Case Report, Clin Oncol Case Rep Vol: 6 Issue: 7
Merkel Cell Carcinoma
Cesar Suarez*
Department of Medical Oncology, Hospital Pasteur Villa Maria CORDOBA, CP 5900, Argentina.
*Corresponding Author: Cesar Suarez,
Department of Medical Oncology
Hospital Pasteur Villa Maria CORDOBA, CP 5900, Argentina.
E-mail: cesarsuarez23@yahoo.es
Received: July 01, 2023; Manuscript No: COCR-23-104199;
Editor Assigned: July 03, 2023; PreQC Id: COCR-23-104199 (PQ);
Reviewed: July 10, 2023; QC No: COCR-23-104199 (Q);
Revised:July 14, 2023; Manuscript No: COCR-23-104199 (R);
Published: July 18, 2023; DOI: 10.4172/cocr.6(7).299
Citation: Suarez C (2023) Merkel Cell Carcinoma. Clin Oncol Case Rep 6:7
Abstract
Merkel carcinoma is very rare skin cancer, with high mortality. The standard treatment until 2017 was surgery, radiotherapy, and chemotherapy. Since 2017 FDA and EC approved Avelumab for the marketing and sale, based in phase II clinical trial. In Argentina was approved registration for the treatment of patients with metastatic Merkel cell carcinoma, controversies in authorizations, leads to delay in the start of treatment, and sometimes requires the intervention of the justice.
Keywords: Merkel carcinoma; Skin cancer; Chemotherapy; Radiotherapy
Introduction
Merkel carcinoma is a primary skin cancer originating in a population of neuroendocrine cells in the skin, called Merkel cells. It is a very rare cancer, constituting less than 1% of all malignant skin tumors [1]. The annual incidence is estimated in the range of 1 to 2 per 500,000 individuals in the Caucasian population. It usually occurs in elderly people, with a slight predominance in the male sex. The average age at diagnosis is between 68 years and 75 years. Reported mortality rates within 5 years range from 55%/84%, localized/metastatic tumor. It has a survival rate of less than 20% at 5 years [1]. The standard treatment until 2018 has been surgery and for non-surgical or metastatic recurrent tumors [2,3], chemotherapy and eventually radiotherapy with response rates on average of 53 to 61%, but without demonstrating benefit in overall survival. In March 2017 the FDA, and in September 2017 the European Commission approved the commercialization of Avelumab for the treatment of this carcinoma [4-6]. In Argentina in August 2018, the ANMAT approved the *registration* of AVELUMAB as monotherapy for the treatment of adult patients with metastatic Merkel Cell Carcinoma (MCC) [7], based on the Javelin Merkel 200 study. Phase II study, discussed by health systems (Health insurance and prepaid).
Case Presentation
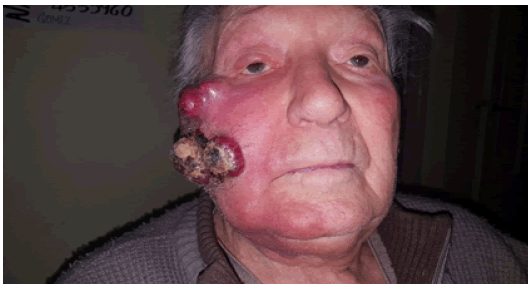
A 95-year-old male patient with PS I EGOG, with a history of heart failure on treatment with diuretics. No other history of significant disease. Oncology consultation in May 2020, with a diagnosis of MERKEL CELL CARCINOMA of the skin of the right cheek with surgical resections in 2019 on 2 occasions and recurred, rapidly growing without new surgical possibility due to size and without the possibility of radiotherapy due to the size and location. He was also unable to undergo chemotherapy due to comorbidities and known adverse events. On physical examination: he presented a 10 cm × 10 cm × 6 cm multilobulated tumor on the skin of the right cheek with involvement of the masseter muscle and difficulty in opening the jaw with pain on palpation and movement of the lower jaw, with no other relevant data on physical examination.
Laboratory tests: elevated ESR, complete blood count, urea, creatinine, liver transaminases and normal proteinogram.Neither IHQ nor PD L-1 was performed (Figure 1).
Treatment discussion: Considering the patient's age and treatment options, it was decided to propose treatment with anti-PD L-1 Avelumab, which is a human immunoglobulin G1 monoclonal antibody directed against Programmed Death Ligand 1 (PD- L1). PDL1 is a protein present on the surface of tumor cells, which works by preventing attack by cells of the immune system. Avelumab binds to this ligand and blocks the interaction between PD-L1 and its receptors. This removes the suppressive effects of PD-L1 on antitumor CD8+ T cells, resulting in restoration of the cytotoxic T cell response [8, 9].
Rejection of the treatment was generated by the health insurance because it is still in phase II of clinical research, and the ambiguity in the ANMAT ultra-rapid evaluation report for the use of the drug, Legal intervention was needed to authorize the treatment, however, treatment was started at the family's expense, with an excellent response and maintained over time, greater than that known up to now (Javelin Merkel 200).
The 7-country multicenter Javelin Merkel 200 research study recruited 204 patients with metastatic Merkel Cell Carcinoma (MCC) between 2014 and 2019 to receive AVELUMAB 10 mg/k every 15 days and plans to end in 2024 [4, 5, 10]. With results of follow-up at one year (date the patient started) with 60% objective and durable response in 1 year [4]. The best confirmed overall response was according to the response evaluation criteria in Solid Tumors version 1.1. Patients who confirmed complete response were treated for a minimum of 6 months and a maximum of 12 months. The RECIST 1.17 criteria establish the following definitions: Complete Response (CR): disappearance of all lesions. Any pathologic lymph node should have short axis reduction greater than 10 mm.
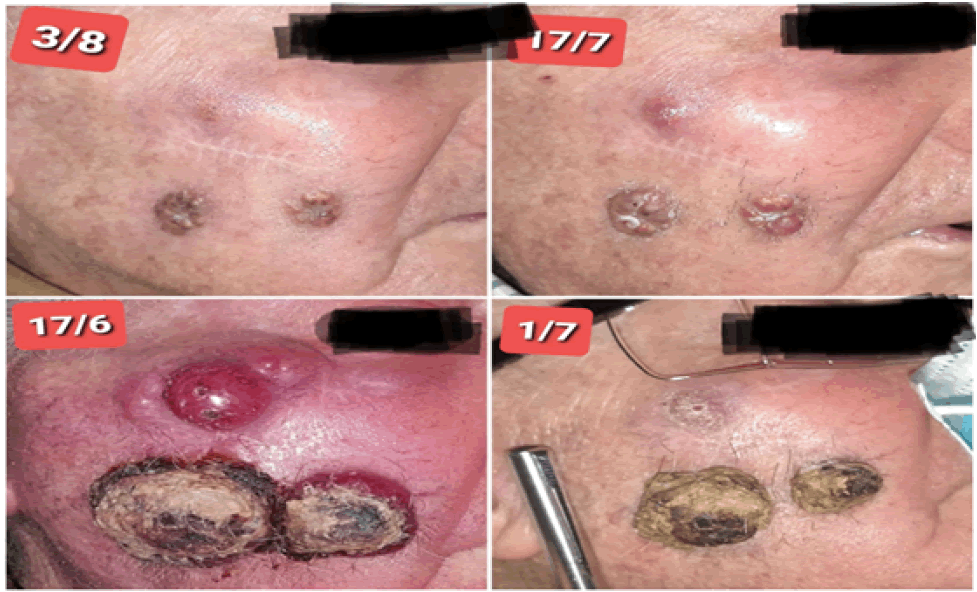
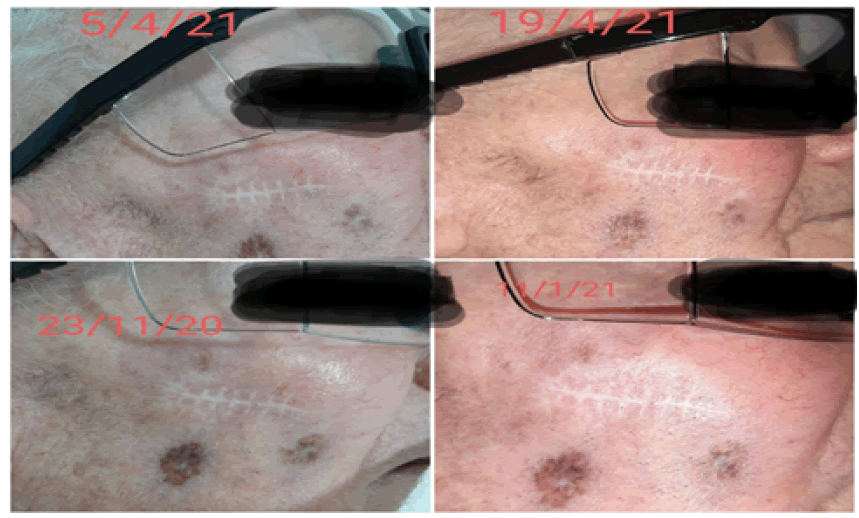
The patient underwent treatment regularly every 15 days without interruption from June 2020 to October 2022, which he suspended due to pneumonia secondary to bronchial aspiration and died, with the best complete response, Bor Recist at 6 months (Figure 2) and maintained for 23 months (Figure 3).
Discussion
Rare diseases are those whose prevalence in the population is equal to or less than 1 in 2,000 people, according to the national epidemiological situation [11]. Since 2012 there is an ANMAT regulation for products intended for the prevention, diagnosis and/ or treatment of rare diseases, for which there is no effective and safe treatment available, these drugs are registered under special conditions based on preclinical and clinical findings, (phase II) and in the benefit-risk ratio, the registration certificate is valid for 1 year. And there is a circular 3/2018 for products that have completed at least one first phase III clinical pharmacology study. For them, the certificate is for 5 years [11].
Clinical trials, in their phases, are the ones that validate new treatments, but in some unusual circumstances such as Infrequent diseases where it is difficult to reach the number of patients, other non-consensus factors must be considered to authorize the marketing of a drug before completing a phase III study.
Conclusion
The problem is the interpretation of the ANMAT authorizations to make it clear to the health system who should cover the costs of drug treatments that are in phase II of the investigation, delaying the start of treatment until the intervention of justice.
References
- Schadendorf D, Lebbe C, Zur Hausen A, Avril MF, Hariharan S, et al. (2017) Merkel cell carcinoma: Epidemiology, prognosis, therapy and unmet medical needs. Europ J Cancer 71: 53-69. [Google Scholar] [Cross Ref]
- Muller-Richter UDA, Gesierich A, Kubler AC, Hartmann S, Brands RC (2017) Merkel cell carcinoma of the head and neck: Recommendations for diagnostics and treatment. Ann Surg Oncol 24: 3430-3437. [Google Scholar] [Cross Ref]
- Freeman RG, Knox JM (2012) Treatment of skin cancer. Springer Sci Business Media. [Google Scholar]
- Kaufman HL, Russell JS, Hamid O, Bhatia S, Terheyden P, et al. (2018) Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after ≥ 1 year of follow-up: Javelin Merkel 200, a phase 2 clinical trial. J Immunother Cancer 6: 1-7. [Google Scholar] [Cross Ref]
- D'Angelo SP, Bhatia S, Brohl AS, Hamid O, Mehnert JM, et al. (2020) Avelumab in patients with previously treated metastatic Merkel cell carcinoma: long-term data and biomarker analyses from the single-arm phase 2 JAVELIN Merkel 200 trial. J Immunother Cancer 8. [Google Scholar] [Cross Ref]
- Merkel cell carcinoma: Epidemiology, prognosis, therapy and unmet medical needs.
- [Google Scholar]
- Butterfield LH, Kaufman HL, Marincola FM (Eds) (2021) Cancer immunotherapy principles and practice. Springer Pub Company. [Google Scholar] [Cross Ref]
- Kroemer G, Zitvogel L (2018) Oncoimmunology: A practical guide for cancer immunotherapy. Springer. [Google Scholar] [Cross Ref]
- Kaufman H, Hamid O, D'Angelo SP, Yuan G, Chin KM, et al. (2015) A phase II, open-label, multicenter trial to investigate the clinical activity and safety of avelumab (MSB0010718C) in patients with metastatic Merkel cell carcinoma. J Clin Oncol 33. [Google Scholar] [Cross Ref]
- Voelker R (2017) Immunotherapy for rare skin cancer. JAMA 317: 1716-1716. [Google Scholar] [Cross Ref]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi