Research Article, Clin Res Orthop Vol: 2 Issue: 1
Medical Treatment of Bone Tissue Metabolism Disorders in Patients with Vitamin-D Resistant Rickets
Martsyniak S* and Kincha-Polishchuk T
State institution, Institute of Traumatology and Orthopedics of the National academy of the medical sciences of Ukraine, Kyiv, Ukraine
*Corresponding Author : Martsyniak S
State institution, Institute of Traumatology and Orthopedics of the National academy of the medical sciences of Ukraine, Kyiv, Ukraine
Tel: +38044 234-99-92
E-mail: stiv25@bigmir.net
Received: December 29, 2017 Accepted: February 27, 2018 Published: March 06, 2018
Citation: Martsyniak S, Polishchuk K (2018) Medical Treatment of Bone Tissue Metabolism Disorders in Patients with Vitamin-D Resistant Rickets. Clin Res Orthop 2:1.
Abstract
Objective: To determine the effect of medical therapy upon genetically determined disorders of bone tissue metabolism in patients with D-resistant rickets.
Materials and Methods: In the consultative out-patient department of the Institute of Traumatology and Orthopedics of the NAMSU, 39 patients who had been diagnosed with VDRR (vitamin D-resistant rickets, phosphate-diabetes), were examined and treated. Medical treatment of the patients was carried out in several stages. Stage 1 ncluded a complete patient examination to determine the level of calcium and phosphorus in blood and urine, calcitriol and calcitriol in blood, parathyroid hormone and osteocalcine parameters as well as the marker of bone formation P1NP and that of osteoresorption B-CTx. At the first stage, children were obliged to undergo a genetic study. The aim of the study was to detect changes (polymorphism) in receptor alleles to vitamin D (VDR) and collagen type 1 (COL1).
The examination at the next stages was conducted entirely, in addition to genetic studies.
Results: Comprehensive study of vitamin D metabolism and biochemical parameters of bone function in patients with VDRR, including the formation of the organic basis of bone, allowed us to examine thoroughly some issues of pathogenesis and the essence of osteomalacia and later osteoporotic changes of different degrees.
Depending on these changes, it was possible to develop different regimens of medical correction of bone metabolism disorders in phosphate-diabetes.
Conclusion: The treatment of orthopedic VDRR should begin with 60 000 units of vitamin D, 12 μg of alpha-calcidol and 18 g of calcium glycerophosphate per month. In 3 months of treatment, a re-examination of bone metabolism should be performed, resulting in alterations of vitamin D or the hormonal form of vitamin D
alpha-calcidol intake. The calcium-phosphorus mixture should be consumed by children in an average dose 600-800 mg per day.
Keywords: D-resistant rickets; Phosphate-diabetes; Hereditary phosphatemia; Rickets; Vitamin D metabolism; Calcidiol; Calcitriol; Deformations of lower extremities in children
Introduction and Background
For the first time, the vitamin D-resistant rickets (VDRR, phosphate-diabetes, hereditary phosphatemia) were separated by McCune in 1935. More deeply, the characteristic features of the disease and its some metabolic aspects were studied and clarified by F. Albright, A.M. Butler, E. Bloomberg in 1937 [1]. The X-linked type of heredity was described by R.W. Winters with co-authors in 1958 [2].
Phosphate diabetes develops due to congenital breakdown of encoding gene phosphate-regulating protein (endopeptidase class) (Grieff M., 1997), that in turn affects the processes of phosphate reabsorption in renal tubules thus causing phosphateuria [3]. Fibroblast growth factor 23 (FGF23) has an inhibiting influence upon 1alpha-hydroxylation, that in turn directly affects formation of calcitriol (1,25(OH)2D3) [4,5]. Also, FGF23 increases reabsorption of the phosphates with urine. Thus, the child’s body does not get sufficient amount of calcium ions and loses phosphorus ions, necessary for bone tissue formation.
There are many different causes of rickets (osteomalacic) syndromes, but they all lead to the deficit of available calcium and phosphorus for the mineralization of the newly created osteoid. Since these disorders have a common trend (defects in mineralization of bones), children with rickets and rickets-like diseases have a very similar clinical picture. This stereotype prompts the doctor, along with clinical or radiological studies, to use widely complex laboratory tests to get detailed the nature of bone metabolism disorders. Some of them have become available only recently [6-8]. The data we received about bone tissue metabolism [9] gave an opportunity to treat bone metabolism disorders in VDRR under biochemical control; the treatment was based on data on the pathogenesis of the disease.
The purpose of our study was to determine the effect of medical therapy upon genetically determined disturbances of bone metabolism in patients with D-resistant rickets.
Materials and Methods
In the consultative out-patient department of the “Institute of Traumatology and Orthopedics of the National academy of medical sciences of Ukraine”, 39 patients with a diagnosis of VDRR were examined and treated. The boys constituted 64%. The age of patients was 2-18 years, 54.3% were admitted at the age 3-9 years, which coincided with the progression of orthopedic manifestations.
Medical treatment of patients with rickets was performed in 4 stages, stage 1 included a complete patient examination referring to calcium, phosphorus in blood and urine, blood calcidiol and calcitriol levels, parathyroid hormone and osteocalcin indices, as well as bone formation marker P1NP and osteoresorbtion marker B-CTx. At the first stage, children were obliged to undergo genetic study to detect changes (polymorphism) in the alleles of receptors to vitamin D (VDR) and collagen type 1 (COL1). The next steps were carried out in full scale, in addition to genetic studies. Distribution of the treated patients is presented in Table 1. The frequency of the examination between stages of treatment was 3-3.5 months.
| Diagnosis | Stages of treatment | |||
|---|---|---|---|---|
| 1 stage | 2 stage | 3 stage | 4 stage | |
| VDRR | 39 | 15 | 5 | 4 |
Table 1: Number of treated patients depending on the nosology and stages of treatment.
Results and Discussion
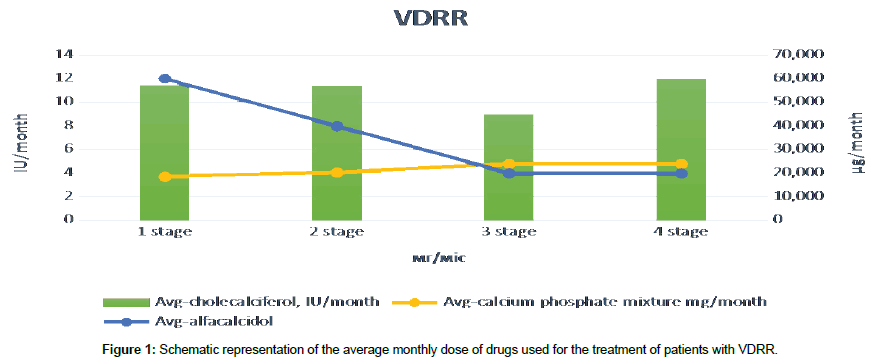
The pathogenesis of the rickets process formation in the VDRR, namely, inhibition of the activity of calcitriol FGF23, by reducing 1α-hydroxylation, with the associated violation of reabsorption of phosphorus in kidneys, prompted us to use in the treatment not only preparations containing calcium, phosphorus and vitamin D but also alfa-calcidol, which can bypass the processes of 1α-hydroxylation in the kidneys, because it has a formula where 1α-hydroxylation has been carried out artificially (Figure 1).
The treatment of orthopedic manifestations of VDRR started with 60 000 units of vitamin D, 12 micrograms of alpha-calcidol and 18 g of calcium glycerophosphate per month. In 3 months of treatment, a re-examination of bone metabolism was performed, resulting in a dose of vitamin D unchanged, and the dose of the hormonal form of vitamin D reduction to 8 micrograms per day (on average, children received 0.25 μg of alfa-calcidol per day). In 6 months of therapy, the dose of alpha-calcidol was reduced to 0.25 μg daily (4 μg/month). Unfortunately, alfa-calcidol is not available in Ukraine in liquid form, so we had some difficulties in the use of this drug in small children. Calcium phosphate mixture was consumed by children at a dose of 600-800 mg per day.
Now let’s have a look how blood and urine parameters changed during bone metabolism in patients with VDRR during pathogenetically substantiated treatment.
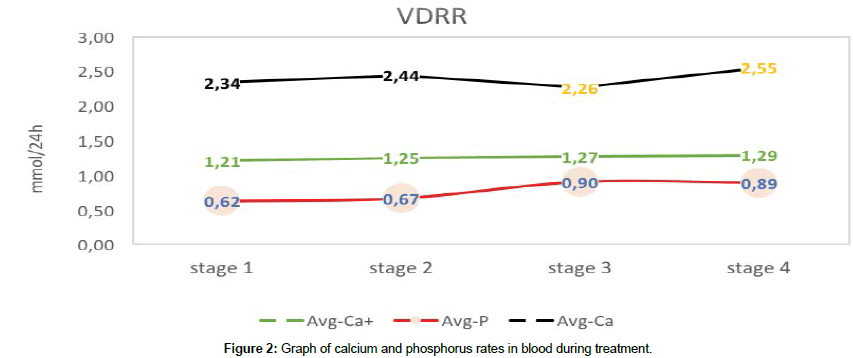
As one can see in the Table 2, after the first stage of treatment, blood indices were significantly improved, while the study of blood phosphorus and excretion of calcium in urine did not have a significant difference. The trend of positive treatment, close to reliability, was found in indices of ionized and total calcium as well as that of phosphorus in urine.
| Stage of treatment | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indices of bone metabolism | Stage 1 M ± m |
Stage 2 M ± m |
Stage 3 M ± m |
Stage 4 M ± m |
||||||||
| Ca+ | 1,21 | ± | 0,01 | 1,25** | ± | 0,02 | 1,27* | ± | 0,0134 | 1,29* | ± | 0,0077 |
| P | 0,62 | ± | 0,03 | 0,67 | ± | 0,07 | 0,90** | ± | 0,0236 | 0,89** | ± | 0,1378 |
| Ca | 2,34 | ± | 0,02 | 2,44** | ± | 0,03 | 2,26** | ± | 0,035 | 2,55** | ± | 0,0262 |
| 25(OH)D | 19,30 | ± | 2,01 | 40,97* | ± | 7,07 | 38,30* | ± | 6,5518 | 52,30* | ± | 18,4309 |
| 1,25(ÞÃÂ)2D | 74,15 | ± | 3,89 | 134,60* | ± | 13,39 | 107,70* | ± | 14,5257 | 127,40* | ± | 24,4436 |
| PTH | 48,66 | ± | 4,59 | 39,01 | ± | 12,86 | 28,03** | ± | 3,9761 | 26,30 | ± | 4,0618 |
| Osteocalcin | 123,35 | ± | 10,27 | 59,11* | ± | 34,05 | 60,40** | ± | 3,0247 | 29,70 | ± | 3,1348 |
| Urine calcium (daily) | 2,42 | ± | 0,25 | 2,54 | ± | 0,94 | 1,20 | ± | 0,2668 | 1,50 | ± | 0,3432 |
| Urine phosphorus (daily) | 34,09 | ± | 1,36 | 26,52** | ± | 3,15 | 18,20 | ± | 0,6064 | 15,10** | ± | 0,7370 |
| P1NP | 815,03 | ± | 59,86 | 576,4 | ± | 205,65 | 450,4** | ± | 25,3347 | 524,6** | ± | 32,3429 |
| B-CTx | 2,16 | ± | 0,11 | 1,69 | ± | 0,46 | 1,80** | ± | 0,1652 | 1,50** | ± | 0,1367 |
| * - significant difference of the parameter comparing to the 1st stage of treatment (p < 0,05) | ||||||||||||
| ** - trend close to significant difference of the parameter comparing to the 1st stage of treatment (0,1 > p > 0,05) | ||||||||||||
Table 2: Average measures of osseous metabolism among examined patients with VDRR.
Study of microelements of blood at the stages of treatment showed a scracely positive dynamics of treatment within the limits of normal age-related parameters of calcium. The blood phosphorus increased slowly, an increase within the norm did not occur (Figure 2).
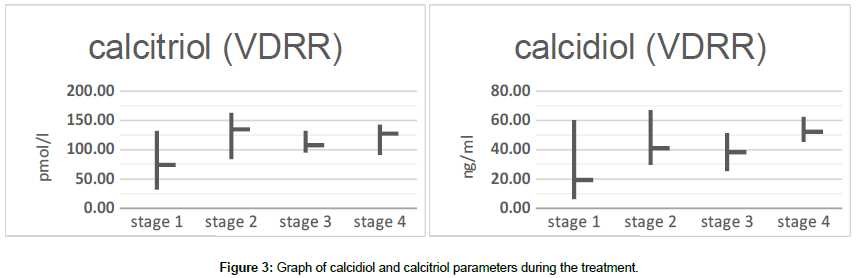
Already at the first stage of treatment, we observed a significant increase of calcidilol and calcitriol indices in the blood, in further calcidiol levels were within the normal age-related range, and calcitriol tended to hold in figures slightly higher than normal (Figure 3).
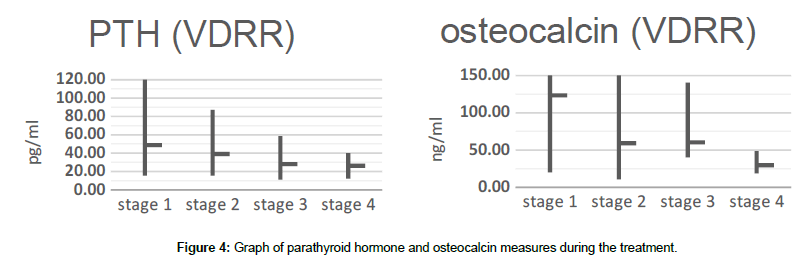
In our study, we observed a decrease in parathyroid hormone levels after the first stage of treatment, which before and after treatment in general did not go beyond the normal age-related limits. The bone metabolism marker reacted more actively with a clear tendency to a significant decrease after 9 months of therapy, normalizing the formation of the hydroxyl-apatite group in the bones (Figure 4).
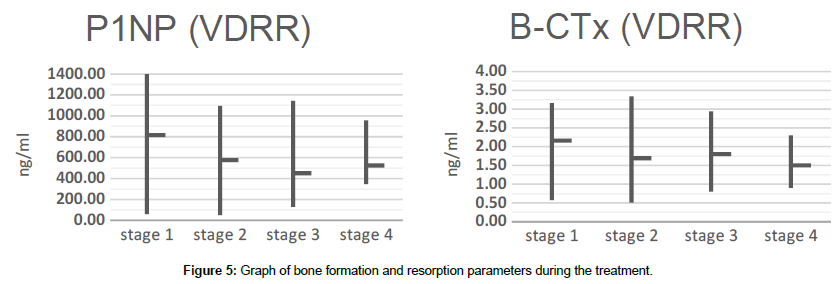
Bone formation and bone destruction have a clear tendency to parameters’ decrease after the initiation of treatment. As one can see at the Figure 5, P1NP and B-CTx have a steady regression, which in turn significantly slows bone turnover, allowing bone tissue to become fully mature.
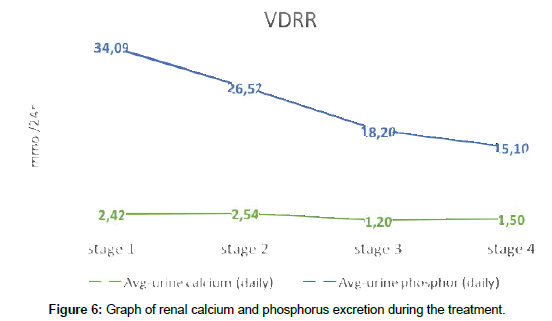
Renal calcium reabsorption was almost unchanged, and the daily calcium in urine was kept within the normal limits, thereby accumulating it in the bloodstream. Phosphate in urine after a first stage of treatment slightly decreased and was kept at the upper limit of excretion after treatment for 1 year (Figure 6).
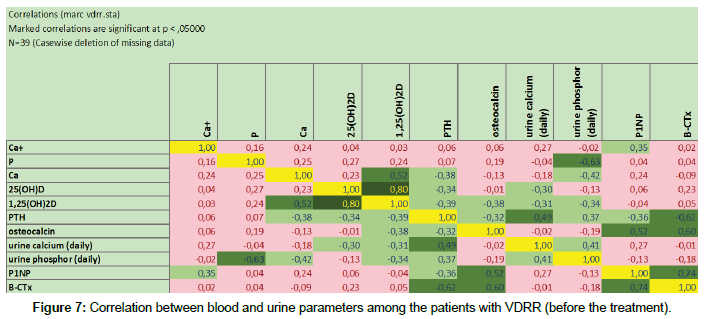
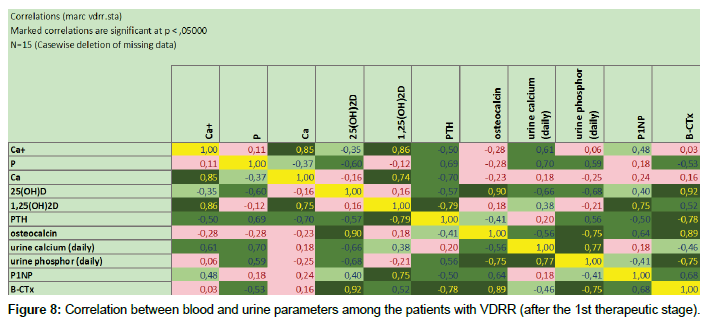
Having studied correlation dependences at the 1st therapeutic stage, we obtained the interesting results. It came about, that in proper pathogenetic therapy were noted correlated increase and reinforcement between vitamin D parameters, including its hormonal form, processes of bone turnover and resorption as well as parathyroid hormone’s level. This observation is clearly presented at the Figure 7 (before treatment) and Figure 8 (after the 1st therapeutic stage). Due to limited number of patients in the groups 3 and 4, correlation dependences between blood and urine parameters were not calculated in this population.
Biochemical parameters of blood serum in patients with VDRR that distinguish VDRR from other forms of rickets, are characterized by low phosphate levels, normal or low levels of calcitriol, normal calcium levels, elevated alkaline phosphatase activity and normal or slightly elevated levels of parathyroid hormone (secondarily).
Physician should be aware of the importance of early diagnosis of PD, since a correct diagnosis at an early stage may be beneficial in early therapy. Given the autosomal dominant type of inheritance, children in affected families should be screened for serum and urine phosphorus levels and serum alkaline phosphatase activity during the first month of life, and in the absence of informative data, again in 3 and 6 months. The given results are an indication for X-ray examination. If rickets is present, therapy begins with vitamin D and calcitriol (alpha-calcidol) and phosphates.
Why a hormonal metabolite and not a normal vitamin D are used? To answer this question, we take into account modern pathophysiological views of the VDRR development. Although PHEX is known to be endopeptidase, the role of PHEX in pathophysiology of PD is still discussed by the different authors. It is known that FGF-23 levels are elevated in PD and this change, namely, increasing concentrations leads to loss of phosphates and a lack of calcitriol concentration in serum. FGF23 suppresses the expression of SLC34A1, thus suppressing the level of NaPiIIa protein, resulting in loss of phosphates. FGF23 also reduces the level of 1α-hydroxylase and increases the level of 25-hydroxyvitamin-D-24-hydroxylase, resulting in reduced synthesis and increased inactivation of 1.25(OH)2D3 [10-12]. Alfa-calcidol (1α(OH)2D3), in contrast to the ready calcitriol, should undergo 25-hydroxylation in the liver and obtain the formula of calcitriol – 1.25(OH)2D3. Thus, we can exclude the effect of FGF23 upon 1α-hydroxylase, and upon calcitriol formation in the body by means of hepatic 25-hydroxylation.
As it has been noted above, treatment necessarily included vitamin D, alpha-calcidol and calcium-phosphorus mixture. Patients required dosing regulation during the treatment. Adequately this is possible only if there is a monitoring of blood and urine indices for three months, while parameters (calcium, phosphorus of blood and urine, determination of the level of calcidiol and calcitriol of blood, parathyroid hormone and osteocalcin indices, as well as the marker of bone formation P1NP and that of osteoresorption B-CTx) do not get closer to the age-related standard. In the future, the examination and correction of treatment can be done once in half a year. The purpose of monitoring is to maintain a normal concentration of Ca and PTH in blood, to avoid hypercalciuria, as well as to reduce the activity of bone destruction processes, whose activity leads to accelerated metabolic processes in the bone.
After 1-2 years of treatment and occasionally later, renal ultrasound should be performed to evaluate the development of nephrocalcinosis. If kidney parameters are stable in this study, the frequency of this study is reduced. We perform X-ray of the lower extremities 1 year after treatment, including operative one, and every 2 years thereafter, during the growth of the child, in order be sure, that the growth zone optimally responds to the treatment. Thus, the radiographic characteristics of epiphyses are important sensors for drug treatment and along with biochemical studies can be important indicators for dose adjustment.
Our therapy allowed reaching age-related normal parameters referring to the duration of fusion after the multi-level corrective osteotomy with intramedullary constructs, including growing one; where in patients with VDRR open growth zones took place. We plan to share this data in our future publications.
Conclusion
The biochemical data from blood serum in patients with VDRR that distinguish VDRR from other forms of rickets are characterized by low levels of phosphates, normal or low levels of calcitriol, normal calcium levels, increased alkaline phosphatase activity, and normal or slightly elevated levels of parathyroid hormone (secondarily).
The treatment of orthopedic VDRR should begin with 60 000 units of vitamin D, 12 μg of alpha-calcidol and 18 g of calcium glycerophosphate per month. In 3 months of treatment, a re-examination of bone metabolism should be performed, resulting in a dose of vitamin D or the hormonal form of vitamin D-alpha-calcidol variations. The calcium-phosphorus mixture should be consumed in an average dose of 600-800 mg per day. The proposed treatment regimen allows to improve the relationships between the participants of the metabolic process in the bone tissue of patients with VDRR, which affects the activity of growth zones, formation of bone deformations and the term of adhesion after osteotomy (p<0.05).
References
- Albright F, Butler AM, Bloomberg E (1937) Rickets resistant to vitamin D therapy. American Journal of Diseases of Children 54: 529-547.
- Winters RW, Graham JB, Mcfalls VW, Burnett CH (1958) A genetic study of familial hypophosphatemia and vitamin D resistant rickets with a review of the literature. Medicine 37: 97-142.
- Grieff M, Mumm S, Waeltz P, Mazzarella R, Whyte MP, et al. (1997) Expression and cloning of the human X-linked hypophosphatemia gene cDNA. Biochemical and biophysical research communications 231: 635-639.
- Hochberg Z (2003) Vitamin D and Rickets. Karger Medical and Scientific Publishers, Switzerland.
- Econs MJ (2002) Bone disease resulting from inherited disorders of renal tubule transport and vitamin D metabolism In: Coe FL, Favus MJ (eds) Disorders of Bone and Mineral Metabolism. Raven Press, New York, USA.
- Fu GK, Portale AA, Miller WL (1997) Complete structure of the human gene for the vitamin D 1α-hydroxylase, P450c1α. DNA and cell biology 12: 1499-1507.
- Holick MF (1990) The use and interpretation of assays for vitamin D and its metabolites. The Journal of Nutrition 120: 1464-1469.
- Tohme JF (1991) Biochemical markers of bone metabolism. Zietschr Rheumatol 50: 133-141.
- Martsyniak S, Guk I, Kinchaya PT, Andrii Z, David GS, et al. (2015) Results of conservative treatment in patients with phosphate diabetes. Bone Abstracts 4: 194.
- Gattineni J, Baum M (2010) Regulation of phosphate transport by fibroblast growth factor 23 (FGF23): implications for disorders of phosphate metabolism. Pediatric Nephrology 25: 591-601.
- Ramon I, Kleynen P, Body JJ, Karmali R (2010) Fibroblast growth factor 23 and its role in phosphate homeostasis. European journal of endocrinology 162: 1-10.
- Strom TM, Juppner H (2008) PHEX, FGF-23, DMP1 and beyond. Curr Opin Nephorol Hypertens 17: 357-362.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi