Case Report, Clin Oncol Case Rep Vol: 2 Issue: 1
Impressive Response to Immunotherapy in a Patient with Metastatic Adrenocortical Carcinoma
Lindsey Addington*, Hagman Thomas, Hawk, Nicole CMA and Pablo Bedano
Community Hospital Cancer Center, 1440 East County Line Road Indianapolis, IN 46227, USA
*Corresponding Author : Lindsey Addington
Community Hospital Cancer Center South 1440 East County Line Road Indianapolis, IN 46227, USA
Tel: 3175072666
E-mail: lindsmyoung79@gmail.com
Received: August 18, 2018 Accepted: February 06, 2019 Published: February 13, 2019
Citation: Addington L, Thomas H, Hawk, Nicole CMA, Bedano P (2019) Impressive Response to Immunotherapy in a Patient with Metastatic Adrenocortical Carcinoma. Clin Oncol Case Rep 2:1.
Abstract
Adrenocortical carcinoma (ACC) is a rare cancer with limited effective treatment options and poor prognosis. ACC incidence is 1 to 2 cases per million making randomized clinical trials difficult to conduct. The current standard first line treatment for metastatic ACC showing tumor response and improved progression free survival (PFS) is etoposide-doxorubicin-cisplatin (EDP) plus mitotane. Multiple efforts to find a targeted treatment for ACC have not shown impressive responses. Immunotherapy agents such as checkpoint inhibitors programmed cell death – 1 receptor (PD-1) and programmed cell deathligand 1 have shown significant responses in multiple malignancies including melanoma, non-small-cell-lung cancer, Hodgkin’s lymphoma to name a few. We present a case of a female patient with metastatic ACC involving the lungs, lymph nodes, and liver who progressed after EDP-mitotane, then had a significant decrease in tumor burden with anti-PD-1 agent pembrolizuamb.
Keywords: Metastatic adrenocortical carcinoma; Immunotherapy; Pembrolizumab
Introduction
ACC has a poor prognosis with limited effective treatment options especially in the metastatic setting where survival is 13% at five years [1,2]. The only approved first line treatment for stage IV ACC is EDP + mitotane; although response rates are dismal at 23% and median PFS of 5 months [3-5]. The rarity of ACC makes it difficult to conduct clinical trials to develop more effective treatments. We present a stage IV ACC patient with metastases to the lungs, lymph nodes and liver that had a dramatic response to anti-PD-1 agent, pembrolizumab.
Case Presentation
A 59 year old female presented with fatigue, hypertension, hyperglycemia, nausea, and vomiting. She underwent a left adrenalectomy of a 14 x 8.7 cm mass. Pathology was consistent with adrenocortical carcinoma. One of one lymph node was positive for metastatic disease and margins were positive.
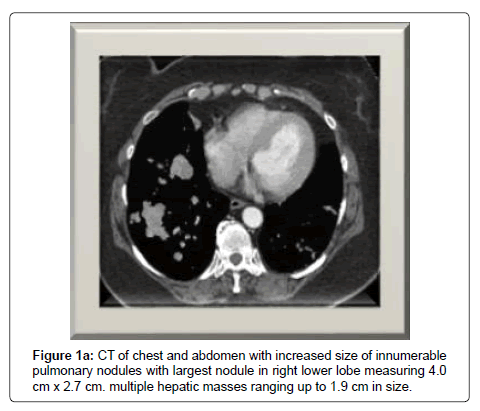
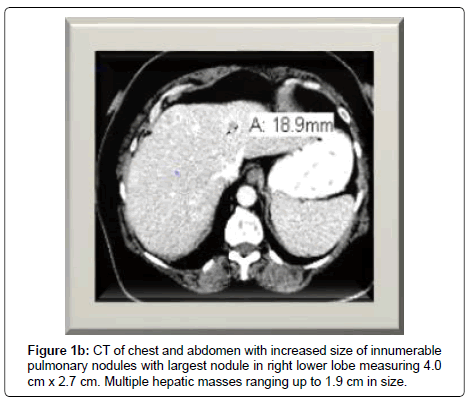
She completed adjuvant radiotherapy and then initiated adjuvant mitotane. Four months after resection, she presented with a persistent cough, and shortness of breath requiring oxygen therapy. A CT of chest revealed increase size and number of pulmonary nodules with new mediastinal and bilateral hilar adenopathy, as well as, evidence of hepatic metastases (Figure 1).
From September to February of 2018, six cycles of EDP + mitotane were administered. Within the first month of treatment, a CT of chest, abdomen, and pelvis demonstrated evidence of interval increase of pulmonary nodules and hepatic metastases. She was referred to a local university for consideration of clinical trial but there were none available for her.
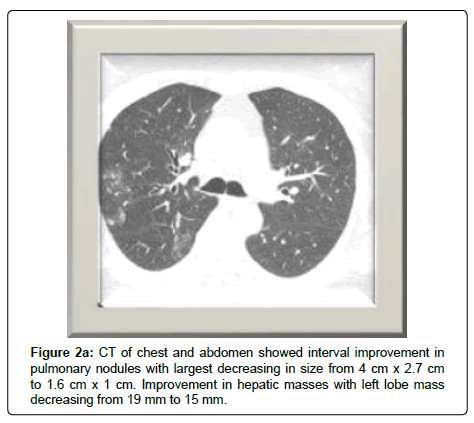
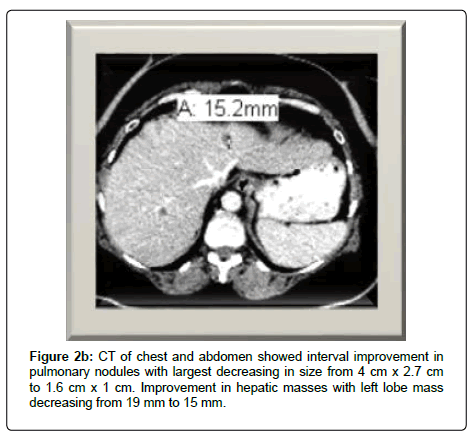
Immunotherapy with pembrolizumab was initiated in March, 2018. A CT scan of the chest, abdomen, and pelvis on week 10 revealed dramatic decrease in size of pulmonary nodules and lymphadenopathy with dominant nodule decreasing from 4 x 2.7 cm to 1.6 x 1 cm. As well as interval improvement of hepatic metastases. Mitotane was discontinued in June, 2018 due to undesirable side effects of hypotension, hypercalcemia, memory loss and acute kidney injury (Figure 2).
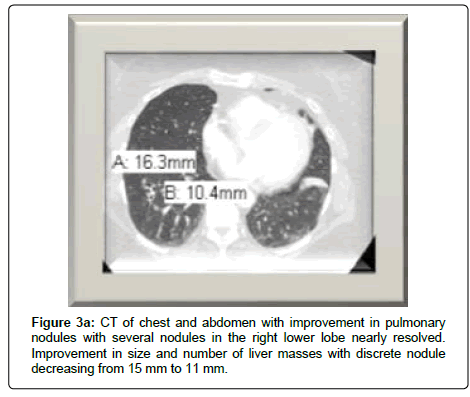
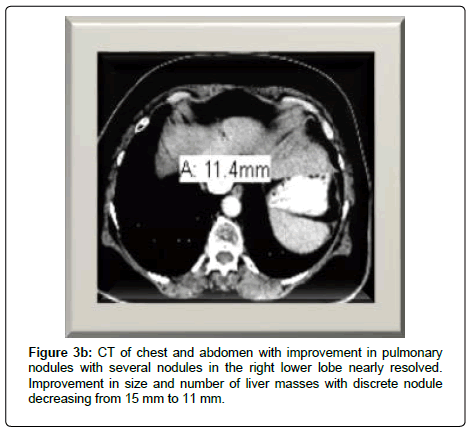
The patient’s clinical status improved and her persistent cough resolved. She no longer needed oxygen therapy and she was also able to enroll in an exercise program. A CT scan at week 18 showed continued improvement in pulmonary metastases with near resolution of several nodules. There was also decrease size and number of hepatic lesions. Pembrolizumab was tolerated well with only a grade I rash to arms (Figure 3).
Interestingly this patient’s tumor was PD-L1 negative. Tumors that express PD-L1 are felt to have better response to therapies that target PD-L1 function by blocking PD-L1 from inhibiting T-cells that attack cancer cells. [6,7].
Discussion
Metastatic ACC has limited treatment options and poor prognosis. The FIRM-ACT trial was the largest, randomized clinical trial to establish standard treatment for advanced disease. The results of this study showed EDP + mitotane had a 23% objective tumor response, median PFS of 5 months but failed to improve overall survival [3]. This chemotherapy regimen has the potential of serious adverse effects including myelosuppression, infection, thrombosis, neuropathy and decline in overall health [4].
The advancement of genomic molecular testing has shown ACC is biologically and genetically a heterogeneous cancer. Multiple early phase clinical trials to target the signaling of these genes have not demonstrated a significant clinical response [8]. Studies using immunotherapy have been recently completed in the setting of metastatic ACC (NCT02720484, NCT 0267333). Immunotherapy with anti-PD-1, PD-L1, and CTLA-4 (pembrolizumab, nivolumab, ipiluzumab) have shown efficacy in multiple advance cancers including melanoma, non-small-cell-lung cancer, and urothelial carcinoma [9-11]. New approvals and indications are occurring at a rapid rate. According to clinicaltrial.gov there are hundreds of open trials for immunotherapy and several that may affect how ACC is treated in the future [12]. Immunotherapy is in general well tolerated with majority of toxicities being grade 1 or 2 [13].
Conclusion
In summary, we present a case in which anti-PD-1 treatment with pembrolizumab made a dramatic impact on this patient’s tumor burden and rapidly improved her clinical status as well as her quality of life. This shows that anti-PD-1 immunotherapy may be effective treatment in metastatic ACC despite no expression of PD-1.
References
- Elfiky AA, Nair HK (2016) Assessment and management of advanced adrenocortical carcinoma using a precision oncology care model. Discovery Med 21: 133.
- Else T, Kim A, Sabolch A (2014) Adrenocortical Carcinoma. Endocr Rev 35: 2.
- Fassnacht M, Terzolo M, Allolio B (2012) Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med. 366: 2189-2197.
- Costa R, Carneiro BA, Tavora F (2016) The challenge of developmental therapeutics for adrenocortical carcinoma. Oncotarget 7: 29.
- Varghese J, Habra M (2017) Update on adrenocortical carcinoma management and future directions. Curr Opin Endocrinol Diabetes 24: 3.
- Grosso J, Horak C, Inzunza D (2016) Association of tumor PD-L1 expression and immune biomarkers with clinical activity in patients with advanced solid tumors treated with nivolumab. J Clin oncol 31: 15.
- Nick J (2018) Quest Diagnostic. FAQ: How does PD-L1 therapy work?
- Creemers S, Hofland L, Korpershoek E (2016) Future directions in the diagnosis and medical treatment of adrenocortical carcinoma. Endocrine-Related Cancer 23: 1.
- Larkin J, Chiarion-sileniv, Gonzalez R (2015) Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373: 1.
- Brahmer J, Reckamp KL, Baas P (2015) Nivolumab verus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373: 2.
- Bellmunt J, Wit R, Vaughn DJ (2017) Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 376: 1015.
- Leon L (2018) National Library of Medicine.
- Yang Y, Ding L, Wang P (2016) Dramatic response to anti-PD-1 therapy in a patient of squamous cell carcinoma of thymus with multiple lung metastases. J Thorac Dis 8: 7.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi