Research Article, Clin Dermatol Res J Vol: 6 Issue: 4
Glycaemic Control and its Relation to Foot Skin Ph in People Living with Type 2 Diabetes Mellitus
Neil Micallef, Stephen Mizzi, Cynthia Formosa*
Department of Health Sciences, Professor of Health Sciences, University of Malta, Malta
*Corresponding Author: Cynthia Formosa PhD
Department of Health Sciences, Professor of Health Sciences, University of Malta, Malta
Tel: +35699861396
E-mail: Cynthia.fomosa@um.edu.mt
Received: June 06, 2021 Accepted: June 21, 2021 Published: June 28, 2021
Citation: Micallef N, Mizzi S, Formosa C (2021) Glycaemic Control and its Relation to Foot Skin Ph in People Living with Type 2 Diabetes Mellitus. Clin Dermatol Res J 6:4.
Abstract
Aim: To determine whether different HbA1c levels, especially poor glycaemic impacts foot skin pH. Research Design and Method: Two hundred and forty-one participants (n=241) were recruited for this study; 180 living with type 2 diabetes mellitus and 61 healthy participants (Group1). Participants living with type 2 diabetes were categorised into three different groups according to their HbA1c levels: HbA1c levels between 2.5-5.9% (Group 2), 6-8% (Group 3) and > 8% (Group 4). Skin pH at predefined sites (plantar, interdigital and dorsal areas of each foot), were measured utilizing a skin pH meter (Apera PH60F™). Results: Mean foot skin pH in all three regions of interest (ROI) between the four groups of participants was found to be significantly different with p-value being lower than 0.05. Group 2 (good HbA1c) presented with the lowest foot skin pH in all ROI, followed by group 1 (healthy), group 3 (fair HbA1c) whilst group 4 (poor HbA1c) presenting with the highest skin pH values. The mean interdigital foot skin pH was also found higher (less acidic in nature), when compared to the other sites for both the left and right foot across all groups. Conclusions: This study concludes that a low skin pH (below 5.7), is associated with optimum skin function and health. Results obtained showed that the higher the HbA1c score, the higher (less acidic) the mean foot skin pH was observed in all ROI, thus possibly contributing to various dermatological conditions.
Keywords: Ph measurements, Skin pH, Foot, Diabetes Mellitus, Glycaemic Control, HbA1c.
Introduction
Among various factors which have been previously investigated, such as trans-epidermal water loss [1], skin roughness [2], stratum corneum hydration [3], skin conductance [3] and skin pH [2], it was suggested that skin pH is increasingly renowned as being a vital key component in the various roles and functions of the human skin [4]. According to various studies [5, 6], skin pH in healthy individuals is acidic in nature. Studies have classified healthy human skin pH in the range of 4.5 to 5.7 on the pH scale [4, 7]. When this acidic environment is shifted to an alkaline level, it was found to increase the presence of various dermatological eruptions, such as acne, bacterial and fungal infections, as well as eczema and skin dryness [8, 9].
According to Ali and Yosipovitch, one condition that has been found to have a major impact on skin health and skin pH fluctuation is diabetes mellitus [1]. Although literature has looked at diabetes and its effects on skin pH which resulted in an increase in skin manifestations and a decrease in wound healing rates [1, 10], no studies have investigated the effect of glycaemic control, in type 2 diabetes population and its association to skin pH, on different anatomical sites within the foot. This study sought to investigate whether different levels of glycaemic control contributed to a change in foot skin pH.
Methods
Study Subjects
Two hundred and forty-one participants (n=241) were recruited for this study, of which 180 were living with type 2 diabetes mellitus and 61 were healthy participants. Convenience sampling was employed for this study, whereby subjects were recruited by a first through the door basis. Recruited participants were subdivided into four groups based on their glycaemic levels. Group 1 consisted of the control group which included healthy participants with no diabetes (n=61, 30 males and 31 females), group 2 had an HbA1c score which fell in the range of 2.5-5.9% (n=60, 30 males and 30 females), group 3 consisted of participants with an HbA1c score of 6%-8% (n=60, 30 males and 30 females) and group 4 involved participants with HbA1c control which was greater than 8% (n=60, 30 males and 30 females
Ethical approval and Consent
All participants were provided with verbal as well as written information on the study and informed consent was obtained. All experiments were conducted in accordance with the Declaration of Helsinki [11].
Experiment tool used
In this study, the Apera PH60F™ Premium Pocket pH Meter was utilised to measure skin pH. It consists of a flat glass electrode and an auto calibration with auto buffer recognition. It also incorporates an Auto Temperature Compensation (ATC), ±0.01 pH, 0.5˚C accuracy with -2.00 to 16.00 pH measuring range, auto recognition and stable sensors which improved consistency [12, 13].
Study procedure
Following informed consent, 241 participants were recruited in this study. The inclusion criteria for group 1 included participants who were not living with diabetes mellitus condition and who were under no medications. While those of group 2, 3 and 4 included participants living with type 2 diabetes and these were categorized according to their HbA1c levels. Participants over 75 years of age, smokers, participants with skin foot pathologies and active foot ulcerations, were excluded from this study. Demographic data which included age, BMI, duration of diabetes, exercise duration and HbA1c levels was collected during the examination. Before every session, the Apera PH60F meter™ was prepared and calibrated according to the manufacturer’s instructions [14]. The tip of the pH electrode was rinsed with distilled water and blot dry with a soft tissue. Calibration was complete when ranges of between 95-105% accuracy were achieved and the instrument exhibited a signal of confirmation. This protocol was performed every morning, before the commencement of participants’ foot skin pH testing, to maintain extreme accuracy and to reduce contamination. The same examination room was utilised throughout the study and was kept at a constant room temperature of 20-22 °C. Participants were instructed to remove footwear and any socks, and lie on the couch in a supine position for 20 minutes, for acclimatization to take place. This was done in accordance with recommendations published by Stefaniak et al. [15] and the European group of efficacy measurement of cosmetics and other topical products (EEMCO) [16], who highlight the best practice for optimum clinical testing of human skin pH. Skin pH was measured at predefined sites including the plantar, interdigital and dorsal areas of each foot, utilizing the pH meter, whereby measurement from a single site was taken three times and a mean value calculated. This was done in both left and right foot of each participant and for each different site. The data required from every participant was obtained in a single clinical session.
Data Analysis
Data was tested for normal distribution to determine normality of data, using the Shapiro-Wilk test. The Kruskal Wallis test was employed to test for significant difference between groups, while the Shapiro-Wilk, Spearman correlation, Mann Whitney and Chi-square tests were used to analyse and compare other variables in relation to foot skin pH. The IBM SPSS® software was used to evaluate and analyse the collected data.
Results
Demographic data
A total of 241 participants (120 males and 121 females) were included in the study, with a mean age of 54 years; ranging from 19 years to 74 years (SD ±12.28). BMI values were the highest in group 4 with mean values of 28 kg/m2 followed by group 3 with a BMI score of 25.4 kg/m2, both classified as overweight. BMI values for group 2 and group 1 classified both groups as ‘normal’ with values of 24.6 kg/m2 and 23.8 kg/m2 respectively. Group 2 had the lowest HbA1c levels (4.81%) while the highest mean HbA1c score was in group 4 (9.97%). Furthermore, a trend was noticed whereby an increase in diabetes duration, reflected a higher score in HbA1c levels, with participants in group 2 who’s diabetes onset was just over 3 years, had the lowest mean HbA1c scores when compared to group 4 who reported a mean duration of 6.78 years and a mean HbA1c score of 9.97%.
Foot Skin pH data
The following regions of interest (ROI) in the foot have been analysed for skin pH (both left and right sides), and included:
• The middle part of the dorsum of the foot.
• The inter-digital space, between the 4th and 5th digits.
• The middle part (arch area) of the plantar aspect of the foot.
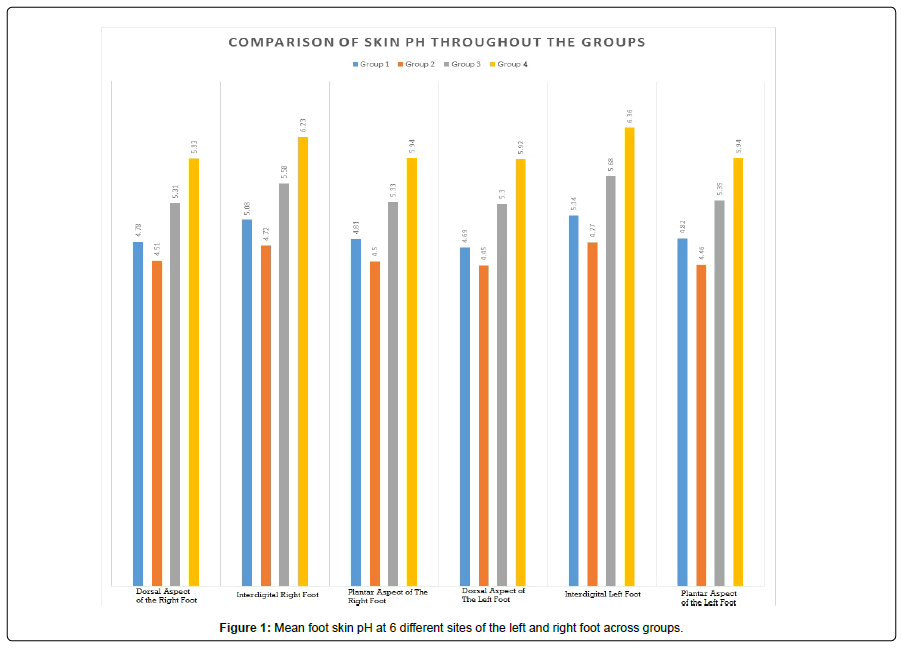
It was observed that group 4 (poor HbA1c) had the highest skin pH (less acidic) in all tested sites with an average pH of 6.05. This was followed by group 3 (fair HbA1c) with an average pH of 5.43 and the control group, group 1 (healthy) with a mean skin pH of 4.89. Group 2 (good HbA1c) had the lowest mean skin pH with a mean pH of 4.57 across all sites. It was also worth noting that the interdigital foot skin pH of both left and right foot of each group was found to be higher when compared to the other measured sites (Figure 1) (Table 1).
| Sites measured | Group 1 | Group 2 | Group 3 | Group 4 |
|---|---|---|---|---|
| (healthy) | (same) | |||
| Mean skin pH | Mean skin pH | Mean skin pH | Mean skin pH | |
| R. Dorsal | 4.78 | 4.51 | 5.31 | 5.93 |
| R. Interdigital | 5.08 | 4.72 | 5.58 | 6.23 |
| R. Plantar | 4.81 | 4.5 | 5.33 | 5.94 |
| L. Dorsal | 4.69 | 4.45 | 5.3 | 5.92 |
| L. Interdigital | 5.14 | 4.77 | 5.68 | 6.36 |
| L. Plantar | 4.82 | 4.46 | 5.35 | 5.94 |
| Mean from all sites | 4.89 | 4.57 | 5.43 | 6.05 |
Table 1: The average of skin pH from the 6 sites across the 4 groups.
This study also observed other results which were associated with skin pH, mainly:
i) Its relationship between the left and right side. No significant difference was found in mean foot skin pH between the left and right foot (mean p-value = 0.59). The Mann-Whitney test was utilised to compare mean scores for each parameter between the two independent groups.
ii) Its relationship between genders. No significant difference was found in mean foot skin pH between the male and female participants at all the ROI (mean p-value = 0.89). Test used to obtain this result was the Mann-Whitney test.
iii) The influence of age, duration of diabetes, BMI and HbA1c on foot skin pH
This study has found that the following four properties had the most impact on foot skin pH values:
• Age of participants (years)
• Duration of diabetes (years)
• BMI of participants (kg/m2)
• HbaA1c levels (%)
The multinomial logistic regression was utilised to assess whether foot skin pH range can be predicted based on age of participants, BMI, HbA1c and duration of diabetes. The test was applied for the four different groups and the three sites (dorsal, plantar and interdigital) were taken separately. The dependant variables used were the pH of the dorsal, interdigital and plantar skin and categorized into three different scales as follows:
• Skin pH less than 4.5
• Skin pH from 4.5-5.7 (normal skin pH range)
• Skin pH greater than 5.7
Results demonstrate that with an increase in HbA1c and duration of diabetes, the probability of a high skin pH (less acidic) increases. On the other hand when an increase in BMI and age was recorded, the probability of a low skin pH (under 5.7) increased. Tables 2, 3 and 4 show the significance and the odds ratio of each site which include the dorsal, interdigital and plantar aspect respectively.
| Parameter Estimates | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dorsal foot skin pH | Variable | B | Std. Error | Wald | df | Sig. | (OR) | 95% Confidence Interval for Exp(B) | |
| Lower Bound | Upper Bound | ||||||||
| less than 4.5 | Age | 0 | 0 | 25 | 1 | <0.001 | 1 | 1 | 1 |
| BMI | 0 | 0 | 16 | 1 | <0.001 | 1 | 1 | 1 | |
| HbA1c | -1 | 0 | 78 | 1 | <0.001 | 0 | 0 | 0 | |
| Years of Diabetes | -0 | 0 | 25 | 1 | <0.001 | 1 | 1 | 1 | |
| 4.5-5.7 | Age | 0 | 0 | 12 | 1 | <0.001 | 1 | 1 | 1 |
| BMI | 0 | 0 | 7 | 1 | 0 | 1 | 1 | 1 | |
| HbA1c | -1 | 0 | 47 | 1 | <0.001 | 0 | 0 | 1 | |
| Years of Diabetes | -0 | 0 | 7 | 1 | 0 | 1 | 1 | 1 | |
| a. The reference category is: greater than 5.7. | |||||||||
Table 2: Predictions for dorsal foot skin pH.
| Parameter Estimates | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Interdigital foot skin pH | Variable | B | Std. Error | Wald | df | Sig. | (OR) | 95% Confidence Interval for Exp(B) | |
| Lower Bound | Upper Bound | ||||||||
| less than 4.5 | Age | 0.1 | 0 | 20 | 1 | <0.001 | 1 | 1.05 | 1.13 |
| BMI | 0.1 | 0 | 14 | 1 | <0.001 | 1 | 1.07 | 1.25 | |
| HbA1c | -1 | 0.1 | 67 | 1 | <0.001 | 0 | 0.31 | 0.49 | |
| Years of Diabetes | -0 | 0.1 | 18 | 1 | <0.001 | 1 | 0.68 | 0.87 | |
| 4.5-5.7 | Age | 0.1 | 0 | 22 | 1 | <0.001 | 1 | 1.05 | 1.13 |
| BMI | 0.1 | 0 | 11 | 1 | 0 | 1 | 1.06 | 1.22 | |
| HbA1c | -1 | 0.1 | 71 | 1 | <0.001 | 0 | 0.32 | 0.49 | |
| Years of Diabetes | -0 | 0.1 | 9.9 | 1 | 0 | 1 | 0.76 | 0.94 | |
| a. The reference category is: greater than 5.7. | |||||||||
Table 3: Predictions for interdigital foot skin pH.
| Parameter Estimates | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Plantar foot skin pH | Variable | B | Std. Error | Wald | df | Sig. | (OR) | 95% Confidence Interval for Exp(B) | |
| Lower Bound | Upper Bound | ||||||||
| less than 4.5 | Age | 0 | 0 | 24.11 | 1 | <0.001 | 1.1 | 1 | 1.1 |
| BMI | 0 | 0 | 19.05 | 1 | <0.001 | 1.2 | 1 | 1.3 | |
| HbA1c | -1 | 0.1 | 82 | 1 | <0.001 | 0.4 | 0 | 0.4 | |
| Years of Diabetes | -0 | 0.1 | 14.62 | 1 | <0.001 | 0.8 | 1 | 0.9 | |
| 4.5-5.7 | Age | 0 | 0 | 10.68 | 1 | 0 | 1.1 | 1 | 1.1 |
| BMI | 0 | 0 | 12.99 | 1 | <0.001 | 1.2 | 1 | 1.3 | |
| HbA1c | -1 | 0.1 | 53.28 | 1 | <0.001 | 0.4 | 0 | 0.6 | |
| Years of Diabetes | -0 | 0.1 | 5.586 | 1 | 0 | 0.9 | 1 | 1 | |
| a. The reference category is: greater than 5.7. | |||||||||
Table 4: Predictions for plantar foot skin pH.
Discussion
This study concludes that there is a significant difference (p < 0.05) in foot skin pH between group 1 (healthy) and the three groups living with type 2 diabetes (group 2,3 and 4). It was also observed that group 2 (good HbA1c) exhibited similar results to group 1 (healthy), with mean foot skin pH values of 4.57 and 4.88 respectively, thus highlighting the fact that when high HbA1c values were present, skin pH was higher. According to previous published literature, normal skin pH observed in a healthy population was found to be in the range of 4.5 to 5.7 [4, 7, 17]. In this study, the mean foot skin pH of group 1 and 2 is congruent to these studies, as participants exhibited similar mean foot skin pH within that range, in all the six tested sites for both left and right foot. It can be observed, that although group 2 participants were living with type 2 diabetes, mean skin pH results were similar to group 1 possibly due to the good glycaemic control exhibited within the study group.
On the other hand, it was observed that participants pertaining to groups with higher HbA1c levels exhibited higher skin pH values, especially in group 4 (poor HbA1c), scoring a mean foot skin pH value of 6.06. Group 3 (fair HbA1c) and group 4 (poor HbA1c) scored a higher mean foot skin pH score in all six tested sites, 5.43 and 6.06 respectively, shifting to a less acidic pH compared to group 1 and 2. One possible reason for this could be that patients with persistent uncontrolled glycaemic levels experience glycosylation of various tissue components in the skin, resulting in skin pH changes and by controlling HbA1c levels, similar to the group 2 participants, these effects can be mitigated [2]. These results were consistent with the research conducted by Yosipovitch et al., on skin pH. Although in their study skin pH reading were taken from different body regions (axilla, inframammary and inguinal regions) when compared to this present study, results obtained were similar since participants living with type 2 diabetes showed to have higher mean skin pH (less acidic) when compared to the control group, both in male (5.67 vs 6.08) and female participants respectively (5.92 vs 6.58) [18].
In contrast, conclusions made by Mackiewicz-Wysocka et al., varied from previous literature and also from this present study. This is because results obtained were quite contradicting in that skin pH of subjects living with diabetes, was found to be lower than that of the control group [10]. It was noted that the more controlled the glycaemic levels were (less than 8%), the higher the skin pH readings were. Participants with HbA1c levels greater than 8% were found to exhibit lower skin pH values when compared to the healthy control group and also to those who had better control on their glycaemic levels (less than 8%). The results obtained when comparing the control group (healthy) against the group living with type 1 DM have showed that the mean dorsal foot skin pH were 5.41 and 5.20 respectively [10]. Both mean values are within the normal range for healthy skin pH, however the healthy control group was found to have a higher mean value, unlike previous studies and also this present study which reported the opposite, whereby skin pH in the healthy control groups were observed to be lower than groups with higher HbA1c values.
This observation might offer a possible explanation for studies compiled by Van Hattem et al in 2008, whereby it was noted that patients with type 2 DM had a higher incidence of skin conditions especially infections, when compared to type 1 DM patients [19]. A possible explanation could be that skin pH being in a more acidic region in type 1 DM, when compared to type 2 DM patients who experience a less acidic skin pH making certain skin manifestation much more common and easier to thrive. Another contributing factor for certain skin conditions, which was revealed in this present study, is the possibility of a different pH environment in the interdigital regions of the foot. Van Hattem et al., selected the cheek, dorsal surface of the forearm and dorsal aspect of the foot as the regions of skin pH measurements, unlike this present study which took into consideration the interdigital areas of the foot, anatomical site which few studies have observed.
Mean skin pH from the interdigital region in all participants from all groups in both the left and right sides, was higher compared to the other sites. Normal sweat production is usually slightly acidic in nature, however in certain cases where moisture is maintained for a prolonged period of time and the environment is anaerobic, bacteria tend to hydrolyse it into ammonia which in itself is slightly alkaline [20]. In contrast to previous studies [21, 22], this present study has shed more light on skin manifestations whereby it was acknowledged that an acidic skin pH in the ranges of 4 to 5.5 functions to create a hostile environment for the colonization of pathogenic microorganisms, raising the possibility that some common skin conditions such as tinea pedis are also pH dependant [21]. This could possibly be a main factor, to why individuals living with type 2 diabetes, especially those with uncontrolled glycaemic levels, tend to be more at risk in acquiring certain skin conditions and take longer to heal [19]. This study has shown that uncontrolled glycaemic levels (high HbA1c) has been found to be associated with a higher (less acidic) skin pH and therefore pose a higher risk of skin conditions especially in the interdigital region. This observation was also discussed by Proksch et al., (2018) whereby skin conditions such as dermatitis, ichtyosis, acne, dry skin and infections all thrive when disruption of skin barrier and an increase in skin pH are present [21].
Therefore, results from this present study are congruent with previous literature and confirm that uncontrolled diabetes disrupts normal skin pH and can increase risk of delayed wound healing, as well as an increase in skin conditions especially in the interdigital areas where tendency for a higher skin pH (less acidic) is present and thus offers an optimum environment for fungal, bacterial and yeast infections. In this regard, one could consider regular monitoring of foot skin pH levels in order to predict such infections from happening and to improve wound healing processes. Skin pH monitoring could form an integral part of diabetes foot screening to help determine the skin pH level in this high risk population.
Strengths and limitations
Although every effort was made in this present study to control most of the confounding variables that might have had an impact on skin pH such as gender, age, duration of diabetes and HbA1c, some other important factors namely U.V. radiation, polycyclic aromatic hydrocarbons (PAH’s) and long term exposure to daily pollutants were difficult to accurately record. To minimise variability of skin environment during the skin pH measurement protocol, participants were instructed to remove footwear and any socks, and lie on the couch in a supine position for 20 minutes, in a room with a temperature of 20 to 22 °C in order for acclimatization to take place [14].
Conclusion
This present study has shed light and has confirmed that uncontrolled glycaemic levels in participants with type 2 diabetes, were associated with a change in skin pH making it less acidic and more prone to possible infections. This study was the first of its kind since it analyzed novel regions of interest in the foot. Although past studies have described altered pH readings as a possible connection to diabetes, none have investigated its effects on foot skin manifestations, or suggested the possibility of its future application to prevent foot complications, especially in people living with type 2 diabetes. Further studies are warranted to establish this possibility further.
Disclaimers
The views expressed in the submitted article are those of all authors and not an official position of the institution or funder.
Acknowledgements
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ali SM, and Yosipovitch G (2013) Skin pH: from basic science to basic skin care. Acta Derm Venereol 93: 261-269.
- Niaz F, Bashir F, Shams N, Shaikh Z, and Ahmed I (2016) Cutaneous manifestations of diabetes mellitus type 2: prevalence and association with glycaemic control. Journal of Pakistan Association of Dermatology 26: 4-11.
- Yosipovitch G, Tur E, Cohen O, and Rusecki Y (1993) "Skin surface pH in intertriginous areas in NIDDM patients: possible correlation to candidal intertrigo." Diabetes Care 16: 560-563.
- Ring J, Eberlein-König B, Schäfer T, Huss-Marp J and Darsow U, et al. (2000) Skin surface pH, stratum corneum hydration, trans-epidermal water loss and skin roughness related to atopic eczema and skin dryness in a population of primary school children: clinical report. Acta Derm Venereol 80: 188-191.
- Korting HC, and Braun-Falco O (1996) The effect of detergents on skin pH and its consequences. Clin Dermatol 14: 23-27.
- Giacomoni PU, Mammone T, and Teri M (2009) Gender-linked differences in human skin. J Dermatol Sci 55: 144-149.
- Lambers H, Piessens S, Bloem A, Pronk H, and Finkel P (2006) Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int J Cosmet Sci 28: 359-370.
- Plasencia I, Norlen L, and Bagatolli LA (2007) Direct visualization of lipid domains in human skin stratum corneum's lipid membranes: effect of pH and temperature. Biophys J 93: 3142-3155.
- Runeman B, Faergemann J, and Larko O (2000) Experimental Candida albicans lesions in healthy humans: dependence on skin pH. Acta Derm Venereol 80: 421-424.
- Mackiewicz-Wysocka M, Araszkiewicz A, Niedzwiedzki P, Schlaffke J, and Zozulinska-Ziolkiewicz D, et al. (2015) Skin pH is lower in type 1 diabetes subjects and is related to glycemic control of the disease. Diabetes Technol Ther 17: 16-20.
- Goodyear MD, Krleza-Jeric K, and Lemmens T (2007) The declaration of Helsinki.
- Kome GK, Enang RK, Yerima BPK and Lontsi MGR (2018) Models relating soil pH measurements in H2O, KCl and CaCl2 for volcanic ash soils of Cameroon. Geoderma Regional, 14, e00185.
- PH60 Series Premium Ph Testers Instruction Manual. Aperainst, (2016).
- Stefaniak AB, Plessis JD, John SM, Eloff F and Agner T, et al. (2013). International guidelines for the in vivo assessment of skin properties in non‐clinical settings: part 1. pH. Skin Res Technol 19: 59-68.
- Parra JL, and Paye M (2003) EEMCO guidance for the in vivo assessment of skin surface pH. Skin Pharmacol Appl Skin Physiol 16:188-202.
- Mleczko, A (2008) Investigation of skin physiological parameters in term neonates and evaluation of the influence of bathing on skin barrier function in newborns during the first four weeks of life: Prospective examination of the following skin parameters: stratum corneum hydration, skin pH, transepidermal water loss and skin surface lipids. Dissertation.
- Yosipovitch G, Tur E, Cohen O and Rusecki Y (1993) "Skin surface pH in intertriginous areas in NIDDM patients: possible correlation to candidal intertrigo." Diabetes Care 16: 560-563.
- Van Hattem S, Bootsma AH and Thio HB (2008) Skin manifestations of diabetes. Cleve Clin J Med 75: 772-774.
- Guinovart T, Bandodkar AJ, Windmiller JR, Andrade FJ, and Wang J (2013) A potentiometric tattoo sensor for monitoring ammonium in sweat. Analyst 138: 7031-7038.
- Rippke F, Schreiner V, Doering T, and Maibach HI (2004) Stratum corneum pH in atopic dermatitis. American journal of clinical dermatology 5: 217-223.
- Surber C, Abels C, and Maibach H (2018) pH of the Skin: Issues and Challenges. Karger Medical and Scientific Publishers.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi