Research Article, Vector Biol J Vol: 3 Issue: 2
Insecticidal activity of Green Synthesized silver Nanoparticles using Coleus aromaticus and Wrightia tinctoria Leaf Extracts against Culex quinquefasciatus
1Annai Vailankanni Arts and Science College, Thanjavur, Tamil Nadu, India
2PG and Research Department of Zoology, Rajah Serfoji Government College, Thanjavur, Tamil Nadu, India
*Corresponding Author : AK Dass
PG and Research Department of Zoology, Rajah Serfoji Govt College (Autonomous), Thanjavur-613005, Tamilnadu, India
E-mail: kdassrsgc1987@gmail.com
Received: July 14, 2018 Accepted: August 13, 2018 Published: August 28, 2018
Citation: Dass AK, Mariappan P (2018) Insecticidalactivity of Green Synthesized silver Nanoparticles using Coleus aromaticus and Wrightia tinctoria Leaf Extracts against Culex quinquefasciatus. Vector Biol J 3:2. doi: 10.4172/2473-4810.1000131
Abstract
Background
Mosquitoes transmit dreadful diseases, causing millions of deaths every year. The use of synthetic insecticides to control vector mosquitoes has caused physiological resistance and adverse environmental effects in addition to high operational cost. The plant extracts seem to be a better alternative to control mosquitoes due to the presence of many bioactive compounds. In addition, the green synthesized silver nanoparticles have a vital application in biological research. The present study addresses the green synthesized silver nanoparticles (AgNPs) of Coleus aromatics and Wrightia tinctoria leaf extract and their larvicidal and pupicidal activity against Culex quinquefasciatus.
Methods
The synthesized AgNPs were characterized by UV-Vis spectrum, Scanning Electron Microscopy (SEM), Fourier Transform Infrared Spectroscopy analysis (FTIR) and XRD measurements. The II, III, IV instar and pupa of C. quinquefasciatus were exposed to different concentration of silver nanoparticles (5 ppm to 50 ppm) for 24 hours.
Results:
LC50 values of II, III, IV instars and pupa of C. quinquefasciatus were 13.37 ppm and 16.26 ppm, 36.07 ppm and 41.47 pp. LC90 values are 35.47 ppm, 53.57 ppm and 67.60 ppm, 76.68 ppm against Coleus aromatics. Likewise, Wrightia tinctoria LC50 values were 14.56 ppm, 18.77 ppm, 42.76 ppm and 51.06 ppm and LC90 values were 46.03 ppm, 62.17 ppm, 79.89 ppm and 93.16 ppm.
Conclusion:
Biosynthesis of silver nanoparticles using leaf aqueous extract of Coleus aromaticus, Wrightia tinctoria provides a potential source for the larvicidal and pupicidal activity against Cx. quinquefasciatus. Between the two plants studied, the LC50 and LC90 values indicate that Coleus aromatics synthesized silver nanoparticles were more effective than Wrightia tinctoria.
Keywords: Larvicidal activity; Culexquin quefasciatus; Green synthesized silver nano-particles
Introduction
Mosquitoes are important group of insects in terms of public health. Malaria, Filaria, Japanese Encephalitis, Dengue Fever, Heamorragic Fever, Yellow Fever, Chikungunya and Zika fever are the most dreadful diseases transmitted by the mosquitoes that lead to millions of deaths every year [1,2]. C. quinquefasciatus is one such mosquito species that spread disease in an estimated population of 120 million, among these 44 million people have chronic manifestation [3,4]. Repeated use of synthetic insecticides in controlling the mosquitoes have disrupted the natural biological control systems and led to resurgences of mosquito population [5-7]. This has also resulted in the development of resistance, undesirable effects on non-target organisms [8-10]. Bio-pesticides are an alternate for chemical insecticides as it is long lasting, low cost, eco-friendly and harmless to non-target organisms [11,12]. In the present study to enhance the activity of plants derived bioactive compound using modern technique adapted by synthesis of nano-particles using silver, copper, gold and zinc due to their unique physical, chemical and biological properties [13]. In this regard, an attempt was made to find out the differences in the larvicidal properties of green synthesized silver nanoparticles of plants such as Coleus aromatics and Wrightia tinctoria leaves against filarial vector C. quinquefasciatus.
Materials and Methods
Collection of plant materials
The leaves of Coleus aromatics (Lamiaceae) and Wrightia tinctoria (Apocynaceae) leaves collected from Karambayam village (10.49°N, 79.30°E), Thanjavur district, Tamilnadu, India.
Preparation of plants extract
Fresh leaves were thoroughly washed with distilled water. 25 g leaves were taken and crushed with 100 ml sterile distilled water. The extract was filtered through Whatman No.1 filter paper (Pore size 25 μm). The filtrate was further filtered using a 0.6 μm sized filters [14].
Synthesis of silver nanoparticles
2.0 mM aqueous solution of silver nitrate (AgNO3) used for the synthesis of silver nanoparticles. 10 ml of leaf extract was added to 90 ml of aqueous solution of 2 mM silver nitrate for reduction of Ag+ ions and kept at room temperature for a period 5 hours. As a result, a brown solution is formed which indicates the formation of silver nanoparticles. The sample was centrifuged at 5000 rpm for 20 min and the resulting suspension was redispersed in 10 ml sterile distilled water. Centrifugation and redispersion process was repeated three times. The purified suspension was freeze dried to obtain dried powder. The dried nanoparticles were used for further analysis [15,16].
Characterization of silver nanoparticles
UV-Vis Spectra Analysis: UV-Vis spectra analysis is an important technique to preview the morphology and stability of nanoparticles. The reduction of pure Ag+ ions was monitored by measuring the UV-Vis spectrum (UV-3024) of the reaction medium after 5 hours diluting a small aliquot of the sample into distilled water.
SEM Analysis of Silver Nanoparticles: Scanning Electron Microscopic (SEM) analysis was done using Vega 3 Tescan SEM machine. Thin film of the sample was made on carbon coated copper grid by just dropping a small amount of the sample, extra solution was removed by using a blotting paper and the film formed on the SEM grid was allowed to dry by keeping under a mercury lamp for 5 min [17].
Fourier transform infrared spectroscopy (FTIR) analysis
FTIR analysis was carried out at the central instrumentation facility of St. Joesph’s College, Tiruchirappalli, Tamilnadu. Two milligram of silver nanoparticle was prepared by mixing with 200 mg of spectroscopic grade KBr. FTIR spectra were recorded using a Nicolet 520 P spectrometer with detector at 4000-400 cm-1 resolution and 20 scans per sample [18].
XRD-measurements
Green synthesized silver nanoparticles were determined by an X-pert pro x-ray diffractometer (Rigaku Ultima III XRD) operated at a voltage of 40 kV and a current of 30 mA with Cu K α radiation in a θ-2θ configuration. The crystallite domain size was calculated from the width of the XRD peaks, assuming that they are free from nonuniform stains, using the Debye Scherrer’s formula.
D=0.94λ/β Cos θ
Where D is the average crystalline domain size perpendicular to the reflecting planes, λ is the X-ray wavelength, β is the full width at half maximum (FWHM), and θ is the diffraction angle [18].
Preparation of stock solution and test concentration
50 mg of green synthesized silver nano-particles first dissolved in 10 ml of distilled water this solution used for experiment and the make the test concentration 5 ppm, 10 ppm, 20 ppm, 30 ppm, 40 ppm and 50 ppm respectively.
Culture of test animal
Filarial vector, C. quinquefasciatus egg rafts were collected from stagnant sewage water of Thanjavur. The hatched larvae were cultured and maintained in the laboratory at room temperature (27°C ± 2°C) and 75%–85% relative humidity. The larvae were fed with dog biscuits and yeast powder in the ratio 3:1. Adults emerged from these larvae were reared in mosquito cage and the females were allowed to feed on avian (pigeon) blood and the males were provided with 10% glucose solution socked in cotton. Sample from this parent population was used to confirm the species by The District Entomologist of Malarial Control Program, Thanjavur. Eggs laid by these adults were cultured in a separate container and larvae developed from these eggs were used for bioassay studies [19,20].
Larvicidal and pupicidal bioassay
Mosquito larvicidal bioassay was carried out according to WHO [21] Standard procedure with slight modification. 200 ml of was it dechlorinated water was taken in a series of 250 ml beakers. The concentration starting 5 ppm to 50 ppm of silver nanoparticles of Coleus aromatics and Wrightia tinctoria were used for the experiment. A control was also maintained separately by adding now the total volume become 202 ml. was it the case with experimental insects also. 10 larvae per concentration were used for all the experiments. The number of dead larvae at the end of 24 h was recorded and the percent corrected mortality was calculated by using Abbott’s formula [22]. The mortality rates of C. quinquefasciatus pupae were estimated in the same manner.
Statistical analysis
LC50 and LC90 data for the mortality and the relationship between concentration and mortality (dose dependent mortality) were subjected to regression analysis and a 95% confidential limited was calculated by probit analysis [23]. ANOVA was performed to find out the relationship between the green synthesized silver nanoparticles and larval mortality. SPSS 16 [24] version was used for doing the statistical analysis (Tables 1 and 2).
| Larval stages | LC50 (ppm) (UCL-LCL) | LC90 (ppm) (UCL-LCL) | x2 | Regression equation |
|---|---|---|---|---|
| Coleus aromatics | ||||
| II instar | 13.37 (17.70–11.65) |
35.47 (39.80–3.75) |
3.250 | Y=1.78+0.18X |
| III instar | 16.26 (19.91–14.95) |
53.47 (39.80 –33.75) |
2.381 | Y=1.42+0.16X |
| IV instar | 36.07 (42.31–34.49) |
67.60 (77.08–4.86) |
.530 | Y=-1.24+0.16X |
| Pupa | 41.47 (51.49–39.21) |
76.68 (82.92–5.10) |
.258 | Y=-1.31+0.12X |
| Wrightia tinctoria | ||||
| II instar | 14.56 (45.28–13.17) |
46.03 (49.75–44.64) |
1.018 | Y=1.62+0.17X |
| III instar | 18.77 (22.41–17.49) |
62.17 (65.81-60.97) |
1.257 | Y=0.99+0.16X |
| IV instar | 42.76 (50.65–41.22) |
79.89 (87.78–78.35) |
.031 | Y=-1.46+0.14X |
| Pupa | 51.06 (59.26–50.04) |
93.16 (101.95–92.14) |
.376 | Y=-1.256+0.10X |
Table 1: The LC50 and LC90 values of Coleus aromaticus and Wrightia tinctoria synthesised silver nano-particle against the II, III, IV instar and pupa of Culex quinquefasciatus after 24 h of exposure.
| Source of variation | SS | df | F | P-Value | F-crit |
|---|---|---|---|---|---|
| Green synthesized silver nanoparticles | 49.60 | 1 | 6.69 | 0.081 | 10.12 |
| Larval stages | 1527.13 | 3 | 68.72 | 0.002 | 9.27 |
| Error | 22.21 | 3 | 2.29 | ||
| Total | 1598.95 | 7 |
Table 2: Two way ANOVA to test the validity of relationship in mortality (LC50) as a function of two plants green silver nano-particles and larval and pupa.
Results
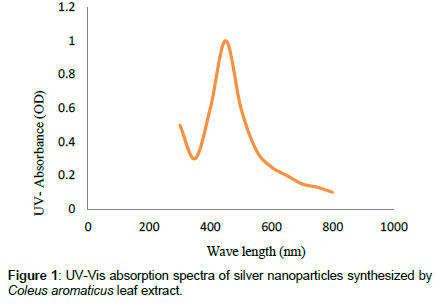
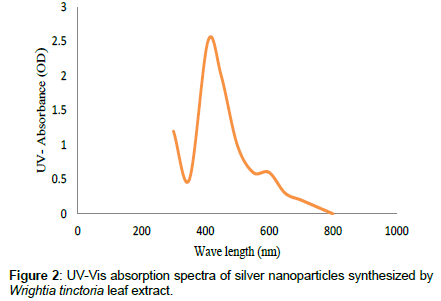
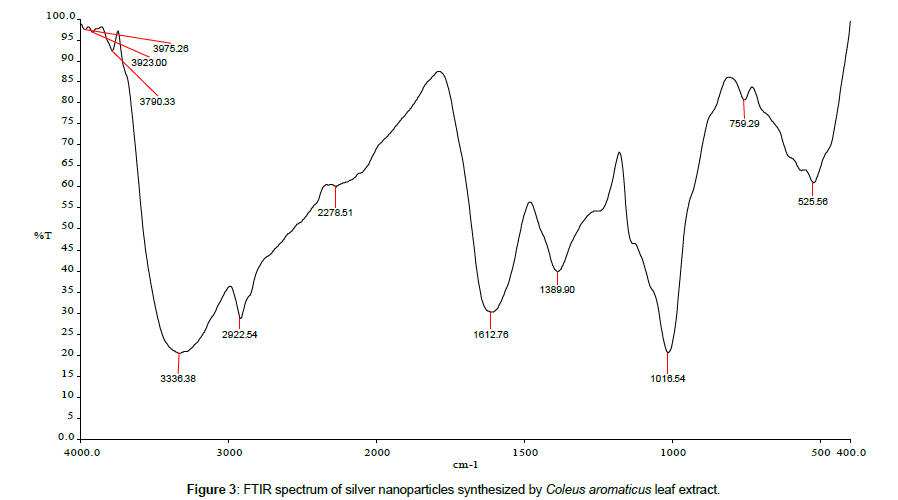
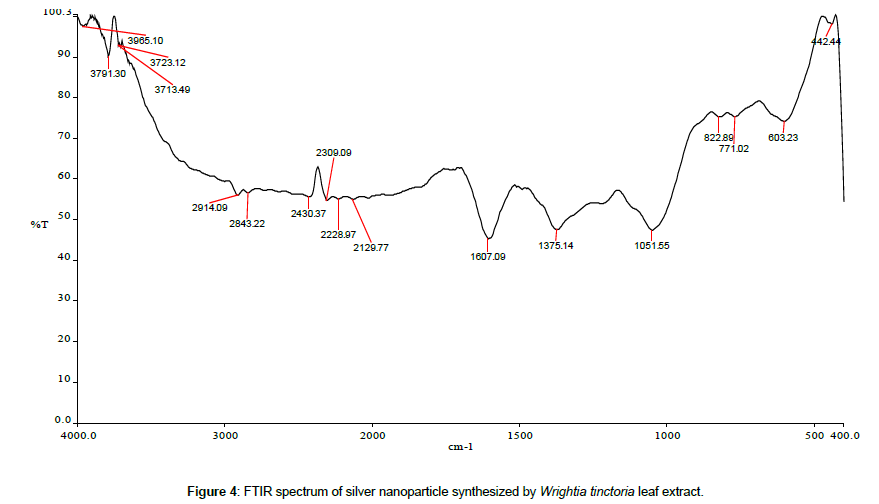
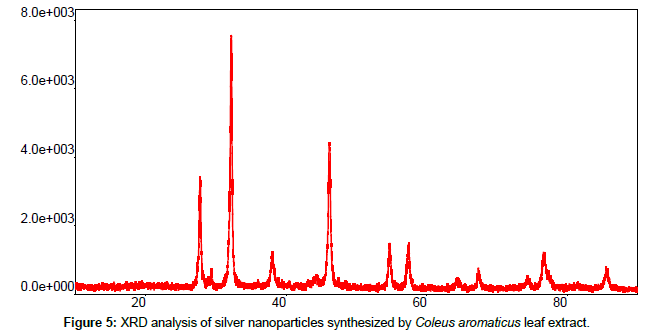
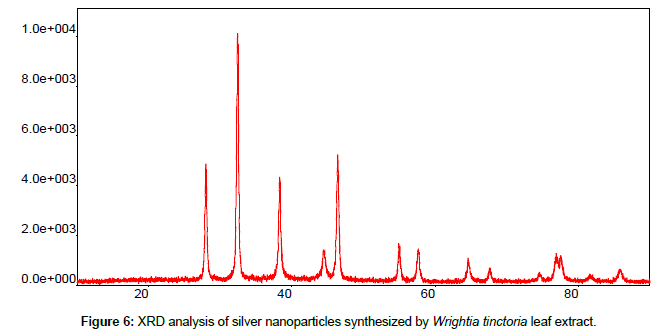
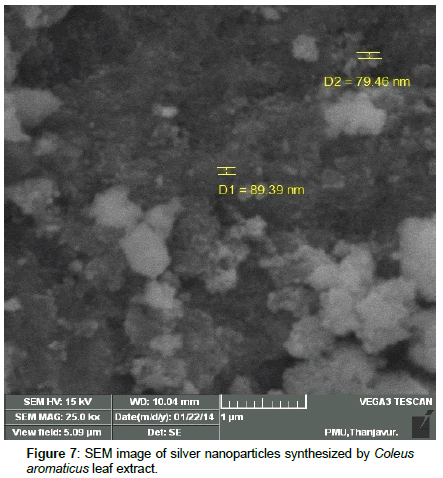
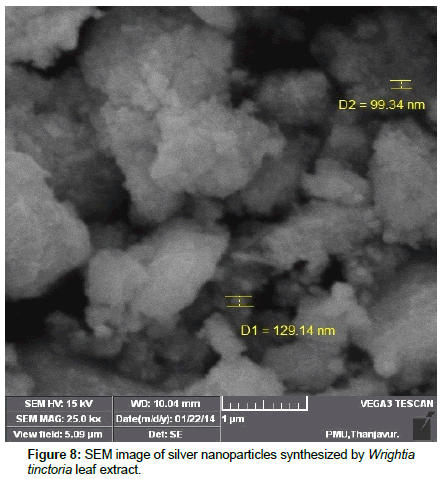
Leaves extracts of C. aromatics and W. tinctoria were filtered and treated with 2 m M AgNO3 solution and incubated at room temperature for 5 hours. The colour of the solution was changed into dark brown that this indicates confirmed that formation of nanoparticles. Further the synthesis of silver nano-particles is confirmed by UV-Visible absorption by spectroscopy. The peak was seen at 441 nm for C. aromatics whereas for W. tinctoria it was obtained at 418 nm (Figures 1 and 2). FTIR of synthesized silver nanoparticles was observed at 3336, 2922, 2278, 1612, 1389, 1016, 759, 525 cm-1 for C. aromatics and 2914, 2843, 2430, 2309, 2228, 2129, 1607, 1375, 1051,822, 771, 603, 442 cm-1 for W. tinctoria (Figures 3 and 4). In X-ray diffraction the characteristic peaks as exhibited between 20 and 80, where a distinct diffraction peak was obtained at 27°, 32°, 46°, 57° at 2θ values indexed to 220, 122, 220, 241 respectively for C. aromatics whereas W. tinctoria the peaks were obtained at 27°, 32°, 38°, 46° and 57° to a corresponding sets of lattice planes to 220, 122, 111, 200, and 241 respectively (Figures 5 and 6). Scanning Electron Microscopic (SEM) image analysis showed a clear shape and size of the AgNPs for C. aromaticus (79.46 nm, 89.39 nm) and W. tinctoria (99.34 nm,129.149 nm) (Figures 7 and 8). XRD analysis reveals the nature of the green synthesized silver nanoparticles (Tables 3 and 4).
| No. | 2-theta (deg) | D (ang.) | Height (cps) | FWHM (deg) | Int. I (cpsdeg) | Int. W (deg) | Asym. factor |
|---|---|---|---|---|---|---|---|
| 1 | 27.747(7) | 3.2125 | 2904 | 0.278 | 1326 | 0.46 | 0.63 |
| 2 | 32.165(4) | 2.7806 | 6550 | 0.283 | 3038 | 0.46 | 0.64 |
| 3 | 46.144(9) | 1.9656 | 3624 | 0.335 | 1910 | 0.53 | 0.68 |
| 5 | 57.39(3) | 1.6043 | 861 | 0.45 | 521 | 0.61 | 0.9 |
Table 3: XRD patterns of Coleus aromaticus synthesized silver nano-particles.
| No. | 2-theta (deg) | D (ang.) | Height (cps) | FWHM (deg) | Int. I (cps deg) | Int. W (deg) | Asym. factor |
|---|---|---|---|---|---|---|---|
| 1 | 27.983(9) | 3.1860 | 3140 | 0.294 | 1296 | 0.41 | 1.10 |
| 2 | 32.400(6) | 2.7610 | 6849 | 0.288 | 2663 | 0.389 | 1.14 |
| 3 | 38.275(14) | 2.3496 | 2812 | 0.305 | 1339 | 0.48 | 1.1 |
| 5 | 46.349(10) | 1.9574 | 3472 | 0.339 | 1512 | 0.44 | 0.74 |
| 7 | 57.58(2) | 1.5994 | 881 | 0.391 | 433 | 0.49 | 0.77 |
Table 4: XRD patterns of Wrightia tinctoria synthesized silver nano-particles.
Results of larvicidal bioassay of C. aromaticus and W. tinctoria synthesized AgNPs against II, III, IV instars and pupa of C. quinquefasciatus are given in Table 1. LC50 values were13.37 ppm and 16.26 ppm, 36.07 ppm and 41.47 ppm and the LC90 values are 5.47 ppm, 53.57 ppm and 67.60 ppm, 76.68 ppm for C. aromatics. Likewise, W. tinctoria synthesized silver nano-particles LC50 values were 14.56 ppm and 18.77 ppm, 42.76 ppm and 51.06 ppm and LC90 values are 46.03 ppm, 62.17 ppm, 79.89 ppm and 93.16 ppm against Cx. quinquefasciatus.
From the present study it is found that II instar of C. quinquefasciatus has more susceptible to green synthesized silver nanoparticles (P<0.05) than other larval stages for two plants. C. aromaticus was more effective than W. tinctoria nano-particles used. A two way analysis of variance was performed to find out the difference among the two plant species and larval stages on mortality and the analysis indicate that there is a significant difference (P<0.05) (Table 2) existing between the larval mortality as a function of larval stages and green synthesized silver nano-particles.
Discussion
The effect of green synthesized silver nanoparticles on the adult emergence was also studied. Though there was a reduction in the rate of adult emergence as a function of concentration, there was no significant difference among the green synthesized silver nanoparticles. Green synthesized nanoparticles are much popular as larvicide in controlling mosquitoes and most of these studies prefer silver nano-particles [25-27] while other metals like Copper [28], Zinc [29], Titanium [30], Magnesium [31] and Gold [32] are also in use.
A comparative study on the larvicidal activity of Nelumbo nucifera leaf crude extract and silver nanoparticles was performed against the malarial and filarial vectors. LC50 values obtained for crude methanol, aqueous, and synthesized silver nanoparticles against the larvae of A. subpictus are 8.89, 11.82, and 0.69 ppm respectively while the LC90 values are 28.65, 36.06, and 2.15 ppm. In the case of C. quinquefasciatus, the LC50 values are 9.51, 13.65, and 1.10 ppm and the LC90 values are 28.13, 35.83, and 3.59 ppm for methanol, aqueous and silver nanoparticles respectively. From this it is known that green synthesized silver nanoparticles more toxic to malarial and filarial vectors than other extracts [33].
Similarly, Gnanadesigan [26] reported that the control Ae. aegypti and C. quinquefasciatus larvae using Rhizophora mucronata F. In this study also green synthesized nanoparticles are toxic to C. quinquefasciatus (LC50=0.585 mg/l) than Ae. aegypti (LC50=0.891 mg/l). Nano-particles synthesized using fungi and bacteria such as Afaricus bisporus, Penicilium spp, E. coli and Vibrio sp were also reported and the results indicate that A. bisporus synthesized nanoparticles is promising in controlling C. quinquefasciatus [34]. To support this work, Borase [17] reported bio-synthesized nanoparticles of Jatropha gossypifolia, Euphorbia tirucalli, Padilanthus tithymaloides and Alseuosmia macrophylla against IV instar of Ae. aegypti and An. stephensi. Of these nanoparticles, nanoparticles synthesized using J. gossypifolia was effective against Ae. aegypti (LC50=4.44 ppm) and An. stephensi (LC50=4.90 ppm).
Green synthesized silver nanoparticle using Adhatoda vasica was tested against C. quinquefasciatus with a LC50 value of 450.03 ppm for III instar [35]. Velayutham and Ramanibai [36] studied the larvicidal activity of green synthesized silver nanoparticles of Annonasquamosa leaves against the larvae of C. quinquefasciatus. The LC50 values obtained for the first to fourth instar larvae are 2.50, 2.78, 3.02, 3.05 μg/ml respectively. Likewise Adesuji [17] studied the effect of biosynthesized silver nanoparticles (AgNPs) of Cassia hirsute aqueous leaf extracts against C. quinquefasciatus where the LC50 value is 4.43 ppm for fourth instar larvae. Larvicidal activity of silver nanoparticles synthesized by using the leaf extracts of Azadirachta indica was tested against C. quinquefasciatus. The results of this study indicates its highest toxicity is LC50=500 ppm [35].
The relationship between concentration and adult emergence is also studied and the results indicate that irrespective of the plant species, when the concentration is increased, there is reduction in adult emergence. Though there is a reduction in the rate of adult emergence as a function of concentration, there is no significant difference among the two plants silver nanoparticles studied. Such studies indicate the enhanced activity of leaf extracts by applying nanotechnology which is reviewed recently by Mondal [27] and Benelli [37]; Elemike [38] and Khader [39].
Conclusion
In the present study, we have synthesized green silver nanoparticles using C. aromaticus and W. tinctoria leaf extract. These green synthesized AgNPs were characterized using UV-Vis, XRD, FTIR and SEM and evaluated for their larvicidal activity against C. quinquefasciatus larvae and pupa. Of the two plant extracts used, C. aromaticus has more effective than W. tinctoria. Hence these green synthesized plant extracts has potential to be used as larvicidal agent in mosquito bio-control program.
References
- Tolle MA (2009) Mosquito-borne disease. Curr Probl Pediatr Adolesc Health Care 39: 97-140.
- Chan FW, Garnet KY, Choi D, Cyril CY, Yip D, et al. (2016) Zika fever and congenital Zika syndrome: An unexpected emerging arboviral disease. J Infect 72: 507-524.
- WHO (2002) Lymphatic filariasis-the disease and its control. World Health Organization,Geneva, Switzerland.
- Bernhard L, Bernhard P, Magnussen P (2003) Management of patients with lymphoedema caused by filariasis in North-eastern Tanzania: Alternative approaches. Physiother 89:743- 749.
- Anyaele OO, AmusanAAS (2003) Toxicity of hexanoic extracts of Dennettiatripetala (G.Baxer) on larvae of Aedes aegypti (L). Afri J Biomed Res 6: 49-53.
- Mohapatra B, Kuriakose S, Mohapatra S (2015) Rapid green synthesis of silver nanoparticles and nanorods using Piper nigrum extract. J Allo Comp 637: 119–126.
- Adesuji ET, Oluwaniyi OO, Adegoke HI, Moodley R (2016) Investigation of the larvicidal potential of silver nanoparticles against Culex quinquefasciatus: A Case of a Ubiquitous Weed as a Useful Bioresource. J Nanomaterials.1-11.
- Brown AWA (1986) Insecticide resistance in mosquito’s pragmatic review. J Am Mosq Control Assoc 2:123-140.
- Govindarajan M (2010) Larvicidal efficacy of Ficusbenghalensis L plant leaf extracts against Culex quinquefasciatus Say Aedes aegypti L and Anopheles stephensi L (Diptera: Culicidae). Eur Rev Med Pharmacol Sci 14:107-111.
- Dass K, Mariappan P (2016) Larvicidal activity of Colocasiaesculendum, Ecliptaprostrataand Wrightiatinctorialeaf extract against Culex quinquefasciatus. Proc Natl Acad Sci India Sect B Biol Sci 86:139-143.
- Pavela R (2009) Larvicidal effects of some Euro-Asiatic plants against Culex quinquefasciatus Say larvae (Diptera: Culicidae). Parasitol Res 105: 887-892.
- Ghosh A, Chowdury N, Chandra G (2012) Plant extracts as potential mosquito larvicides. Indian J Med Res 35: 581-598.
- Nikhil SS, Bule M, Bhambure R, Singha S, Singh SK, et al. (2009) Biosynthesis of silver nanoparticles using aqueous extract from the compaction producing fungal strain. Process Biochem 44:939-943
- Salunkhe RB, Patil SV, Patil CD, Salunke BK (2011) Larvicidal potential of silver nanoparticles synthesized using fungus Cochliobolus lunatus against Aedes aegypti (Linnaeus, 1762) and Anopheles stephensi Liston (Diptera; Culicidae). Parasitol Res 109: 823-831.
- Suganya A, Murugan K, Kovendan K, Kumar MP, Hwang JS (2013) Green synthesis of silver nanoparticles using Murrayakoenigii leaf extract against Anopheles stephensi and Aedes aegypti. Parasitol Res 112:1385-1397.
- Ramanathan A, Shanmugam V, Raghuchander T, Samiyappan, R(2002) Induction of systematic resistance in ragi against blast disease by Pseudomonas flourescens.Ann Pc Port Soc 10: 313-318.
- Borase HB, Patil CD, Salunkhe RB, Narkhede CP, Salunke BK, Patil SV(2013)Phyto-synthesized silver nanoparticles: A potent mosquito biolarvicidal agent. Nanomed. Biothera Dis 3:1-7.
- Rajkumar G, Rahuman AA (2011) Larvicidal activity of synthesized silver nanoparticles using Ecliptaprostrataleaf extract against filariasis and malaria vector. Acta Trop 188: 196-203.
- Kuppusamy C, Murugan K (2009) Mosquitocidal effect of Andrographis paniculatanees against the malaria vector, Anopheles stephensi Liston (Diptera: Culicidae). Inter J Integrative Biolo5: 75- 81.
- Arivoli S, John Ravindran K, Raveen R, Tennyson, S (2012) Larvicidal activity of botanicals against the filarial vector Culex quinquefasciatus say (Diptera: culicidae). Inter J Res Zoo 2: 13- 17.
- WHO (1992) Vector resistance to pesticides, Fifteenth report of the WHO Expert Committee of Vector Biology and Control. WHO Tech Rep Ser 818:1-62.
- Abbott A (2012) A method of computing the effectiveness of insecticides. J Econ Entomol 18: 267-269.
- Finney DJ (1971) In: Probit Analysis. Cambridge University Press, London, United Kingdom.
- SPSS (2007) SPSS for windows, version 11.5. SPSS Chicago, USA.
- Prakash A, Seema S, Naheed A, Ashok G, Preety S (2011) Synthesis of AgNPs by Bacillus cereus bacteria and their antimicrobial potential. J Biomaterial Nanobiotech 2:156-162.
- Gnanadesigan M, Anand M, Ravikumar S, Maruthupandy M, Vijayakumar V, et al. (2011) Biosynthesis of silver nanoparticles by using mangrove plant extract and their potential mosquito larvicidal property. Asian Pac J Trop Med 799-803.
- Mondal NK, Chowdhury A, Dey U, Mukhopadhya PP, Chatterjee S, et al. (2014) Green synthesis of silver nanoparticles and its application for mosquito control. Asian Pac J TropDis 4: 204-210.
- Mondal NK, Hajra A (2016) Synthesis of copper nanoparticles (CuNPs) from petal extracts of marigold (Tagetes sp.) and sunflower (Helianthus sp.) and their effective use as a control tool against mosquito vectors. J Mosq Res 6: 1-9.
- Vijayakumar S, Vinoj G, Malaikozhundan B, Shanthi S, Vaseeharan B (2015) Plectranthusamboinicus leaf extract mediated synthesis of zinc oxide nanoparticles and its control of methicillin resistant Staphylococcus aureus biofilm and blood sucking mosquito larvae. Spectrochim Acta A Mol Biomol Spectrosc. 25; 137:886-891.
- Murugan K, Dinesh D, Kavithaa K, Paulpandi M, Ponraj T, et al. (2016) Hydrothermal synthesis of titanium dioxide nanoparticles: Mosquitocidal potential and anticancer activity on human breast cancer cells (MCF-7). Parasitol Res 115:1085-1096.
- Rao KG, Ashok CH, Rao KV, Chakra CH, Akshaykranth A (2015) Eco friendly synthesis of MgO nanoparticles from orange fruit waste. Inter J Adv Res Phy Sci 2:1-6
- Soni N, Prakash S (2012) Synthesis of gold nanoparticles by the fungus Aspergillus niger and its efficacy against mosquito larvae. Rep Parasitol 2:1-
- Santhoshkumar T, Rahuman AA, Rajakumar G, Marimuthu S, Bagavan A (2011) Synthesis of silver nanoparticles using Nelumbonucifera leaf extract and its larvicidal activity against malaria and filariasis vectors. Parasitol Res 108:693-702.
- Dhanasekaran D, Thangaraj R (2013) Evaluation of larvicidal activity of biogenicnanoparticles against filariasis causing Culex mosquito vector. Asian Pac J Trop Dis 3:174-179
- Subashini K, Ramesh N, Jeyasankar A(2015) Larvicidal activity of silver nanoparticle synthezed by the leaf extracts of Adhatodavasica against Culex quinquefasciatus (Say) (Diptera: Culicidae). Inter Res J Nat Appl Sci 2:32-51
- Velayutham K, Ramanibai R (2016) Larvicidal activity of synthesized silver nanoparticles using isoamyl acetate identified in Annonasquamosa leaves against Aedes aegypti and Culex quinquefasciatus. J Basic Appl Zool 74: 16-22.
- Benelli G,Caselli A, Canale A (2017) Nanoparticles for mosquito control: Challenges and constraints. J King Saudi Univ Sci 29 :424-435
- Elemike EE, Onwudiwe DC, Ekennia AC, Sonde C, Ehiri RC (2017) Green synthesis of Ag/Ag2O nanoparticles using aqueous leaf extract of eupatorium odoratum and Its antimicrobial and mosquito larvicidal activities. Molecules 2017; 22:1-15
- Khader SZA, Ahmed SSZ, Sathyam J, Manboob MR, Venkatesh KP, et al.(2018) comparative study on larvicidal potential of selected medicinal plants over green synthesized silver nano particle. Egypt J Bas App Sci 5: 54-62.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi