Case Report, Clin Oncol Case Rep Vol: 5 Issue: 12
Improved Survival Outcome with Flat Dosing Pembrolizumab (Pembroxim®) in Late-Stage Non-Small Cell Lung Cancer- A Case Report
Vijayakumar Narayanan*, Shane Ali Dungersi, James Mbogo
Department of Oncology, DrVJ's Oncology Associates Pvt Ltd, Nairobi, Kenya
*Corresponding Author: Vijayakumar Narayanan
Department of Oncology,DrVJ’s Oncology Associates Pvt Ltd, Nairobi, Kenya.
E-mail: drvijay@drvjoncologyassociates.co.ke
Received: December 16, 2022; Manuscript No: COCR-22-83774;
Editor Assigned: December 19, 2022; PreQC Id: COCR-22-83774 (PQ);
Reviewed: December 24, 2022; QC No: COCR-22-83774 (Q);
Revised: December 27, 2022; Manuscript No: COCR-22-83774 (R);
Published: December 29, 2022; DOI: 10.4172/cocr.5(12).266
Citation: Narayanan Vk, Dungersi SA, Mbogo J,Improved Survival Outcome with Flat Dosing Pembrolizumab (Pembroxim®) in Late-Stage Non-Small Cell Lung Cancer-A Case Report . Clin Oncol Case Rep 5:12
Abstract
Historically, advanced and metastatic non-small cell lung cancer had dismal prognosis. These patients were subjected to best supportive care which included, inter alia, symptomatic management and advance care planning to get the best out of whatever time that is remaining for them. However, the past decade has seen important advances in treatment and diagnosis which have translated into improved survival outcomes in such cases. The Introduction of targeted therapy and immunotherapy for selected patients had dramatically changed the outcome of such patients, both in life expectancy and quality of life.
Keywords: Non-small-cell lung cancer; Immunotherapy; Quality of life
Introduction
Global cancer statistics in 2020 reported 14,35,943 newly diagnosed cases of lung cancer among men worldwide and 770,828 cases among women. This amounts to 14.3% of all newly diagnosed cancers among men and 8.45% among women in 2020.Lung cancer remains the leading cause of cancer deaths , not only in many low to middle income countries but also in most of high-income regions like North America, Europe and Australia [1]. 85% of the newly diagnosed lung cancers were Non-small Cell lung cancer and 25%-30% are adenocarcinoma [2]. The median survival time of stage IV NSCLC patients was 10 months (9.5 months to 10.5 months). The median survivals of single or multiple metastasis were 11 months vs 7 months [3]. However ,widespread use of tyrosine kinase inhibitors in patients with specific gene mutations has made the survival outcomes better [4-6]. Advanced lung cancer treatment witnessed a lot of new development in the last one decade. In fact, the therapy underwent a paradigm shift with the advent of the small tyrosine kinase inhibitors and other targeted therapeutic drugs and the immunotherapeutic agents, thanks to better understanding of the tumour biology. Immune Checkpoint Inhibitors (ICIs) have shown tremendous benefit in the treatment of Non-Small Cell Lung Cancer (NSCLC) .They are now being used as first-line therapies in metastatic disease, consolidation therapy following chemoradiation in unresectable locally advanced disease, and adjuvant therapy following surgical resection and chemotherapy in resectable disease [7]. NCCN guidelines Version 2022, suggests the following molecular testing for advanced metastatic NSCLC adenocarcinoma: EGFR mutation, ALK, PD-L1 (all category 1), KRAS, ROS1, BRAF, NTRK1/2/3, METex14 skipping, RET, ERBB2 (HER2) [8].
Case Report
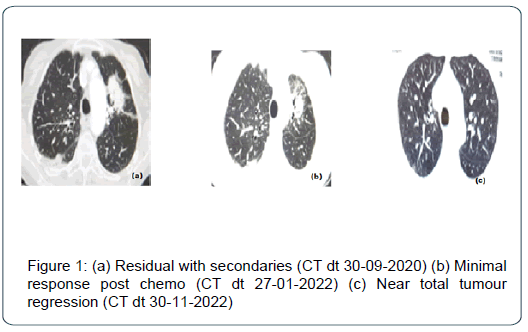
79-year-old lady, spinster, non-smoker, presented with productive cough, laboured breathing and left sided chest pain to a university hospital in Nairobi. She was diagnosed with left upper lobe adenocarcinoma by a CT guided biopsy in February 2020. Patient was subjected to metastatic work up and clinically staged s cT3 N2M1 ( Stage IV- UICC/AJCC 7th ed). The tumour was EGFR mutated, ALK negative , PD-L1-1%.The primary team put her on first line (Gefitinib 250 mg) Tyrosine Kinase Inhibitor (TKI) therapy which she could not tolerate due to excessive dermatological toxicity. The TKI changed to second line (Erlotinib 150 mg) and then on to third generation (Osimertinib 80 mg), all within a period of three months. Patient poorly tolerated the drug and progressed on the disease, sustained humerus fracture from skeletal secondary. She decided to change the primary team and reported to us. The pathological fracture was managed conservatively. She had moderate pleural effusion which was drained. Following radiotherapy to upper arm, she was given six cycles of Carboplatin Bevacizumab doublet therapy. Patient responded well with minimal side effects. On completion of therapy there was a 4.5 cm × 2.9 cm tumour on right upper lobe which was symptomatic, hence hypo fractionated focal radiotherapy was given. Patient asked for a therapy holiday for 3 months. Incidentally , she contracted with SARS-CoV-2 infection, but recovered well. She was started on ICI therapy with flat dosing Pembrolizumab 200 mg (Pembroxim® - Beacon Pharma limited). She completed 1 year therapy without any major adverse reactions. Post therapy assessment revealed near total tumour regression. Patient is currently on therapy holiday and enjoying her Christmas with family abroad (Figure 1).
Discussion
Histologic subtype is mandatory for any patient with recurrent or metastatic non-small cell carcinoma to select the best possible treatment. The selection of standard cytotoxic chemotherapy is dependent on the evidence-based recommendations, patient choices, possible side effects, tolerability and the prior treatments accessed by patient.
All patients with metastatic non-squamous NSCLC and NSCLC Not Otherwise Specified (NOS) should be subjected to molecular testing.
Squamous cell NSCLC has very few actionable mutations compared to non-squamous histology. Targeted therapies are recommended for patients with metastatic NSCLC with specific oncogenic drivers like EGFR exon 19 deletions, EGFR L858R, ALK. Such therapy yields higher response rates (e.g., Osimertinib, 80%). Targeted therapies are well tolerated [9].
Use of targeted therapy for eligible patients would decrease tumour burden, relieve the patient from symptoms thus improving the quality of life. The recommended targeted therapy for first line usage in patients with specific mutations are Afatinib, Alectinib, Brigatinib, Capmatinib, Ceritinib, Crizotinib, Dacomitinib, Entrectinib, Erlotinib, Gefitinib, Lorlatinib, Osimertinib, Parlsetinib, Selpercatinib and Tepotinib [10]. Some newer agents that are recommended for second line usage in patients with specific driver mutations are Amivantamab, Mobocertinib and Sotorasib. This list is expanding. EGFR TKI therapy provides the patients longer progression free survival, improvement of performance status, and the therapy response is better, as per the findings of ASPIRATION trial [11].
The most common treatment-related AEs included mild-tomoderate gastrointestinal symptoms (e.g., diarrhoea, nausea) and dermatological toxicity (e.g., rash, pruritus), decreased appetite, hypertension and weight loss. A patient who is on EGFR TKI progresses on the disease, change of treatment strategy is not always warranted if the patient is clinically stable. Even the documented tumour growth as per RECIST criteria is not enough to change the strategy. A decision to change the therapy should be taken carefully. Patient’s tolerance to therapy is very important. In advanced, metastatic lung cancer, patient satisfaction should be looked into. Symptomatic progression of the disease cannot be ignored.
The patient in the discussed case had disease progression. While making therapeutic decisions in any advanced stage disease, patient should be an integral part in the decision-making process. At one point patient remarked: “You are eighty years old, why should you be treated further? Why can’t you leave it to the nature?” was the question posed to me by my treating physician- But I did not want to give up. Life is beautiful. I am full of it. I did not want to spare any chance for my life to get prolonged. Hence, I decided to opt the therapy”.
She did not want hair loss, excess skin reactions which can alter her body image; she expressed her displeasure with the excessive skin reactions, corneal erosion and eyelash growth while on TKI therapy. Carboplatin Bevacizumab doublet was found to be an appropriate choice for the patient. Post therapy maintenance therapy with ICI was decided after elaborate discussion with the patient.
The introduction of agents that promote tumour recognition by the immune system by inhibiting signalling between the programmed death-1 receptor and Programmed Death Ligand 1 (PD-L1) and Programmed Death Ligand 2 (PD-L2) has been an important recent advance in the treatment of NSCLC [12]. Pembrolizumab is a monoclonal antibody that binds the programmed death-1 receptor and blocks its interaction with PD-L1 and PD-L2. Antitumour activity and acceptable toxicity of pembrolizumab monotherapy in patients with advanced NSCLC treatment naive and previously treated were first demonstrated in the phase I b KEYNOTE-001 study. Five-year outcomes from the KEYNOTE-001 study demonstrate that pembrolizumab provides long-term overall survival benefit and durable responses with tolerable safety for treatment naive and previously treated patients with advanced PDL1- expressing NSCLC [13]. The data from KEYNOTE-001 study paved the way for a novel management of selected patients with stage 4th advanced non-small cell lung cancer with Pembrolizumab monotherapy, which resulted in improved long-term outcome.
Conclusion
With the advent of newer therapeutic agents, there is a true paradigm shift in cancer therapy especially in late stage. Improvements in survival outcome and good quality of life can be expected. Appropriate selection of cytotoxic drugs, systemic targeted therapy, immunotherapy is the key in reaching this goal. There should be an attitudinal change in addressing the issues of a patient with advanced disease.
Acknowledgement
This report was supported by grant from Beacon Medicare Ltd, Dhakka, Bangladesh.
References
- Globocan U. (2020) New Global Cancer Data. [Google Scholar]
- Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, et al. (2012) Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 62: 220-241. [Google Scholar] [Cross Ref]
- Hong NG, Meili MA, Baohui HAN (2011) Survival analysis of 1,742 patients with stage IV non-small cell lung cancer. Zhongguo Fei Ai Za Zhi 14. [Google Scholar] [Cross Ref]
- Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, et al. (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13: 239-246. [Google Scholar] [Cross Ref]
- Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, et al. (2018) Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 378:113-125. [Google Scholar] [Cross Ref]
- Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, et al. (2014) First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 371: 2167-2177. [Google Scholar] [Cross Ref]
- Mamdani H, Matosevic S, Khalid AB, Durm G, Jalal SI (2022) Immunotherapy in lung cancer: Current landscape and future directions. Front Immunol 13: 823618. [Google Scholar] [Cross Ref]
- Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, et al. (2022) Non-small cell lung cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 20: 497-530. [Google Scholar] [Cross Ref]
- Mazieres J, Drilon A, Lusque A, Mhanna L, Cortot AB, et al. (2019) Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: Results from the IMMUNOTARGET registry. Ann of Oncol 30: 1321-1328. [Google Scholar] [Cross Ref]
- Hanna N, Johnson D, Temin S, Baker Jr S, Brahmer J, et al. (2017) Systemic therapy for stage IV non-small-cell lung cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. [Google Scholar] [Cross Ref]
- Park K, Ahn M, Yu C, Kim S, Lin M, et al. (2014) ASPIRATION: First-line erlotinib (E) until and beyond RECIST progression (PD) in Asian patients (pts) with EGFR mutation-positive (mut+) NSCLC. Ann Oncol 25: 426. [Google Scholar] [Cross Ref]
- Brahmer JR, Govindan R, Anders RA, Antonia SJ, Sagorsky S, et al. (2018) The society for immunotherapy of cancer consensus statement on immunotherapy for the treatment of non-small cell lung cancer (NSCLC). J Immunotherapy Cancer 6: 1-15. [Google Scholar] [Cross Ref]
- Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, et al. (2019) Five-year overall survival for patients with advanced non-small-cell lung cancer treated with pembrolizumab: Results from the phase I KEYNOTE-001 study. J Clin Oncol 37: 2518. [Google Scholar] [Cross Ref]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi