Review Article, Clin Oncol Case Rep Vol: 5 Issue: 12
Hormone Therapy and Prostate Cancer: Reminder and Latest News
Fabien Dutheil*,Youssef Slama
Department of Radiotherapy Oncology,Sainte-Clotilde Clinic, 127 route de Bois de Nefles, Saint-Denis 97400, La Reunion, France.
*Corresponding Author: Fabien Dutheil
Department of Radiotherapy Oncology,Sainte-Clotilde Clinic, 127 route de Bois de Nefles, Saint-Denis 97400, La Reunion, France.
E-mail:fabien.dutheil@clinifutur.net
Received: December 16, 2022; Manuscript No: COCR-22-84038;
Editor Assigned: December 18, 2022; PreQC Id: COCR-22-84038 (PQ);
Reviewed: December 26, 2022; QC No: COCR-22-84038 (Q);
Revised: December 28, 2022; Manuscript No: COCR-22-84038 (R);
Published: December 30, 2022; DOI: 10.4172/cocr.5(12).267
Citation: Dutheil F, Slama Y (2022) Hormone Therapy and Prostate Cancer: Reminder and Latest News. Clin Oncol Case Rep 5:12
Abstract
Androgen deprivation is a key treatment in the management of patient with locally advanced and metastatic prostate cancer. A better comprehension of the castration resistance's mechanisms allowed the development of second-generation hormonal therapies that are generally better tolerated than chemotherapy and can improve the survival and quality of life of patients with prostate cancer.
Keywords: Prostate cancer; Recommendations; Treatment; Hormone therapy
Introduction
Androgen suppression or hormone therapy is a standard treatment for the management of locally advanced and metastatic prostate cancer. After a phase of tumour disease control with first generation hormone therapy, castration resistance mechanisms developed by the tumour cells appear. The development of second generation hormone therapy resulting from a better understanding of castration resistance mechanisms has led to improved management of patients with metastatic prostate cancer.
Review of Literature
Testosterone synthesis and androgenic suppression
Definition
Charles Brenton Huggins, a North American physician, established a method for assessing prostate function by altering hormonal parameters. He discovered that surgical or biological castration leads to atrophy of the prostate gland. Re-administration of androgens reverses the process. In 1941, the beneficial e ffect of androgen suppression on metastatic prostate cancer was realised when Huggins and Clarence Hodges treated patients with either surgical castration or estrogen therapy [1]. They monitored prostate size and therapeutic efficacy by me asuring se rum prostatic ac id phosphatase levels. Huggins was the first to use a systemic approach to treating prostate cancer.
Androgen suppression (surgical or medical) remains the first-line treatment for metastatic prostate cancer to this day. According to the European Association of Urology (EAU), surgical castration is still considered to be the most effective technique for achieving effective, rapid and durable castration. It remains the least expensive technique. Androgen suppression improves symptoms but does not significantly prolong survival. Newer testosterone assay methods are more accurate and castration is achieved when the testosterone level is < 20 ng/dL (1 nmol/L) but some jurisdictions still use the value of 50 ng/dL (1.7 nmol/L) which was determined in the 1970s [2].
Neuroendocrine mechanisms
Testosterone secretion is mainly regulated by the hypothalamicpituitary axis (Figure 1). LH-RH, a hypothalamic hormone, is secreted in a pulsatile manner and acts on the pituitary gonadotropic cells. The latter will produce and release the gonadotropins, LH and FSH, into the venous system in the same way. LH is able to control the production and secretion of testosterone via its receptor on the surface of Leydig cells. It induces the transcription of genes encoding many enzymes involved in testosterone biosynthesis. Androgens exert a negative feedback on the secretion of gonadotropins at the hypothalamic-pituitary level. Surgical castration suppressing testosterone production leads to increased blood levels of FSH and LH. Alternatively, administration of an LH-RH analogue causes continuous and transient stimulation of its receptors on the pituitary. After an initial peak in secretion, desensitisation of the LH-RH receptors takes place, resulting in a decrease in blood levels of LH and testosterone.
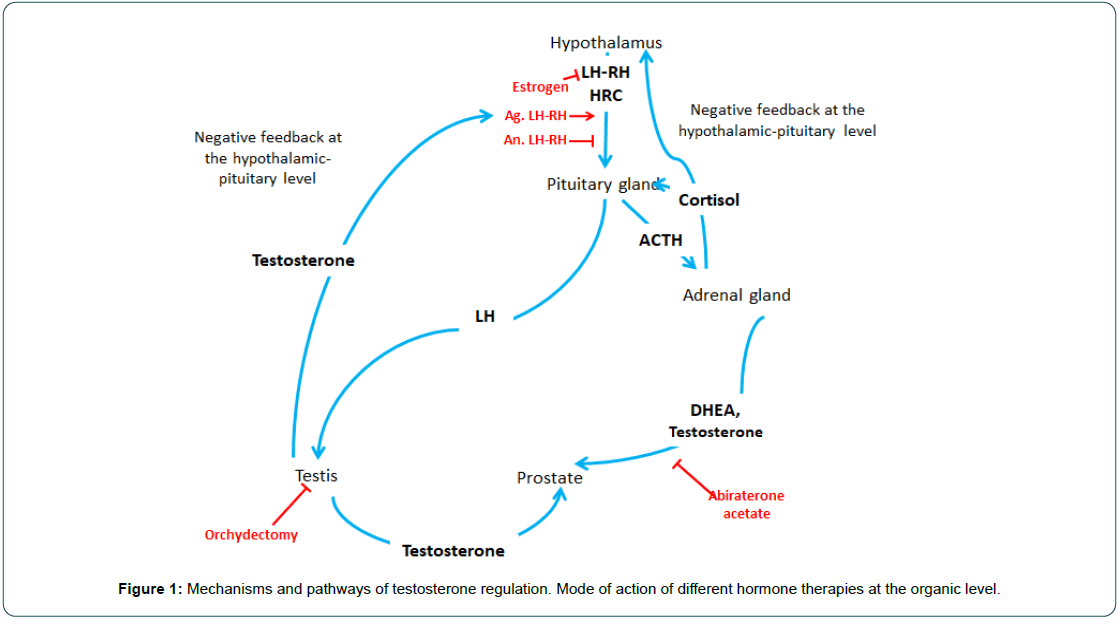
Figure 1: Mechanisms and pathways of testosterone regulation. Mode of action of different hormone therapies at the organic level.
The biosynthesis of testosterone, a cholesterol derivative, takes place 95% of the time in the Leydig cells of the testis and to a lesser extent by the adrenal glands. Before being synthesised into testosterone, cholesterol is metabolised in the mitochondria and smooth endoplasmic reticulum of the cells into a series of metabolites by various enzymatically active proteins belonging to the cytochrome P450 family (CYPs). CYP17A1 is involved in biochemical synthesis by successively converting progesterone into 17 β-OH-progesterone (25-17 β-hydroxylase activity) and then into androstenedione (C17-20-lyase activity). Finally, androstenedione is converted to testosterone by another enzyme. Testosterone can act directly on the androgen receptor or indirectly via its role as a pro-hormone. The action of type 2, 5α-reductase, which predominates in the prostate in contrast to type 1, 5α-reductase, converts testosterone into an active metabolite, dihydrotestosterone, which has five times more affinity for the androgen receptor than testosterone (Figure 2). In the prostate, but especially in prostate tumour cells, testosterone derivatives will promote cell growth and survival as well as an increase in blood PSA levels.
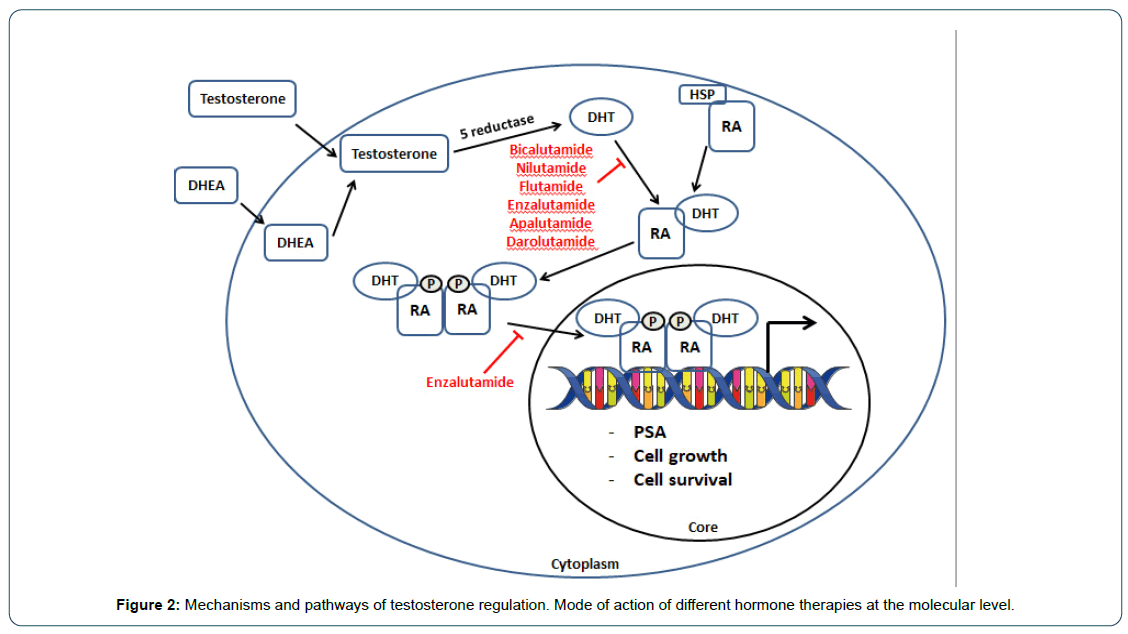
Figure 2: Mechanisms and pathways of testosterone regulation. Mode of action of different hormone therapies at the molecular level.
The figure illustrates the mechanisms of testosterone synthesis and action and the regulatory pathways (Figure 3).
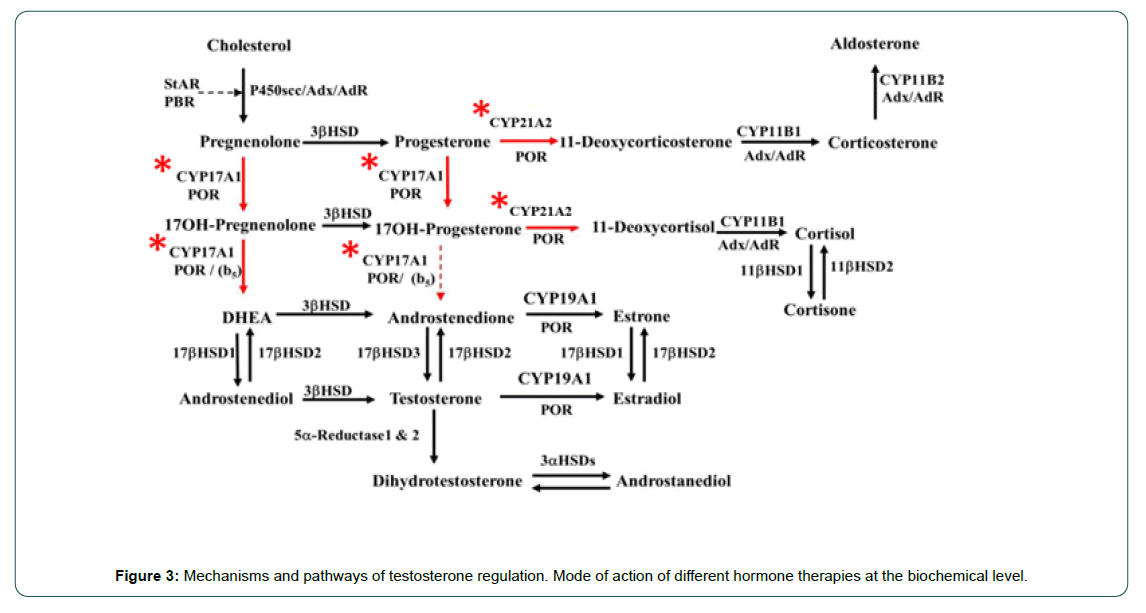
Figure 3: Mechanisms and pathways of testosterone regulation. Mode of action of different hormone therapies at the biochemical level.
The surgical technique
Bilateral orchiectomy, or subcapsular pulpectomy, performed under local or general anaesthesia depending on the country, is the irreversible, simple, inexpensive and generally uncomplicated surgical procedure. It remains the fastest method of achieving castration, usually in less than 12 hours. Even if the installation of prostheses to replace the testis allows the correction of aesthetic after-effects, the use of oral or injectable hormonal treatments is currently preferred.
Drug-induced androgen suppression
LH-RH agonists or antagonists
LH-RH agonists: Long-acting LH-RH agonists are currently the most widely used synthetic hormones in Europe. These synthetic LHRH analogues are administered by injection, the main characteristic of which is a sustained release of LH-RH over a period of 1 months, 2 months, 3 months or 6 months. These agonists, which are more potent than endogenous LH-RH, will block the pulsatile secretion of LH-RH. On the first injection, a transient increase in LH and FSH leading to the "flare up" effect starts 2 days to 3 days after administration and lasts for about a week. In patients with locally advanced or metastatic prostate cancer, this biological increase may have adverse clinical effects such as: acute urine retention which may be complicated by acute obstructive renal failure, exacerbated bone pain, neurological disorders secondary to spinal cord compression and haemostasis disorders such as hypercoagulability [3]. Concomitant treatment with an anti-androgen decreases the incidence of clinical relapses, but does not completely eliminate the risk. It is recommended that an anti-androgen be administered 15 days before the initiation of the antagonist and that the anti-androgen be continued for a further 15 days after administration. Although one study suggests a benefit in progression-free survival with the use of leuprorelin as a once-monthly injection [4]. Although one study suggests a benefit in progression-free survival with the use of leuprorelin as a oncemonthly injection, no study has demonstrated its superiority over other LHRH analogues.
The LH-RH antagonist: In order to avoid this exacerbation of symptoms secondary to LH-RH agonists, an LH-RH antagonist, degarelix, was developed. The advantage of this product is the very rapid onset of biological castration in an average of 3 days, which is durable in about 95% of patients. A loading dose of 240 mg should be initiated followed by a monthly subcutaneous injection of 80 mg.
Anti-androgens
A distinction is made between steroidal, non-steroidal and third generation anti-androgens.
Steroidal anti-androgens: Cyproterone acetate, a progesterone derivative, has a peripheral action by binding to the androgen receptor, competing directly with dihydrotestosterone. Cyproterone acetate also has a central inhibitory effect. This anti-gonadotropic effect results in a reduction in testosterone levels by inhibiting testosterone synthesis by the testes.
In monotherapy, the optimal dosage has not been determined. Cyproterone acetate is usually given in two or three daily doses of 100 mg each. In a randomised trial, this anti-androgen appeared to have poorer overall survival compared to some LHRH analogues [5]. In addition, the various side effects, particularly thrombo-embolic and hepatic, mean that this product is used less than other therapies.
Other steroidal anti-androgens (megestrol acetate and medroxyprogesterone acetate exist but their efficacy remains controversial.
Non-steroidal anti-androgens: Bicalutamide and nilutamide are two of the most widely used non-steroidal anti-androgens. Like steroidal anti-androgens such as bicalutamide, these compounds have a peripheral action by competing directly with dihydrotestosterone at the androgen receptor, thus preventing entry of the androgen receptordihydrotestosterone complex into the nucleus and activation of target genes. They are very rarely used as monotherapy. Only bicalutamide taken three times daily (150 mg/d) is an interesting alternative to castration in patients with locally advanced prostate cancer for whom immediate hormonal treatment is indicated [6].
On the other hand, when initiating treatment with LHRH analogues, the use of these anti-androgens to prevent the "flare up" effect should be systematic.
Third generation anti-androgens: Two third-generation molecules are currently marketed in France and others are being evaluated in clinical trials.
Abiraterone acetate is an inhibitor of androgen synthesis by blocking CYP17A1 which has 17α-hydroxylase and C17, 20-lyase activity. The 17α-hydroxylase activity allows the synthesis of 17OHpregnenolone from pregnenolone. C17, 20-lyase activity allows the synthesis of androstenedione from 17 β-OH-progesterone. Malikova et al. have furthermore shown that abiraterone acetate also has an inhibitory action on CYP21A2 which is involved in the synthesis of 11-deoxycorticosterone and 11-deoxycorticol from progesterone and 17OH-progesterone respectively [7]. The dual inhibition of CYP17A1 and CYP21A2 by abiraterone acetate partly explains the reasons for the disturbance of cortisol synthesis.
The dosage is 4 mg × 250 mg tablets or 2 mg × 500 mg tablets taken daily with meals (at least 2 hours after a meal and 1 hour before the next food intake) combined with 5 mg or 10 mg of oral prednisone or prednisolone. The addition of corticosteroids is intended to compensate for the decrease in cortisol and to block the compensatory increase in ACTH [8].
In the COU-AA-302 study, abiraterone acetate showed a survival benefit of 34.7 months vs 30.3 months for placebo (HR=0.81; 95% CI 0.70-0.93; p= 0.0033) in patients with metastatic, hormone-refractory, chemotherapy-naive prostate cancer [9].
Enzalutamide has a dual action. It inhibits the androgen receptor by binding to the active site and prevents translocation of the receptor from the cytoplasm to the nucleus. The dosage is four 40mg capsules taken at the same time each day.
Results from the AFFIRM study showed a survival benefit of 18.4 months in patients who received enzalutamide versus 13.6 months in those who did not [10, 11]. In the TERRAIN (Enzalutamide versus Bicalutamide in Castrate Men with Metastatic Prostate Cancer) study, in patients with hormone-refractory metastatic prostate cancer who were chemotherapy-naive, enzalutamide very significantly improved progression-free survival compared with bicalutamide in both patients under 75 years of age (HR=0.38 ; 95% CI 0.27-0.52; p< 0.0001) and in patients over 75 (HR=0.59; 95% CI 0.37-0.92; p=0.018) [12].
Apalutamide, is structurally and pharmacologically similar to enzalutamide. It is a competitive selective androgen receptor antagonist with the ability to cross the blood-brain barrier [13, 14]. It has a 5-10 fold greater affinity for the androgen receptor than bicalutamide [15].
