Research Article, J Fashion Technol Textile Eng S Vol: 0 Issue: 4
Functionalization of Textiles with Thermoresponsive Polymers
Lübben JF1*, Keck A1, Kemajou CT1, Bräuning M2, Frick JE2 and Melnikov J2
1Albstadt-Sigmaringen University, Material and Process Engineering, Poststraße 6, 72458 Albstadt, Germany
2Albstadt-Sigmaringen University, Clothing and Textile Technology, Poststraße 6, 72458 Albstadt, Germany
*Corresponding Author : Joern Felix Luebben
Albstadt-Sigmaringen University, Material and Process Engineering, Poststraße 6, 72458 Albstadt, Germany
Tel: +497571 / 732-9565
E-mail: luebben@hs-albsig.de
Received: December 11, 2017 Accepted: February 28, 2018 Published: March 05, 2018
Citation: Lübben JF, Keck A, Kemajou CT, Bräuning M, Frick JE, Melnikov J (2018) Functionalization of Textiles with Thermoresponsive Polymers. J Fashion Technol Textile Eng S4:016. doi:10.4172/2329-9568.S4-016
Abstract
Functionalization of textiles with thermoresponsive polymers is the aim of this research work. Therefore, suitable textile substrates had been selected and characterized with the aid of a skin model regarding the water vapor resistance. A novel modified cup method was optimized for detecting the thermoresponsive behavior of the synthesized polymer coatings. Using free-radical polymerization allows synthesizing thermoresponsive copolymers with different properties, e.g. differing lower critical solution temperatures (LCSTs). Knife coating was found to be a suited method for the application of thermoresponsive polymer layers matching the pore sizes of the textile substrates. The monomer ratio in thermoresponsive copolymers could be determined by Raman spectroscopy as alternative to NMR, and correlated with the LCST. Investigating the repeatability of the thermoresponsive switching behavior, control of haptics and hygrothermal characterization of local properties such as thickness and elasticity were identified as future tasks.
Keywords: Thermoresponsive polymers; Smart textiles; Hygrothermal behavior; Energy and mass transfer; Comfort; Water vapor resistance; Raman spectroscopy
Introduction
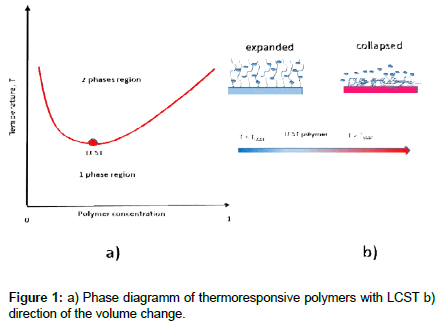
The development of new materials whose shape, color, or function changes with external stimuli, will play an important role in the elucidation of future societal challenges. The book intelligent stimuli-responsive materials provides an up-to-date overview of such called "stimuli-reactive" or "intelligent" materials [1]. Due to their ability to respond to internal and / or external chemical or physical (temperature, pH, etc.) stimuli, stimuli-responsive polymers are gaining a lot of attention for many applications [2]. Therefore thermoresponsive polymers (TRPs) have many application fields ranging from controlled drug delivery via filtration and separation to tissue engineering and increase of wearing comfort of textiles. This broad range of applications is due to their property to change their volume in a solvent when exceeding or falling below a critical temperature or trigger temperature [3]. Most water soluble synthetic polymers become more soluble when heated, some other separate from solution upon heating. This inverse temperature-dependent solubility is characteristic of polymers which dissolve when cooled and phase separate when heated above the phase transition temperature, known as lower critical solution temperature in their phase diagram (LCST) (Figure 1a).
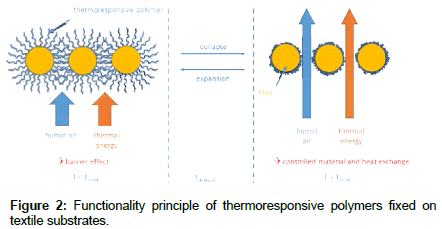
TRPs with a LCST collapse when they are heated in a humid environment and they expand when cooled down again (Figure 1b). The associated changes in shape make them ideal candidates for the production of smart filters and membranes, e.g. for heat and moisture control in textiles. Textile surfaces functionalized with TRPs, e.g. smart protective clothing systems, exhibit adaptive properties. Namely, reversibly collapsing and expanding TRP coatings can adapt the heat and material flow through textile pores corresponding to the change of body and environmental temperatures [4].
In this contribution it will be demonstrated based on latest investigations and representative examples how TRPs can be synthesized and fixed on fibers and textiles. In particular, copolymerization and variation of monomer ratios lead to different TRPs with different trigger points. As one example, the synthesized copolymer poly(N-isopropylacrylamide-co-N-tert-butylacrylamide) will be discussed in more detail.
Up to now, TRPs are mostly studied in liquid environments. Relatively little is known about the thermoresponsive behavior of polymers under hygrothermal conditions. Therefore, water vapor resistances and relative time diffusion constants are measured quantitatively by means of a skin model [5] and a novel modified cup method, which will be described in more detail in a subsequent publication.
Materials and Methods
Textile substrates
Well-defined precision woven substrates consisting of polyethylene terephthalate PET (Sefar, Switzerland), as well as commercial PET (Flying TEX CO, LTD, Taiwan) were chosen as reference materials. Woven substrates have well-defined mechanical properties and pore sizes. For this reason, the team focuses on this type of textile material and dispenses with knitwear and nonwovens. Two different weave structures were chosen, plain and twill: A plain weave is the simplest and tightest way of crossing yarns, where the warp yarn is alternately running on and under the weft yarn. In this case both sides of the fabric look similar. Twill is a type of textile weave with a pattern of diagonal parallel ribs. This is done by passing the weft thread over one or more warp threads then under two or more warp threads and so on, with a step or offset, between rows to create the characteristic diagonal pattern (Figure 2).
Important parameters for the project are the pore sizes and the open area showed in Table 1. The parameters were taken from the manufacturer, pore sizes were additionally measured by microscopy (Zeiss Axio Imager, Germany) and analyzed with an open source software (Gwyddion).
| Material | Weave | Pore size [µm] | Open area [%] |
|---|---|---|---|
| PET, Sefar | Twill | 1 | 2 |
| PET, Sefar | Twill | 10 | 2 |
| PET, Sefar | Plain | 30 | 21 |
| PET, Sefar | Plain | 100 | 32 |
| PET, Flying TEX | Plain | 40 | < 0.5 |
| PET, Flying TEX | Plain, Coated | 40 | < 0.5 |
Table 1: Woven textile substrates.
Selection and synthesis of thermoresponsive materials
Mainly polymers exhibiting a LCST in the physiological range are interesting in this project. Because of their solubility in water and their commercial availability pluronics, poly(vinylmethylether) (PVME) [3], and poly(N-isopropylacrylamide) (PNIPAAM) were selected. To have a more tunable polymeric system, the copolymer poly(Nisopropylacrylamide- co-N-tert-butylacrylamide) was synthesized using radical polymerization.
N-Isopropylacrylamide (NIPAAM) (TCI Chemicals, Tokyo, Japan, >98 %), N-Tert-Butylacrylamide (TBAAM) (Thermo Fisher, Karlsruhe, Germany, 97 %) and 2,2’-Azobis(2-methylpropionamidine) dihydrochloride (AAPH) (Sigma-Aldrich, St. Louis, USA, 97 %) were used without further purification. Acetone (Carl Roth, Karlsruhe, Germany, ≥99.5 %), petrol ether (Carl Roth, Karlsruhe, Germany, boiling range 40-60 °C) and hydrochloric acid (Merck, Darmstadt, Germany, 37 %) were used as received. Deionized water (κ<10 μS.cm-1) was bubbled with nitrogen for 20 min. To receive a 0.1 M monomer solution preweighed amounts of monomer were added and stirred until complete dissolution, while being kept under a nitrogen atmosphere. After heating the monomer solution to 70 °C in an oil bath, a 40 mM solution of AAPH (0.1 mol% of total monomer content) was added through a septum. The polymerization was conducted under constant nitrogen flow and reflux of evaporated solvent. After 7 h the polymerization was stopped by quenching the solution in an ice bath. For purification the polymer solution was heated rapidly in a water bath (60 °C) and concentrated hydrochloric acid was added dropwise until coagulation. After washing with hot deionized water until pH-neutrality the polymer was dissolved in 5 mL of acetone and poured in a 5-fold excess of petrol ether. The precipitate was dried under vacuum at 40 °C. The last purification step was repeated 2 times. The purified and dried polymer was stored at 4 °C.
LCST determination by means of turbidity measurements
LCST was determined by heating a 0.1 wt% polymer solution by 1 °C steps in a Julabo water bath with a recirculated thermostat, starting at 10 °C. After each step the polymer solution was allowed to be conditioned for 5 min and turbidity was checked with a laser beam (0.5 mW, 638 nm).
Polymer deposition
The layers were deposited by coating with an air knife coater. The thickness of the coated layers after doctor blading is in the range from 50 to 100 μm. Afterwards, the layers were dried in a programmable oven (Mathis Labdryer, Switzerland) resulting in layer thicknesses in the range of the pore diameters. During our research project, the polymer deposition is planned to be optimized to match the thickness of the textiles pore diameters, ranging from 1 to 100 μm diameter, as shown in Table 1.
For experiments presented in this paper, the material PET (Flying Tex, Taiwan), with a pore diameter of 40 μm and an estimated calculated open area smaller than 0.5 % was chosen as textile substrate.
Measurement of water vapor resistance and relative diffusion time constants
The Permetest (Sensora, Czech Republic), a commercial measuring instrument for the non-destructive determination of water vapor resistance or permeability of textile fabrics, nonwovens, foils or paper sheets, was used as a skin model [5]. Water vapor resistance is measured under isothermal conditions according to the ISO 11092 (Textiles – Physiological effects – Measurement of the thermal and water-vapor resistance). The sample is placed on a sintered plate, supplied water passes through the membrane as vapor. Conditioned air flows over a channel parallel to the sample surface. It determines the water vapor partial pressure difference between the two surfaces of a material divided by the resulting evaporation heat flux per unit area along the partial pressure gradient.
Additionally, an assigned cup method was created for the determination of the characteristic times needed for the water vapor diffusion through the pristine and functionalized textile substrates. The cup method is the method used to test water vapor permeability independently based on a simple and perspicuous principle. In our cup method, a certain pressure difference is maintained on two sides of the specimen. The characteristic time constant of water vapor diffusion under specified temperature and relative humidity conditions are related to the water vapor resistance of the specimen.
Raman spectroscopy
Raman spectroscopy, a scattering technique, measures the shift of frequency as a result of the exchange of energy between the incident photon and the material vibrations or rotations. Raman cross sections tend to be strong for symmetric, electron rich moieties; therefore, Raman spectroscopy can beneficially be applied in aqueous media. Raman spectroscopy is an analytical method with a sufficiently rapid response in the order of seconds on polymer composition and conformational changes, which is advantageous for continuous observation of the temperature-dependent TRP collapse and expansion.
In this paper, a Horiba XploRa One (Horiba, Japan) Raman spectrometer was used for the determination of how the changing NIPAAM/TBAAM-ratio in the produced polymers correlates with the LCST.
Results and Discussion
Microscopic results
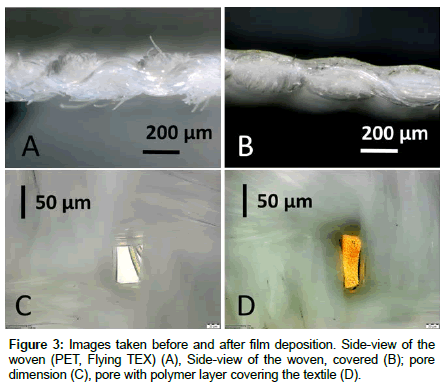
PET woven (Flying Tex) was covered with a thermoresponsive polymer hydrogel layer as described in section Polymer deposition. The thickness of the substrate was measured to equal 200 μm, approximately (see Figure 3A). From the cross-sectional view (Figure 3B) it can be seen that after film deposition, a dense film with ca. 50 μm thickness was formed.
Imaging the substrate from the back side before and after film deposition, a clear distinction of open (Figure 3C) and closed pores (Figure 3D) is possible.
Water vapor resistance and relative diffusion time constants
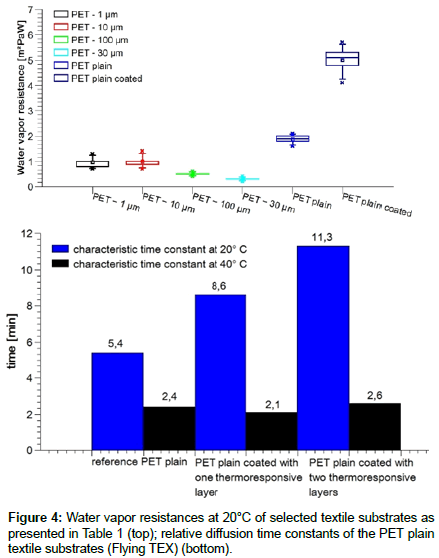
As expected, the water vapor resistances of the first five nonfunctionalized textile reference substrates demonstrate that the open areas shown in Table 1 are almost inversely proportional to the water vapor resistances (Figure 4, top), measured at 20 °C and 50 % relative humidity. The sixth coated sample shows a significantly larger resistance compared to the unfunctionalized ones. Deviations have to be explained in terms of wettability properties of the filament surfaces, the fineness of the filaments and capillary forces. For some applications, a low resistance might be useful, in our case it is really compulsory in order to determine the extent of the thermoresponsivity with highest accuracy. In the ideal case, the water-vapor transmission will be tunable between 0 (closed pores) to 100% (open pores).
A relative diffusion time constant obtained for our cup method indicates the time in which the increasing relative humidity, following an exponential saturation curve, reaches 63.2% of its final value. As can be seen in Figure 4 (bottom), water vapor diffuses through the open pores faster (within 5.4 s) than through the closed ones (8.6 and 11.3 s), so the observed relative time constants of open pores are lower. The graph shows that the relative diffusion constant of the coated samples at 20 °C increase with the layer thickness, while at 40 °C the relative diffusion time constant values remain about the same as the reference value (2.1-2.6 s). Below the temperature trigger point, the pores remain almost closed and fewer vapors diffuses, while above the trigger point the pores are opened and water vapor diffuses almost equally fast.
To emphasize the positive information extracted from Figure 4: Rising the temperature to 40 °C in the chamber experiments clearly shows an opening effect due to TRPs volume collapse: the time constant of an uncovered reference sample with t = 2.4 s is obtained for a bare (t = 2.1 s), a single and a double layered substrate (t = 2.6 s). At 20 °C, diffusion time is clearly slower, showing a dependency on film thickness. Half the film thickness leads to a diffusion time which is nearly twice faster ((11.3-5.4)/(8.6-5.4) ~ 2.). Temperaturedependent control of the water vapor transmission is possible!
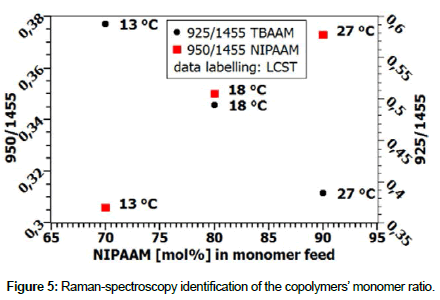
Identification of the monomer ratio within the copolymer poly(N-isopropylacrylamide-co-N-tert-butylacrylamide)
The composition of the synthesized polymer was analyzed by Raman spectroscopy with a confocal Raman microscope configuration. Spectra were recorded with a 638 nm laser in a wave number range from 800 cm-1 to 1500 cm-1. All spectra were baseline corrected and normalized to the peak that is associated with CH3 bending deformation in the polymers. The 1455 cm-1 peak is used as reference and its intensity is related to the intensities of the CH3-rock vibration of the tert-butyl and the iso-propyl group respectively [6,7].
Copolymers with different NIPAAM content have been synthesized and characterized using the peak intensity ratios (Figure 5). A linear correlation between the NIPAAM content in the monomer feed and the peak ratios was found and, by measuring the changing LCST, it was concluded that the increasing LCST corresponds to an increasing amount of NIPAAM in the copolymer. Because of the fact that no absolute monomer ratios can be identified by Raman spectroscopy solely, NMR and GPC measurements shall be done.
Conclusion and Outlook
A decisive progress was achieved with the synthesis of a copolymer which allows tuning of the LCST in the temperature range from 13-32 °C. Methods for the in situ characterization of the opening of the pores are developed, such as a dedicated cup method providing relative diffusion time constants, as presented in this paper.
The repeatability of the switching behavior is of special interest and will be investigated in near future experiments. Both water-vapor resistance and relative time constants for diffusion will be examined in dependence on layer thickness and pore sizes. Hygrothermal characterization of local properties such as thickness, adhesion and elasticity by means of atomic force microscopy (AFM) is useful to understand conformational changes of macromolecules on the nanoscale and will be performed both as shown [3].
In the next step, thermoresponsive copolymers have to be fixed permanently on suited substrates with well-defined pore sizes. For this purpose, both wet-chemical methods as well as plasma-technology are still under investigation and will be compared in terms of ease of application as well as economic and ecological issues.
Efforts have to be put into the complementary characterization of the synthesized polymers by means of nuclear magnetic resonance (NMR), gel permeation chromatography (GPC) and scattering techniques. The strength of the chemical coupling to the surface will be tested using washing tests and thin film spectroscopy (e.g. ATR-IR technology). An optimized compromise between haptic requirements and thermoresponsivity will be found. The functionalized materials will be used to design and develop new innovative smart textiles and clothing that can adapt to different surroundings actively.
Acknowledgement
The German Federal Ministry of Education and Research (BMBF) promotes interdisciplinary research and development projects in the disciplines of engineering, natural sciences and economics, where universities of applied sciences cooperate with partners in business and science. The ongoing research project RespothermTex (support code (FKZ): 13FH060PX4) started in September 2015 and will last to August 2018. The project team works together with the research company “Forschungsgesellschaft für Textiltechnik Albstadt (FTA)“ and the technical universities of Aachen and Dresden.
References
- Li Q (2013) Intelligent stimuli-responsive materials: From well-defined nanostructures to applications.
- Galaev I, Mattiasson B (2007) Smart polymers: applications in biotechnology and biomedicine.
- Lübben JF, Crespy D, de Geus M, Heuberger M (2012) Monitoring the hygrothermal response of poly (vinyl methyl ether) submicron films using AFM. European Polymer Journal 48: 209-216.
- Bräuning M, Lübben JF, Frick JE, Keck A, Melnikov J, et al. (2016) Thermoresponsive polymere für den ein- satz in smarten textilien: Das Projekt Respotherm Tex.
- Bogusławska Bączek M, Hes L (2013) Effective water vapour permeability of wet wool fabric and blended fabrics. Fibres & Textiles in Eastern Europe 21: 67-71.
- Gibbons O, Carroll WM, Aldabbagh F, Yamada B (2006) Nitroxideâ€Âmediated controlled statistical copolymerizations of Nâ€Âisopropylacrylamide with Nâ€Âtertâ€Âbutylacrylamide, J Polym Sci A: Polymer Chemistry 44: 6410-6418
- Lynch I, Blute IA, Zhmud B, Mac Artain P, Tosetto M, et al. (2005) Correlation of the adhesive properties of cells to n -isopropylacrylamide/ n - tert -butylacrylamide copolymer surfaces with changes in surface structure using contact angle measurements, molecular simulations, and raman spectroscopy. Chem Mater 17: 3889-3898.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi