Case Report, Clin Oncol Case Rep Vol: 6 Issue: 2
FLOT in Advanced Gastric Cancer - A Single Center Experience
Arif Khan*, Vamshi Krishna
Department of Medical Oncology, Asian Institute of Gastroenterology, Hyderabad, India
*Corresponding Author: Arif Khan
Department of Medical Oncology,
Asian Institute of Gastroenterology, Hyderabad, India.
E-mail: arif.dr.khan.1981@gmail.com
Received: February 01, 2023; Manuscript No: COCR-23-88394;
Editor Assigned: February 03, 2023; PreQC Id: COCR-23-88394 (PQ);
Reviewed: February 12, 2023; QC No: COCR-23-88394 (Q);
Revised: February 16, 2023; Manuscript No: COCR-23-88394 (R);
Published: February 18, 2023; DOI: 10.4172/cocr.6(2).275
Citation: Khan A, Krishna V (2023) FLOT in Advanced Gastric Cancer - A Single Center Experience. Clin Oncol Case Rep 6:2
Abstract
Treatment of advanced gastric cancer not amenable for surgery has undergone constant refinements in recent past. The plethora of choices available makes the choice of first line treatment highly complicated. Despite recent advances in systemic therapy survival has marginally improved. FLOT has improved survival markedly in locally advanced gastric cancer amenable for surgery. FLOT is quite active in advanced gastric cancer and reasonably safe. This regimen needs to be further explored in advanced gastric cancer.
Keywords: Gastric cancer; Systemic therapy; Cancer; Surgery; Chemotherapy
Introduction
Advanced gastric cancer including locally advanced unresectable or metastatic gastric cancer has dismal prognosis and very poor 5 year survival rate [1]. FLOT chemotherapy (docetaxel, oxaliplatin, 5 Flurouracil) improves overall survival as compared to ECF/ECX regimen (epirubicin, cisplatin, 5 Flurouracil/capecitabine) when used as perioperative chemotherapy in locally advanced gastric cancer [2]. Though addition of docetaxel to CF (5 Flurouracil, cisplatin) improves outcomes in advanced gastric cancer, it is associated with increased toxicity and is cumbersome to administer requiring long hospital stay. Oxaliplatin based regimens are well tolerable and have similar efficacy as opposed to cisplatin regimens [3]. Doublet chemotherapy is the standard first line option for advanced gastric cancer. There is an ongoing GASTFOX study comparing FOLFOX (5 Flurouracil, leucovorin, oxaliplatin) with TFOX (Docetaxel, 5 Flurouracil, oxaliplatin) whose results are awaited. FLOT chemotherapy is active and has favourable toxicity profile in advanced gastric cancer [4]. Here in we report the cases of advanced gastric cancer treated in our institution with FLOT regimen in frontline setting.
Materials & Methods
It is retrospective analysis of prospective maintained dataset of all advanced gastric or gastroesophageal junction cancer patients either metastatic or locally advanced unresectable treated with first line chemotherapy with FLOT regimen in Asian institute of gastroenterology, Hyderabad, India between march 2019 and December 2020. Initial work up with upper gastrointestinal endoscopy and biopsy of growth was done for confirmation of malignancy. PET with contrast enhanced CT scan was done in all patients as part of staging procedure. Patients with diagnosis of adenocarcinoma of stomach were assessed by surgeons for resectability. The locally advanced unresectable and metastatic patients not eligible for curative treatment were considered for palliative chemotherapy.
Baseline fitness for chemotherapy was assessed with complete blood counts, liver function and kidney function tests along with electrocardiogram and echocardiography. The performance status was assessed using Eastern Cooperative Oncology Group (ECOG) performance scale. The biopsy samples were sent for biomarker analysis by Immunohistochemistry (IHC) for Human Epidermal Growth Factor Receptor (HER2), Programmed Death Ligand (PDL-1 ) and Microsatellite Instability (MSI). The choice of first line palliative chemotherapy was as per treating physician’s discretion.
Treatment Details: Those with ECOG performance score between 0 to 2 and adequate organ function were given FLOT regimen every 2 weeks with prophylactic growth factor support. The doses given were Docetaxel 50 mg/m2 , Oxaliplatin 85 mg/m2, leucovorin 200 mg/ m2 , 5 Flurouracil 2600mg/m2 over 24 hours. Response was assessed with PET CT or Contrast enhanced CT scans using RECIST criteria version 1.1. Patients received FLOT chemotherapy for maximum of 8 cycles and those patients who had not progressed after 8 cycles were put on maintenance tegafur uracil. The toxicity of chemotherapy was monitored and graded according to CTAC AE version 5.
Statistical Analysis: Duration of response was calculated from date of documented response till progression or death. Progression Free Survival (PFS) was calculated from the date of starting chemotherapy to date of clinical or radiological progression or death or last follow up. Overall survival (OS) was calculated from start of treatment to date of death from any cause or last follow up. Kaplan Meier analysis was used to calculate the progression free and overall survival.
Results
Between March 2019 and Dec 2020, 9 patients were treated with FLOT chemotherapy in first line. Among them 7 were male and 2 were female. All patients had metastatic disesase except one had locally advanced unresectable disease. One patient had HER2 positive disease (IHC her 2-3+) and received trastuzumab as part of frontline therapy, one had deficient MMR (mismatch repair protein) status and 4 patients had PDL-1 Combined Positive Score (CPS) ≥ 1%. Median no of cycles of FLOT chemotherapy given were 7 cycles. The response seen with FLOT chemotherapy was 7 patients had partial response, one had stable disease and one had progressive disease. Median duration of response seen was 7 months. Median PFS (Figure 1), and median OS (Figure 2) , are 7 months (95% CI 1.15 to 12.84),s and 13.5 months (95% CI 13.25 to 13.74) respectively (Table 1)
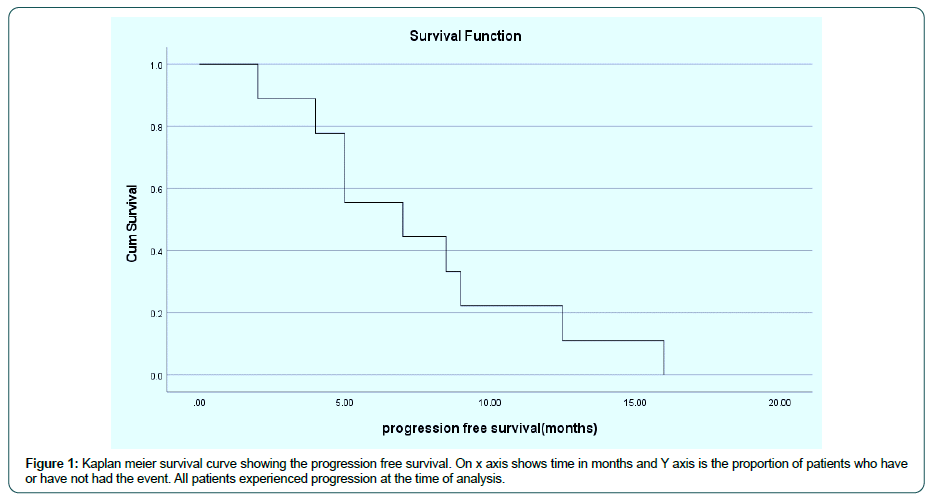
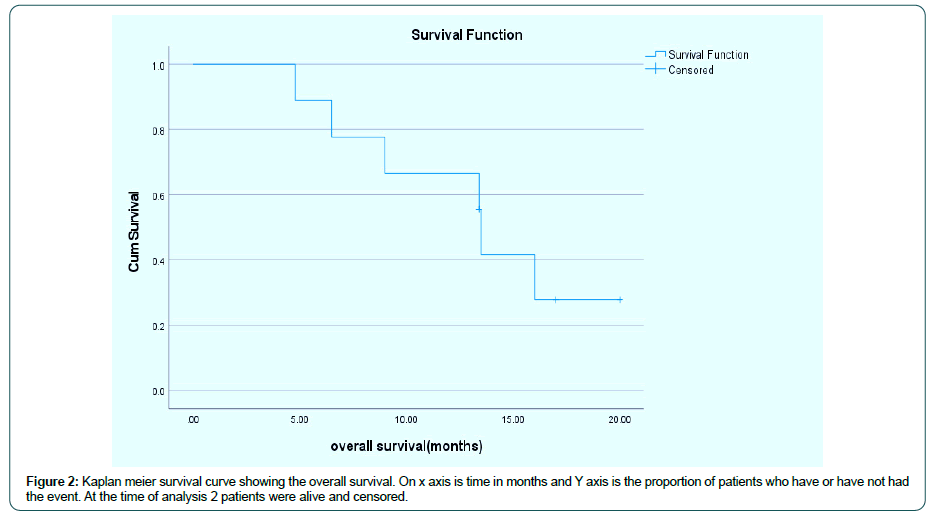
Table 1: The above table depicts baseline demographic and clinical charactersitics of the patients of all the patients included in this study.
<| Characteristic | Number |
|---|---|
| Age (Year) median (range) | 44 (26-65) |
| Gender | |
| Male | 7 |
| Female | 2 |
| Disease status | |
| Locally advanced unresectable | 1 |
| Metastatic | 8 |
| Sites of metastatic disease | |
| Peritoneum | 5 |
| Liver | 2 |
| Other organs | 3 |
| Molecular status | |
| HER2 positive | 1 |
| MSI (deficient MMR) | 1 |
| PDL-1 (CPS) â?¥ 1% | 4 |
| ECOG PS | |
| 0 | 2 |
| 1 | 3 |
| 2 | 4 |
| Response rate | |
| CR | 0 |
| PR | 7 |
| SD | 1 |
| Median PFS | 7 months |
| Median OS | 13.5 months |
| Subsequent therapy | |
| Maintenance tegafur uracil | 4 |
| FOLFIRI + ramucirumab | 2 |
| Paclitaxel | 2 |
| EOX | 1 |
Toxicity
Grade III/IV toxicity was seen in 3 patients. There were no deaths due to toxicity (Table 2)
Table 2: All patients had grade 1 and 2 toxicities according to CTC AE v 5. Only those patients who had grade 3/4 toxicties are included in this table.
| Toxicity | N=9 |
| Fatigue | - |
| Neutropenia | - |
| Febrile neutropenia | - |
| Peripheral neuropathy | - |
| Mucositis | 1 |
| Diarrhea | 1 |
| Nausea and vomiting | 1 |
Discussion
The treatment of advanced gastric cancer is continuously evolving. With recent advancements treatment is getting highly specific, individualised and tailored based on biomarker status. The survival has marginally improved despite the therapeutic advancements like targeted and immunotherapy. Triplet chemotherapy is more efficacious as compared to doublet chemotherapy in first line treatment of advanced gastric cancer [5]. In a meta analysis, taxane based regimens improved responses as compared to non taxane regimens at the cost of increased toxicity [6]. The DCF (Docetaxel, Cisplatin, 5 Flurouracil) regimen requires prolonged hospital stay associated with increased toxicity hindering the dose intensity. Despite modifications of DCF regimen more than half of patients experience grade 3 to grade 4 toxicities [7]. A meta analysis showed that oxaliplatin based regimens improved remissions and were better in tolerability as compared to cisplatin [8]. So triplet chemotherapy with least toxicity seems to be reasonable first line choice of treatment of advanced gastric cancer.
Majority of the patients in our study had peritoneal metastasis. Peritoneal metastasis is associated with response rates of less than 14% and median survival of approximately 7 months. Taxanes have better bioavailability in peritoneum and have been used as repeated intraperitoneal chemotherapy for treatment of refractory peritoneal metastases [9]. Paclitaxel used in weekly schedule improves clinical benefit response in malignant ascites [10]. Due to cumulative toxicity associated with chemotherapy, continuation of first line therapy till progression is not feasible. So maintenance treatment helps in extending the favourable results achieved with induction therapy. A chinese study showed that maintenance capecitabine prolongs PFS as compared to no maintenance [11]. In our study most of patients who did not progress after frontline chemotherapy received maintenance tegafur uracil.
In our study majority of patients had partial response after 4 cycles of induction chemotherapy and response was maintained for a long duration in two of our patients delaying the start of second line chemotherapy. FLOT is quite effective in controlling the symptom burden and delaying the start of second line chemotherapy.
Management of advanced gastric cancer is complicated and it is imperative to use best chemotherapy regimen at first line for rapid control of disease and provide early symptomatic relief which would translate into better survival. Most of the patients after progression on first line are not eligible for second due to deteriorating performance status. So the most effective regimen has to be used frontline to improve outcomes. FLOT regimen which consists of docetaxel, oxaliplatin and shorter duration 5 Fluorouracil infusion should be preferred choice in first line in fit patients with high tumour burden. Ongoing studies like GASTFOX study as mentioned above would answer the question that whether TFOX is better than FOLFOX.
Conclusion
The prognosis of advanced gastric cancer remains grim despite advent of targeted and immunotherapy. Those with peritoneal metastasis and high tumour burden need dose intense chemotherapy. FLOT regimen is tolerable and efficacious choice of first line therapy and should be further explored in advanced gastric cancer.
References
- Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics 2022. CA: A Cancer J Clin 72: 7-33. [Google Scholar] [Cross Ref]
- Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J (2019) Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 393: 1948-1957. [Google Scholar] [Cross Ref]
- Al-Batran SE, Hartmann JT, Probst S, Schmalenberg H, Hollerbach S, et al. (2008) Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: A study of the arbeitsgemeinschaft internistische onkologie. J Clini Oncol 26: 1435-1442. [Google Scholar] [Cross Ref]
- Al-Batran SE, Hartmann JT, Hofheinz R, Homann N, Rethwisch V, et al. (2008) Biweekly fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) for patients with metastatic adenocarcinoma of the stomach or esophagogastric junction: A phase II trial of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol 19: 1882-1887. [Google Scholar] [Cross Ref]
- Guo X, Zhao F, Ma X, Shen G, Ren D, et al. (2019) A comparison between triplet and doublet chemotherapy in improving the survival of patients with advanced gastric cancer: A systematic review and meta-analysis. BMC Cancer 19: 1-14. [Google Scholar] [Cross Ref]
- Chen XL, Chen XZ, Yang C, Liao YB, Li H, et al. (2013) Docetaxel, cisplatin and fluorouracil (DCF) regimen compared with non-taxane-containing palliative chemotherapy for gastric carcinoma: A systematic review and meta-analysis. PLoS ONE 8: 60320. [Google Scholar] [Cross Ref]
- Shah MA, Janjigian YY, Stoller R, Shibata S, Kemeny M, et al. (2015) Randomized multicenter phase II study of modified docetaxel, cisplatin, and fluorouracil (DCF) versus DCF plus growth factor support in patients with metastatic gastric adenocarcinoma: A study of the US Gastric Cancer Consortium. J Clini Oncol 33: 3874-3879. [Google Scholar] [Cross Ref]
- Zhang F, Zhang Y, Jia Z, Wu H, Gu K (2019) Oxaliplatin-based regimen is superior to cisplatin-based regimen in tumour remission as first-line chemotherapy for advanced gastric cancer: A meta-analysis. J Cancer 10: 1923. [Google Scholar] [Cross Ref]
- Kitayama J, Ishigami H, Yamaguchi H, Sakuma Y, Horie H, et al. (2018) Treatment of patients with peritoneal metastases from gastric cancer. Ann Gastroenterolo Surg 2: 116-123. [Google Scholar] [Cross Ref]
- Sakamoto J, Morita S, Yumiba T, Narahara H, Kinoshita K, et al. (2003) A phase II clinical trial to evaluate the effect of paclitaxel in patients with ascites caused by advanced or recurrent gastric carcinoma: A new concept of clinical benefit response for non-measurable type of gastric cancer. Jap J Clin Oncol 33: 238-240. [Google Scholar] [Cross Ref]
- Qiu MZ, Wei XL, Zhang DS, Jin Y, Zhou YX, et al. (2014) Efficacy and safety of capecitabine as maintenance treatment after first-line chemotherapy using oxaliplatin and capecitabine in advanced gastric adenocarcinoma patients: A prospective observation. Tumor Biol 35: 4369-4375. [Google Scholar] [Cross Ref]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 