The Selective Prostate Androgen Receptor Targeting (SPARTAN) with RNA-509 phase 3 randomised controlled trial evaluated the efficacy of apalutamide in patients with non-metastatic hormoneresistant prostate cancer. The analysis of 1207 patients showed that there was a highly significant increase in metastasis-free survival, being 40.5 months in the apalutamide group versus 16.2 months in the placebo group (HR=0.45; 95% CI 0.32- 0.63; p < 0.001) [16].
Darolutamide, like enzalutamide and apalutamide, is also a competitive selective androgen receptor antagonist. Its structure gives it fewer side effects. It is known to cross the blood-brain barrier less. Its low affinity for Gamma-Aminobutyric Acid (GABA) type A receptors also reduces the risk of seizures. The ARAMIS study is an international, randomised, double-blind, placebo-controlled, phase 3 study to evaluate the efficacy and safety of darolutamide in men with high-risk, non-metastatic castration-resistant prostate cancer. The Primary endpoint was metastasis-free survival (PFS). Secondary endpoints were Overall Survival (OS) and time to pain progression, first cytotoxic chemotherapy and symptomatic skeletal events. Quality of life (QoL) was measured using validated prostate tools. Safety was recorded throughout the study of the 1509 patients enrolled, 469 had PSADT > 6 months (darolutamide n = 286; placebo n = 183) and 1040 had PSADT of 6 mo (darolutamide n = 669; placebo n = 371). Baseline characteristics were balanced between the subgroups. Darolutamide significantly prolonged PFS compared with placebo in both subgroups (unstratified hazard ratio [95% confidence interval]: PSADT > 6 months, 0.38 [0.26-0.55]; PSADT 6 months, 0.41 [0.33-0.52]). OS and other efficacy and quality of life outcomes favoured darolutamide with significant improvement over placebo in both subgroups. The incidence of adverse events, events commonly associated with androgen receptor blockers (fractures, falls, hypertension and mental retardation), and dropouts due to adverse events were low and similar to placebo. Study limitations include small subgroup populations [17].
In CPC patients with PSADT > 6 months (maximum 10 months), darolutamide provided a favourable benefit/risk ratio, characterised by significant improvements in FMS, OS, treatment efficacy and other clinically relevant endpoints, maintenance of quality of life and favourable tolerability.
Estrogenic derivatives
The efficacy of these products has been demonstrated in previous publications, but estramustine and diethylstilbestrol are no longer or rarely used because of extremely deleterious side effects such as thrombo-embolic and cardiovascular complications [18].
Estramustine: Estramustine is indicated in hormone-resistant prostate cancer. It has a mixed hormonal activity with an antigonadotropic and antineoplastic effect of the spindle poison type. The dosage is 2 daily doses of 2 capsules of 140 mg to be taken at a distance from meals (at least 1 hour before or 2 hours after meals). In case of insufficient efficacy, the daily dose can be increased to 5 capsules or 6 capsules (without exceeding 15 mg/kg) in 2 doses or 3 doses.
The French multicentre randomised controlled GETUG 12 trial studied the efficacy of hormonal goserelin treatment with and without docetaxel and estramustine in patients with high-risk localised prostate cancer (stage T3-T4, Gleason score ≥ 8, PSA > 20 ng/mL or positive pathological lymph node) [19]. 207 patients were included in the goserelin plus docetaxel and estramustine arm and 206 in the goserelin alone arm. The median follow-up was 8.8 years. Recurrence-free survival at 8 years was 62% (95% CI 55-69) in the triple-therapy arm versus 50% (95% CI 44-57) in the goserelin arm alone (HR=0.71, 95% CI 0.54-0.94, p=0.017). Among patients treated with radiotherapy and for whom data were available, 31 (21%) out of 151 in the goserelin-docetaxel-estramustine arm versus 26 (18%) out of 143 in the goserelin-alone arm experienced urodigestive toxicity of grade 2 or higher (p=0.61).
Diethylstilbestrol: Diethylstilbestrol, a synthetic oestrogen, can be used as a second-line treatment for patients with metastatic castration-resistant prostate cancer. Diethylstilbestrol exerts a negative feedback control on the hypothalamic-pituitary axis limiting the pulsatile secretion of FSH and LH, and thus inhibiting testosterone synthesis [20]. The dosage is 3 daily doses of 1 tablet of 1 mg. This dosage could then be reduced to 1 mg per day.
A retrospective English study investigated the efficacy and toxicity of diethylstilbestrol in 231 patients with hormone-resistant prostate cancer. The biological response rate (PSA level) was 28.9%. The median duration of biological response was 4.6 months. 9.9% of patients experienced thromboembolic complications [18].
These molecules, initially classified as second-line drugs for patients with castration-resistant prostate cancer who cannot receive chemotherapy, have been progressively replaced by third-generation anti-androgens.
The main indications for hormone therapy
In combination with surgery: There is no indication for neoadjuvant hormone therapy prior to prostatectomy. A metaanalysis showed no improvement in overall survival or progressionfree survival [21].
In the adjuvant setting, no study has shown a benefit to the use of hormone therapy in the case of lymph node involvement. The French GETUG 20 multicentre, phase III, randomised study evaluating the benefit of adjuvant hormonal treatment with leuprorelin acetate (Eligard® 45 mg) for 24 months after total prostatectomy in patients at high risk of recurrence will make it possible to propose a therapeutic strategy.
The retrospective study by Abdollah et al. investigated the role of radio-hormone therapy on specific mortality in 1107 patients with node-positive prostate cancer. This study showed a decrease in treatment-specific mortality for two subgroups. The first one corresponds to patients with 1 positive nodes or 2 positive nodes, a Gleason score ≥ 7 and classified pT3 or R1, and the second group corresponds to patients with 3 positive nodes to 4 positive nodes [22]. This data needs to be confirmed by prospective trials.
In combination with radiotherapy: For patients with low-risk localised prostate cancer, there is no indication to combine it with hormone therapy.
In contrast, according to the AFU and EAU recommendations, for intermediate and high risk groups, hormone therapy should be started 2 months before radiation for a total duration of 6 months (intermediate unfavourable group, ISUP 3) to 2 years to 3 years (high risk groups, ISUP 4 and 5) [23].
Combined radiotherapy and LHRH analogue hormone therapy has been shown to be superior to radiotherapy alone followed by delayed sequential hormone therapy in relapse in several randomised phase 3 trials [24-27]. These trials mainly included high-risk patients, mainly due to locally advanced disease (T3-T4 N0-X). The EORTC 22863 study is one of the most robust studies that has established radio-hormone therapy as a preferred treatment option for patients with high-risk prostate cancer [28].
One of the parameters interfering with the duration of hormone therapy is cardiovascular risk. In the RTOG 9910 trial, 1579 intermediate-risk patients were randomised. Patients received LHRH antagonist therapy 2 months prior to irradiation and then for 8 weeks or 28 weeks. Prolonged androgen suppression did not significantly improve overall survival or recurrence-free survival. The equivalence study, EORTC 22961, investigated in 970 high-risk patients the combination of radiotherapy (70Gy) and LHRH agonist therapy for 6 months or 3 years. With a median follow-up of 6.4 years, cancerspecific mortality and overall mortality were significantly lower in the long-term hormone therapy group [23]. The EORTC 22991 study investigated overall survival and recurrence-free survival in 819 patients with predominantly intermediate prognosis treated with radiotherapy alone (70 Gy-78Gy) or combined with 6 months of androgen suppression [29]. A benefit in clinical recurrence-free survival and a significant decrease in the occurrence of metastases but no benefit on overall survival was observed. The benefit of this hormone therapy is all the more marked as the dose of irradiation is high.
Hormone therapy alone: According to the EAU, in highrisk patients who cannot benefit from surgery or radiotherapy, hormone therapy alone is considered if the patient is symptomatic or asymptomatic but has a poorly differentiated tumour, with a PSA doubling time of less than 12 months and a PSA level > 50 ng/ml. This is only for a minority of patients. Even in unfit patients, treatment with radio-hormone therapy is preferred, using hypofractionated radiotherapy and hormone therapy adapted to the comorbidities.
In case of isolated biochemical recurrence after curative treatment, hormonal treatment may be discussed. The vast majority of studies remain controversial about the conditions for initiating treatment. Patients eligible for hormone therapy after curative treatment are those with a strong suspicion of metastatic recurrence (lymph node involvement, seminal vesicle invasion, Gleason score ≥ 8, early recurrence, PSADT < 10 months). In the absence of metastases, hormone therapy may be proposed in patients with a short doubling time (≤ 12 months) [30]. The study by Boorjian et al. showed a survival benefit to early introduction of hormone therapy in high-risk patients with a long life expectancy and a PSA doubling time < 6 months [31].
In patients with metastatic prostate cancer, continuous early hormone therapy is the gold standard. There is no indication for routine complete androgen blockade in the treatment of metastatic patients. Androgen blockade is mainly defined by radiobiochemical criteria; clinical criteria alone are not sufficient. According to the AFU, a testosterone level < 50 ng/dl or 1.7 nmol/l combined with either biochemical progression: three PSA increases resulting in two 50% increases above nadir with a PSA > 2 ng/mL, or radiographic progression defined by the appearance of at least two new lesions on bone scan or progression of a lesion measurable according to the Response Evaluation Criteria in Solid Tumours (RECIST) constitutes resistance to castration [32]. After an extension work-up (bone scan and thoraco-abdomino-pelvic CT scan with or without choline PET scan) and the discovery of metastatic lesion(s), additional systemic treatment must be initiated, especially if the patient is symptomatic.
In metastatic patients: According to the AFU, "first-line hormonal-chemical therapy for the treatment of metastatic hormonoand chemo-naive prostate cancer is the standard treatment for patients whose health status is compatible with the use of docetaxel. The choice of first-line hormonal-chemotherapy, discussed collegially in the PCR, should be based on a shared decision with the patient and an assessment of the risk/benefit balance (level 1, grade A).
It has been established that in metastatic patients with no visceral involvement, little or no symptoms, treatment with apalutamide, darolutamide, abiraterone acetate or enzalutamide is preferred as first-line therapy. On the other hand, in patients with symptomatic bone metastases, visceral metastases, or with an undifferentiated anatomopathological tumour profile, chemotherapy should be started as early as possible, especially if there is early hormonal escape.
Abiraterone acetate is currently indicated for the treatment of newly diagnosed, high-risk, hormone-sensitive metastatic prostate cancer, the treatment of metastatic castration-resistant prostate cancer in patients with little or no symptoms, after failure of androgen suppression therapy and for whom chemotherapy is not yet clinically indicated, the treatment of castration-resistant metastatic prostate cancer in patients whose disease has progressed during or after docetaxel-based chemotherapy.
Between 2015 and 2022, many major studies such as chaarted, stampede, lattitude, titan, enzamet, spartan, prosper, propel, magnitude, aramis, peace-1/getug-21, arasens have changed therapeutic attitudes by improving the survival of metastatic patients, whether in the hormonally sensitive or hormonally resistant phase.
hormonally resistant phase standard first-line treatment for metastatic hormone-induced prostate cancer. Several phase 3 studies have demonstrated an overall survival benefit (lattitude, titan, peace-1/getug-21) by adding NGHT to androgen suppression. Historically, a benefit of adding chemotherapy to androgen suppression has been seen (CHAARTED, STAMPEDE) [32-34]. The study of the evaluation of double hormonal bocage with docetaxel chemotherapy (triplet therapy) then became a natural choice. The PEACE-1 study evaluated the triplet of abiraterone acetate and androgen suppression ± chemotherapy [35]. This study showed a benefit in both progression-free survival and overall survival. Between 2013 and 2018, 1173 patients were enrolled (one patient subsequently withdrew consent for analysis of his data) and assigned to receive standard of care (n=296), standard of care plus radiotherapy (n=293), standard of care plus abiraterone (n=292), or standard of care plus radiotherapy plus abiraterone (n=291). Median follow-up was 3 years to 5 years (IQR 2-8-4-6) for radiographic progression-free survival and 4.4 years (3-5-5-4) for overall survival. Adjusted Cox regression modelling revealed no interaction between abiraterone and radiotherapy, enabling the pooled analysis of abiraterone efficacy. In the overall population, patients assigned to receive abiraterone (n=583) had longer radiographic progression-free survival (Hazard Ratio (HR) 0-54, 99.9% CI 0-41-0-71; p < 0-0001) and overall survival (0-82, 95-1% CI 0-69-0-98; p=0-030) than patients who did not receive abiraterone (n=589). In the androgen deprivation therapy with docetaxel population (n=355 in both with abiraterone and without abiraterone groups), the HRs were consistent (radiographic progression-free survival 0-50, 99-9% CI 0-34-0-71; p< 0-0001; overall survival 0-75, 95-1% CI 0-59-0-95; p=0-017). In the androgen deprivation therapy with docetaxel population, grade 3 or worse adverse events occurred in 217 (63%) of 347 patients who received abiraterone and 181 (52%) of 350 who did not; hypertension had the largest difference in occurrence (76 [22%] patients and 45 [13%], respectively). Addition of abiraterone to androgen deprivation therapy plus docetaxel did not increase the rates of neutropenia, febrile neutropenia, fatigue, or neuropathy compared with androgen deprivation therapy plus docetaxel alone.
Following on from this study, the ARASENS trial, a phase 3, randomised, double-blind, placebo-controlled study, evaluated ODM-201 (darolutamide) versus placebo, in combination with standard androgen deprivation therapy and docetaxel, in patients with hormone-sensitive metastatic prostate cancer. 1,306 patients were randomised, 651 to darolutamide and 655 to placebo, in combination with androgen deprivation therapy and docetaxel. As with PEACE-1, ARASENS showed a significant improvement in overall survival in the darolutamide [36]. The primary analysis involved 1306 patients (651 in the darolutamide group and 655 in the placebo group); 86.1% of the patients had disease that was metastatic at the time of the initial diagnosis. At the data cutoff date for the primary analysis (October 25, 2021), the risk of death was significantly lower, by 32.5%, in the darolutamide group than in the placebo group (hazard ratio 0.68; 95% confidence interval, 0.57 to 0.80; P< 0.001). Darolutamide was also associated with consistent benefits with respect to the secondary end points and prespecified subgroups. Adverse events were similar in the two groups, and the incidences of the most common adverse events (occurring in ≥ 10% of the patients) were highest during the overlapping docetaxel treatment period in both groups. The frequency of grade 3 adverse events or 4 adverse events was 66.1% in the darolutamide group and 63.5% in the placebo group; neutropenia was the most common grade 3 adverse events or 4 adverse event (in 33.7% and 34.2%, respectively).
Studies of new combination therapies in the management of patients with metastatic castration-resistant prostate cancer include the PROpel and MAGNITUDE studies.
These two studies evaluated the efficacy of PARP inhibitors (PARPi) in first-line castration-resistant metastatic cancer. PARPi (poly-ADPribose- polymerase-1) acts on the DNA repair system in synergy with the loss of BRCA function by tumour cells, causing significant genetic instability leading to cell death. More than 30% of mCRPC patients have genetic alterations including BRCA1/2. This phenotype is associated with a poor prognosis, however, it is the prerequisite for PARPi to be effective. The results of the PROpel study are the most significant [37]. Phase III PROpel study evaluates the efficacy and safety of olaparib and abiraterone acetate in the first line. PROpel is a randomized, double-blind, placebo-controlled Phase 3 trial in patients with mCRPC undergoing first line treatment after failure of primary androgen deprivation therapy, enrolled independent of Homologous Recombination Repair (HRR) status. Patients were randomized 1:1 to receive olaparib (300 mg twice daily) or placebo, and abiraterone acetate (1000 mg once daily) + prednisone or prednisolone (5 mg). The primary endpoint was investigator-assessed rPFS with multiple secondary endpoints, including Overall Survival (OS). 796 pts were randomized to olaparib + abiraterone acetate (n=399) or placebo + abiraterone acetate (n=397). In this planned interim analysis, first line treatment with olaparib + abiraterone acetate significantly prolonged rPFS versus placebo + abiraterone in patients with mCRPC irrespective of HRR status (24.8 months vs 16.6 months; HR 0.66, 95% CI 0.54-0.81; P < 0.0001). Predefined subgroup analyses showed rPFS improvement across all subgroups, including patients with (HR 0.54, 95% CI 0.36-0.79) and without (HR 0.76, 95% CI 0.59-0.97) HRR mutations detected by circulating tumor DNA testing. A sensitivity analysis of rPFS by blinded independent central review was consistent with the primary analysis (HR 0.61, 95% CI 0.49-0.74; P=0.004). OS is currently immature with 228 deaths (28.6%). A trend in OS favouring olaparib + abiraterone acetate was observed (HR 0.86, 95% CI 0.66- 1.12). Secondary endpoints of time to first subsequent treatment (HR 0.74, 95% CI 0.61-0.90) and time to second progression-free survival or death (HR 0.69, 95% CI 0.51-0.94) were supportive of long-term benefits. The most common grade ≥ 3 Adverse Event (AE) reported was anemia (15.1% vs 3.3%) for olaparib + abiraterone acetate versus placebo + abiraterone acetate; 13.8 vs 7.8% pts, respectively, discontinued olaparib/placebo because of an AE. The rate of AEs leading to abiraterone acetate discontinuation were similar in both arms (8.5% vs 8.8%). At interim analysis, PROpel met its primary objective, demonstrating significant improvement in rPFS for olaparib + abiraterone acetate versus placebo + abiraterone acetate in patients with newly detected mCRPC who had not received prior first line therapy, irrespective of HRR status. The safety and tolerability profile of olaparib + abiraterone acetate was consistent with the known safety profiles of the individual drugs. Patient follow-up is ongoing for the planned OS analysis.
The main side effects of hormone therapy
As hormone therapy is usually taken over a longer or shorter period of time, the assessment and management of the various side effects must be established so as not to interfere with the quality of life of the patients. The consequences for the long-term use of LHRH agonists and antagonists as well as steroidal and non-steroidal antiandrogens are well known. A clinical-biological assessment should be carried out before the introduction of hormonal treatment and during treatment.
A clinical examination with a search for cardiovascular risk factors, osteoporosis, falls, mood disorders (mini-GDS) and sexuality disorders as well as an assessment of blood pressure, weight, height, body mass index and abdominal circumference should be carried out before introducing hormone therapy. A biological check-up including a blood count, fasting blood sugar, investigation of lipid abnormalities and the determination of 25OH-vitamin D3 should also be prescribed.
Apart from the "flare up" effect, LHRH antagonists have the same side effects as agonists. The most commonly cited are:
• Neuro-cognitive disorders: asthenia, memory, attention, sleep, libido and mood disorders with the risk of a depressive syndrome.
• Vasomotor disorders that can be disabling.
• Body changes: weight gain, fat gain and muscle loss, hair loss, atrophy of the external genitalia and erectile dysfunction.
• Heart rhythm disorders with a risk of QT prolongation.
• Bone demineralisation with the risk of osteoporosis.
• Biological changes: anaemia, dyslipidaemia, insulin resistance, etc.
With non-steroidal anti-androgens, erectile dysfunction, hypospermia and decreased libido are present in the majority of cases. Gynecomastia is also observed in about 50% of cases. Other disturbances such as asthenia, digestive disorders and headaches may also be encountered. This class of drugs can cause liver problems.
The most recent hormone therapies (abiraterone acetate, enzalutamide, apalutamide, darolutamide) are mostly cardiotoxic [38]. A meta-analysis of 7 studies and 8660 patients receiving one of the 2 products showed an increased cardiovascular risk of any grade (RR = 1.36; 95% CI 1.13-1.64, p = 0.001) and more particularly for high grade events (RR = 1.84; 95% CI 1.21-2.80, p = 0.004). The risk of high-grade hypertension was also increased (RR = 2.26; 95% CI 1.84-2.77, p = 0.004).
Abiraterone acetate may also cause edema of the lower limbs, urinary tract infection, haematuria, allergic alveolitis, digestive disorders, skin rash, non-pathological fractures and laboratory disturbances such as hypokalaemia, hepatic cytolysis and hypertriglyceridaemia.
The most common side effects of enzalutamide include fatigue, diarrhoea and headache. It should be used with caution in patients with a history of neurological disorders such as stroke and seizures. It may cause seizures, increase the rate of falls and be associated with Posterior Reversible Encephalopathy Syndrome (PRES syndrome) characterised by headache, confusional syndrome and visual disturbances. Apalutamide toxicities are mainly cutaneous with up to 25% of patients affected. Measures should be taken with a revision of the dosage of the drug according to the grade of the severity of the skin involvement.
Patients with prostate cancer are often very old and polypathological; the risk of drug interactions must also be clearly assessed to limit iatrogenic risks.
Conclusion
Hormone therapy is a standard treatment for the management of patients with locally advanced and metastatic prostate cancer. A better understanding of castration resistance has led to the development of new molecules, but the identification of molecular alterations such as variant 7 for the androgen receptor should lead to the development of new therapeutic strategies to continue the therapeutic progress in the management of this cancer. Similarly, the encouraging results of iPARPs are strengthening the search for new molecules targeting DNA repair proteins. The potentiation of different drug classes offers good therapeutic prospects in this neoplastic disease but a better understanding of the side effects must be carried out.
References
- Huggins C, Hodges CV (2002) Studies on prostatic cancer: The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol 168: 9-12. [Google Scholar] [Cross Ref]
- Morote J, Planas J, Salvador C, Raventos CX, Catalan R, et al. (2009) Individual variations of serum testosterone in patients with prostate cancer receiving androgen deprivation therapy. BJU Int 103: 332-335. [Google Scholar] [Cross Ref]
- Bubley GJ (2001) Is the flare phenomenon clinically significant? Urology 58: 5-9. [Google Scholar] [Cross Ref]
- Crawford ED, Tombal B, Miller K, Boccon-Gibod L, Schröder F, et al. (2011) A phase III extension trial with a 1-arm crossover from leuprolide to degarelix: comparison of gonadotropin-releasing hormone agonist and antagonist effect on prostate cancer. J Urol 186: 889-897. [Google Scholar] [Cross Ref]
- Moffat LE (1990) Comparison of zoladex, diethylstilbestrol and cyproterone acetate treatment in advanced prostate cancer. Eur Urol 18: 26-27. [Google Scholar] [Cross Ref]
- Iversen P, Tyrrell CJ, Kaisary AV, Anderson JB, Van Poppel H, et al. (2000) Bicalutamide monotherapy compared with castration in patients with nonmetastatic locally advanced prostate cancer: 6.3 years of follow-up. J Urol 164: 1579-1582. [Google Scholar] [Cross Ref]
- Malikova J, Brixius-Anderko S, Udhane SS, Parween S, Dick B, et al. (2017) CYP17A1 inhibitor abiraterone, an anti-prostate cancer drug, also inhibits the 21-hydroxylase activity of CYP21A2. J Steroid Biochem Mol Bio 174: 192-200. [Google Scholar] [Cross Ref]
- Auchus RJ, Yu MK, Nguyen S, Mundle SD (2014) Use of prednisone with abiraterone acetate in metastatic castration-resistant prostate cancer. Oncologist 19: 1231-1240. [Google Scholar] [Cross Ref]
- Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PFA, et al. (2015) Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): Final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 16: 152-160. [Google Scholar] [Cross Ref]
- Fizazi K, Scher HI, Miller K, Basch E, Sternberg CN, et al. (2014) Effect of enzalutamide on time to first skeletal-related event, pain, and quality of life in men with castration-resistant prostate cancer: Results from the randomised, phase 3 AFFIRM trial. Lancet Oncol 15: 1147-1156. [Google Scholar] [Cross Ref]
- Penson DF, Armstrong AJ, Concepcion R, Agarwal N, Olsson C, et al. (2016) Enzalutamide versus bicalutamide in castration-resistant prostate cancer: The STRIVE trial. J Clin Oncol 34: 2098-2106. [Google Scholar] [Cross Ref]
- Siemens DR, Klotz L, Heidenreich A, Chowdhury S, Villers A, et al. (2018) Efficacy and safety of enzalutamide vs bicalutamide in younger and older patients with metastatic castration resistant prostate cancer in the TERRAIN trial. J Urol 199: 147-154. [Google Scholar] [Cross Ref]
- Catalona WJ, Smith DS, Ratliff TL, Dodds KM, Coplen DE, et al. (1991) Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med 324:1156-1161. [Google Scholar] [Cross Ref]
- Pokuri VK, Nourkeyhani H, Betsy B, Herbst L, Sikorski M, et al. (2015) Strategies to circumvent testosterone surge and disease flare in advanced prostate cancer: Emerging treatment paradigms. J Natl Compr Canc Netw 13: e49-55. [Google Scholar] [Cross Ref]
- Schulze H, Senge T (1990) Influence of different types of antiandrogens on luteinizing hormone-releasing hormone analogue-induced testosterone surge in patients with metastatic carcinoma of the prostate. J Urol 144: 934-941. [Google Scholar] [Cross Ref]
- Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, et al. (2018) Apalutamide treatment and metastasis-free survival in prostate cancer. Eng J Med 378: 1408-1418. [Google Scholar] [Cross Ref]
- Smith MR, Shore N, Tammela TL, Ulys A, Vjaters E, et al. (2021) Darolutamide and health-related quality of life in patients with non-metastatic castration-resistant prostate cancer: An analysis of the phase III ARAMIS trial. Eur J Cancer 154: 138-146. [Google Scholar] [Cross Ref]
- Wilkins A, Shahidi M, Parker C, Gunapala R, Thomas K, et al. (2012) Diethylstilbestrol in castration-resistant prostate cancer. BJU Int 110: E727-735. [Google Scholar] [Cross Ref]
- Fizazi K, Faivre L, Lesaunier F, Delva R, Gravis G, et al. (2015) Androgen deprivation therapy plus docetaxel and estramustine versus androgen deprivation therapy alone for high-risk localised prostate cancer (GETUG 12): A phase 3 randomised controlled trial. Lancet Oncol 16: 787-794. [Google Scholar] [Cross Ref]
- Tostain J, Rossi D, Martin PM (2004) Physiology of androgens in adult men. Prog Urol 14: 639-660. [Google Scholar] [Cross Ref]
- Shelley MD, Kumar S, Wilt T, Staffurth J, Coles B, et al. (2009) A systematic review and meta-analysis of randomised trials of neo-adjuvant hormone therapy for localised and locally advanced prostate carcinoma. Cancer Treat Rev 35: 9-17. [Google Scholar] [Cross Ref]
- Abdollah F, Karnes RJ, Suardi N, Cozzarini C, Gandaglia G, et al. (2014) Impact of adjuvant radiotherapy on survival of patients with node-positive prostate cancer. J Clin Oncol 32: 3939-3947. [Google Scholar] [Cross Ref]
- Bolla M, de Reijke TM, Van Tienhoven G, Van den Bergh ACM, Oddens J, et al. (2009) Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med 360: 2516-2527. [Google Scholar] [Cross Ref]
- Bolla M, Van Tienhoven G, Warde P, Dubois JB, Mirimanoff R-O, et al. (2010) External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol 11: 1066-1073. [Google Scholar] [Cross Ref]
- Denham JW, Steigler A, Lamb DS, Joseph D, Turner S, et al. (2011) Short-term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer: 10-year data from the TROG 96.01 randomised trial. Lancet Oncol 12: 451-459. [Google Scholar] [Cross Ref]
- Pilepich MV, Winter K, Lawton CA, Krisch RE, Wolkov HB, et al. (2005) Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma- long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys 61: 1285-1290. [Google Scholar] [Cross Ref]
- Roach M, Bae K, Speight J, Wolkov HB, Rubin P, et al. (2008) Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: Long-term results of RTOG 8610. J Clin Oncol 26: 585-591. [Google Scholar] [Cross Ref]
- Tran E, Paquette M, Pickles T, Jay J, Hamm J, et al. (2013) Population-based validation of a policy change to use long-term androgen deprivation therapy for cT3-4 prostate cancer: impact of the EORTC22863 and RTOG 85-31 and 92-02 trials. Radiother Oncol 107: 366-371. [Google Scholar] [Cross Ref]
- Bolla M, Maingon P, Carrie C, Villa S, Kitsios P, et al. (2016) Short androgen suppression and radiation dose escalation for intermediate- and high-risk localized prostate cancer: Results of EORTC trial 22991. J Clin Oncol 34: 1748-1756. [Google Scholar] [Cross Ref]
- Parker PA, Davis JW, Latini DM, Baum G, Wang X, et al. (2016) Relationship between illness uncertainty, anxiety, fear of progression and quality of life in men with favourable-risk prostate cancer undergoing active surveillance. BJU Int 117: 469-477. [Google Scholar] [Cross Ref]
- Boorjian SA, Karnes RJ, Viterbo R, Rangel LJ, Bergstralh EJ, et al. (2011) Long-term survival after radical prostatectomy versus external-beam radiotherapy for patients with high-risk prostate cancer. Cancer 117: 2883-2891. [Google Scholar] [Cross Ref]
- Rischke HC, Schultze-Seemann W, Wieser G, Kronig M, Drendel V, et al. (2015) Adjuvant radiotherapy after salvage lymph node dissection because of nodal relapse of prostate cancer versus salvage lymph node dissection only. Strahlenther Onkol 191: 310-320. [Google Scholar] [Cross Ref]
- Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, et al. (2015) Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med 373: 737-746. [Google Scholar] [Cross Ref]
- Gill D, Gaston D, Bailey E, Hahn A, Gupta S, et al. (2017) Efficacy of eplerenone in the management of mineralocorticoid excess in men with metastatic castration-resistant prostate cancer treated with abiraterone without prednisone. Clin Genitourin Cancer 15: e599-602. [Google Scholar] [Cross Ref]
- Fizazi K, Foulon S, Carles J, Roubaud G, McDermott R, et al. (2022) Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): A multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet 399: 1695-1707. [Google Scholar] [Cross Ref]
- Smith MR, Hussain M, Saad F, Fizazi K, Sternberg CN, et al. (2022) Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N Engl J Med 386: 1132-1142. [Google Scholar] [Cross Ref]
- Saad F, Armstrong AJ, Thiery-Vuillemin A, Oya M, Loredo E, Procopio G, et al. (2022) PROpel: Phase III trial of olaparib (ola) and abiraterone (abi) versus placebo (pbo) and abi as first-line (1L) therapy for patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol 40: 11-11. [Google Scholar] [Cross Ref]
- Iacovelli R, Ciccarese C, Bria E, Romano M, Fantinel E, et al. (2018) The cardiovascular toxicity of abiraterone and enzalutamide in prostate cancer. Clin Genitourinary Can 16: e645-e653. [Google Scholat] [Cross Ref]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 