can cause acute skin failure, putting life, limb, or vital organ function at risk. Immediate medical attention is necessary to address these generalized skin disorders [1]. Identifying histologic changes specific to acantholytic skin diseases is crucial for early diagnosis [2]. Suprabasal acantholysis, commonly found in various inflammatory skin diseases, promotes cell growth in the suprabasal layers to facilitate the repair process [3]. As we know, Hormonal changes, medications, illnesses, diet, pregnancy, and emotional stress can trigger induced immune responses that cause fatal skin reactions [4]. However, it’s important to understand that Depot Medroxy Progesterone Acetate (DMPA) is a reliable and effective method for preventing ovulation and can also function as an emergency contraceptive by halting the release of a dominant follicle [5]. DMPA exerts its contraceptive effects primarily through its progestational activity. It inhibits ovulation by suppressing the release of gonadotropins, thereby preventing follicular maturation and the subsequent release of an egg from the ovary. Additionally, DMPA alters cervical mucus consistency, rendering it less penetrable to sperm. The contraceptive effect lasts for approximately three months, after which a repeat injection is required. While DMPA is generally well-tolerated, it is associated with potential side effects. Irregular menstrual bleeding is common, with many users experiencing amenorrhea or spotting. Weight gain is another reported side effect, although the clinical significance of this remains debated. Bone mineral density loss has been observed with long-term DMPA use, but it is largely reversible upon discontinuation. Knowing these details is critical to understanding the link between depot contraceptives and fatal skin lesions. We present a rare report on their association [6].
Case Presentation
A 25-year-old, South-Asian female, married for 4 years, housewife, mother to two children, resident of Tharparkar, with no known comorbidities, presented to us in medicine OPD with hypertrophic, crusted and painful skin lesions covering her whole body for 8 months. The woman was unable to provide her medical history, so her husband shared the information. According to him, she was in good health 8 months ago and was receiving Depot Provera, a form of Intra Muscular (IM) contraception, from a local hospital in Tharparkar. She had received three IM injections over the course of three months. Following her most recent dose, she experienced a low-grade fever measuring 100°F with intermittent recurrence and oral ulcers that developed 15 days later. Despite taking antipyretic medication, the fever persisted and was accompanied by an uncomfortable burning sensation in the upper abdomen. The oral ulcers were painful and had unclear borders, located on the gums and inner cheeks towards the back of her mouth. The patient's husband reported that she had lost her appetite due to painful swallowing, suggesting that ulcers may have formed on her pharyngeal mucosa as well. After a few days, she developed oral ulcers, followed by ulcerative lesions on the inner surface of her lips. After seeking medical attention at a local clinic, she was prescribed antipyretic and Non-Steroidal Anti-Inflammatory Drugs (NSAID) medication. Although we do not have the specific brand names, we understand that the course was given for one month. Regrettably, 15 days after completing the prescribed treatment, fluidfilled bullae appeared on the palms of both her hands, which gradually spread to her upper arms and lower limbs.
However, her head and neck region, as well as her torso, were spared from these bullae. Unfortunately, the bullae tended to break easily, causing further peripheral spread of the lesions, leading to large, crusted, and ulcerative wounds. Patient’s husband took her to see another local doctor who diagnosed her with Stevens-Johnson Syndrome (SJS), and she was prescribed a three-month course of medication. Her medication course was Tab Ecasil (Linezolid) 400 mg, Tab Rapicort (Prednisolone) 5 mg ,Tab Fexet (Fexofenadine) 120 mg, Tab Becefeland Cap Novelep 200 mg.
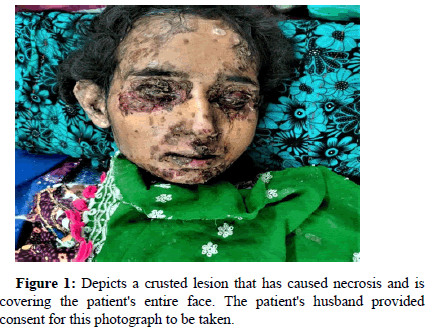
The patient experienced a remitting remission pattern of bullae during the course of treatment. The bullae disappeared while she was taking medication, but reappeared when she stopped. Unfortunately, the course did not aid in the remission of the disease and instead aggravated her condition. Her husband reported that the treatment worsened her symptoms. She developed bullae on her head and neck area in addition to her torso region. These bullae broke and resulted in crusted, painful, and ulcerative lesions on various parts of her body including her eyelids, cheeks, neck, and chest (Figure 1).
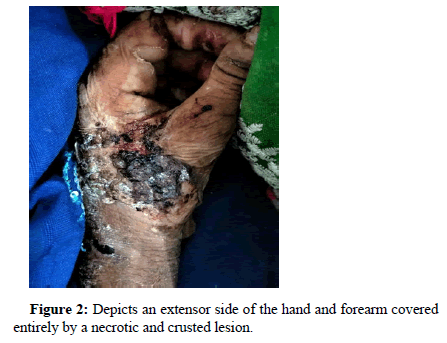
During the physical examination, we observed two types of lesions that caught our attention: Hypertrophic and Necrotic. These lesions appeared as black, patchy spots distributed in various areas such as both eyelids, nasolabial folds (excluding cheeks), both lips, neck, and extensor surfaces of both hands and arms. Fingers and flexor surfaces were not affected and mucosal lesions as shown in (Figure 2).
Discussion
It is possible for dermatologists to encounter life-threatening skin conditions in their office. These conditions can be caused by immediate reactions like anaphylaxis or angioedema, as well as severe acute infections from bacteria, viruses, fungi, or parasites. In our case report highlights a critical observation: A patient developed a fatal skin lesion following a course of depot injections. Despite multiple treatments, the patient's condition worsened rapidly, and unfortunately, we were unable to save her. We took immediate action by sending all necessary reports, but we could not perform a biopsy due to time and resource constraints. However, we remain hopeful that medical research advancements will prevent such tragic outcomes in the future. Our team is dedicated to ongoing research and providing better patient care.
Conclusion
Our case reports provide undeniable evidence linking depot contraceptives to fatal dermatological conditions. It is imperative that researchers take this information into serious consideration when investigating the association between these two conditions. We must work together to ensure that safer contraceptive options are promoted and made readily available.
Limitations
Our case report has limitations as we were unable to perform a biopsy on the fatal dermatological lesion and could not evaluate the cause of the fatal condition due to limited resources and the patient's deteriorating health.
Ethical Statement
The authors of the case report claim to have adhered to ethical guidelines, but they did not seek approval from an IRB as it was a case report. However, they did obtain informed consent from the patient's husband.
Informed Consent
The consent was obtained from the patient's guardians and will be made available to the journal upon their request.
References
- Maria Salavastru C, Livia Severin E, Moisa M, Fritz K, George Tiplica S (2014) Extreme dermatology–the intensive care skills of dermatologists in three case presentations of acute skin failure. Acta Dermatovenerol Croat 22: 44.
- Saggini A, Cota C, Cerroni L (2020) Incidental acantholysis in hailey-hailey disease (Microscopic nikolsky sign): An underappreciated histologic sign. Am J Dermatopathol 42: E61–4.
- Pierard Franchimont C, Pierard GE (1983) Suprabasal acantholysis. A common biological feature of distinct inflammatory diseases. Am J Dermatopathol 55: 421–6.
- Tavakolpour S (2018) Pemphigus trigger factors: special focus on pemphigus vulgaris and pemphigus foliaceus. Arch Dermatol Res 310: 95–106
- Schickler R, Crabtree-Sokol D, Patel J, Bender N, Nelson AL et al. (2021) The potential for intramuscular depot medroxyprogesterone acetate as a self-bridging emergency contraceptive. Contracept X.18; 3
- Bernstein DI, Wanner M, Borish L, Liss GM (2004) Twelve-year survey of fatal reactions to allergen injections and skin testing: 1990-2001. J Allergy Clin Immunol 113(6): 1129-36
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi