Case Report, Clin Oncol Case Rep Vol: 2 Issue: 3
Pancreatic Extraskeletal Ewing Sarcoma/Primitive Neuroectodermal Tumor: Case Report of a Rare Pathology
Jasmine L Martin1*, Heather N Swedberg1, Evita Henderson-Jackson2, Kun Jiang2, Damon R Reed3 and Warren Brenner4
1Charles E Schmidt College of Medicine, Florida Atlantic University, Boca Raton, Florida, United States
2Department of Pathology, Moffitt Cancer Center, Tampa, Florida, United States
3Adolescent Young Adult Program, Sarcoma Department, Moffitt Cancer Center, Tampa, Florida, United States
4Center for Hematology and Oncology, Lynn Cancer Institute, Boca Raton, Florida, United States
*Corresponding Author : Jasmine L Martin
Charles E Schmidt College of Medicine, Florida Atlantic University, 777 Glades Rd, Boca Raton, Florida, 33431, USA
Tel: 561-419-5566
E-mail: jasmart10@gmail.com
Received: July 06, 2019 Accepted: July 22, 2019 Published: August 05, 2019
Citation: Martin JL, Swedberg HN, Jackson HE, Jiang K, Reed DR , et al.(2019) Pancreatic Extraskeletal Ewing Sarcoma/Primitive Neuroectodermal Tumor: Case Report of a Rare Pathology. Clin Oncol Case Rep 2:3.
Abstract
Ewing sarcoma, characterized by an 11:22 translocation, commonly occurs in bone and soft tissue sites. We present a patient with a history of both chest wall desmoid tumor and a subsequent adenosquamous pancreatic cancer treated with distal pancreatectomy, adjuvant chemotherapy, and radiation. Two years later the patient developed lymphadenopathy, which was biopsied and sent for the tissue of origin testing. Results revealed a sarcoma with 90% certainty, primitive neuroectodermal tumor subtype. The original pancreatic tumor was sent for next-generation sequencing, which revealed an EWSR1/FLI1 fusion as the sole genetic mutation suggestive of an underlying Ewing sarcoma. Additionally, the same fusion gene was detected on the supraclavicular lymph node biopsy. Our case affirms a change in diagnosis that was not suspected clinically with a resultant change in therapy. Though the pancreas is an unusual site of extraskeletal Ewing sarcoma (EES), emerging technologies such as next-generation sequencing can aid in management.
Keywords: Peripheral primitive neuroectodermal tumor; Ewings sarcoma; Pancreatic neoplasm
Introduction
The most common primary tumor of the pancreas is adenocarcinoma, which comprises about 85% of all cases with primary mesenchymal tumors of the pancreas being comparatively rare. Of these, Ewing sarcoma/Primitive Neuroectodermal Tumors (PNETs) are an infrequent occurrence with few such case reports existing in the medical literature. Our case discusses an unexpected diagnosis of a morphologically atypical EES found in the unusual location of the pancreas.
Case
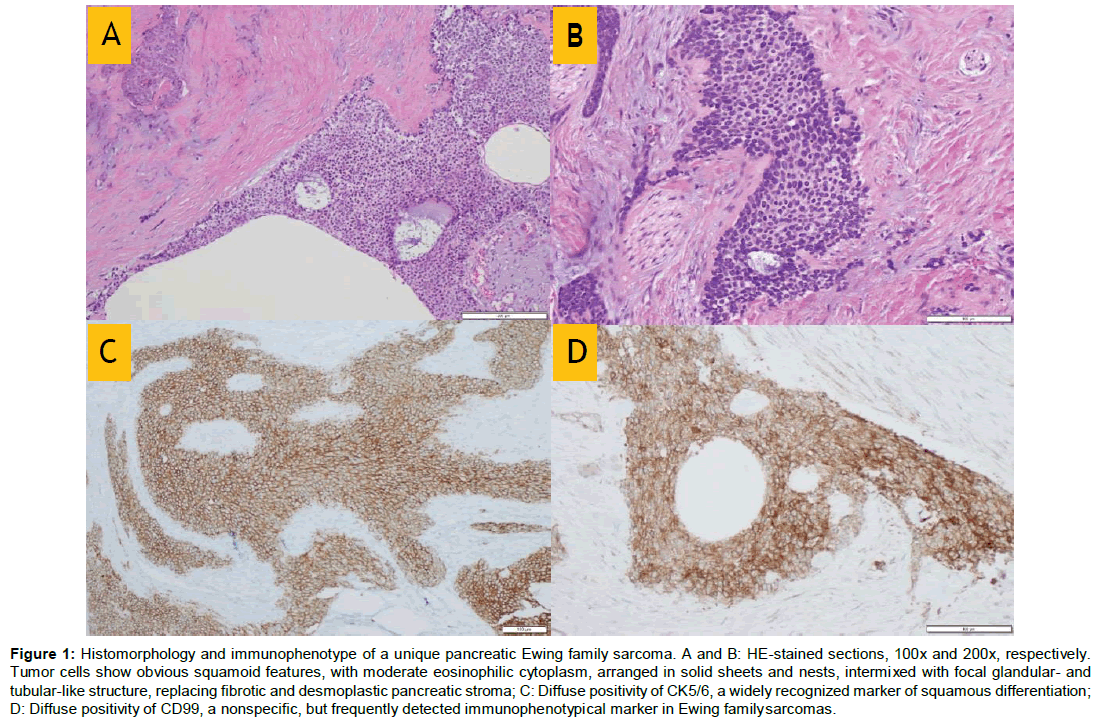
We present a case of a 58-year old female who was undergoing surveillance for a symptomatic desmoid tumor of the chest wall previously treated with sorafenib who was incidentally found to have a 1.9 cm distal pancreatic mass. Initial biopsy suggested a tumor with neuroendocrine features. The patient subsequently underwent distal pancreatectomy with negative margins and lymph nodes. Surprisingly, the tumor was morphologically and immunophenotypically most compatible with an adenosquamous tumor evidenced by medium- sized cells growing in sheets and nests intermixed with atypical glands (Figure 1A and B), diffuse and strong labeling for squamous markers CK5/6 (Figure 1C) and P63 (not shown here). This unique, but not entirely classic histology and immunohistochemical analysis with broad keratin positivity led to a final diagnosis of a stage IIA, T3 N0 adenosquamous cell cancer of the pancreas. In addition to extrapancreatic tumor extension, perineural and lymphovascular invasion were also confirmed.
Figure 1: Histomorphology and immunophenotype of a unique pancreatic Ewing family sarcoma. A and B: HE-stained sections, 100x and 200x, respectively. Tumor cells show obvious squamoid features, with moderate eosinophilic cytoplasm, arranged in solid sheets and nests, intermixed with focal glandular- and tubular-like structure, replacing fibrotic and desmoplastic pancreatic stroma; C: Diffuse positivity of CK5/6, a widely recognized marker of squamous differentiation; D: Diffuse positivity of CD99, a nonspecific, but frequently detected immunophenotypical marker in Ewing family sarcomas.
The patient received adjuvant chemotherapy with gemcitabine and 5-fluorouracil and radiation. On routine follow up two years later new supraclavicular lymphadenopathy was noted, which was restaged with PET CT showing FDG avid enlarged aortocaval, preaortic, and left axillary lymph nodes and a paraspinal soft tissue mass. These findings were suspected to be a recurrence of pancreatic cancer. The patient subsequently underwent ultrasound-guided left supraclavicular lymph node biopsy with pathology initially suggestive of a pure squamous component. Based on the unusual pattern of recurrence and the morphology that was different than the primary, Tissue of Origin (Cancer Type ID, Molecular Cancer Classifier, BioTheranostics) testing was sent, which surprisingly reported a sarcoma with 90% certainty, primitive neuroectodermal tumor subtype.
The patient was started on gemcitabine and nab-paclitaxel initially as this was suspected to be recurrent adenosquamous cell cancer of the pancreas despite the tissue of origin testing results, and additional testing on the original pancreatic tumor was sent for next- generation gene sequencing. This revealed an EWSR1/FLI1 fusion as the sole genetic mutation suggestive of an underlying Ewing sarcoma. Additionally, the same fusion gene without additional genetic mutations was detected on the supraclavicular lymph node biopsy confirming this as recurrence of her original pancreatic tumor.
Our patient was started on doxorubicin, vincristine, cyclophosphamide, and mesna with pegfilgrastim support, alternating with etoposide and ifosfamide. She completed 7 cycles and her most recent PET CT shows no evidence of metabolically active disease.
Discussion
Ewing sarcoma was first characterized to have the 11:22 translocation in 1993 [1]. Subsequently, Ewing sarcoma and Ewing- like tumors have been further characterized but novel translocations are still being identified [2-5]. Importantly, there is no agreement regarding which tumors are Ewing sarcoma, Ewing family or Ewing- like tumors. For clinical trials, exclusion criteria are increasingly being based on translocation type as it is recognized that some translocations portend a poorer prognosis such as CIC/DUX4 translocation [6-8]. Historical terms for these tumors include Primitive Neuroectodermal Tumors (PNET) and Askin tumors of the chest wall. While the tissue of origin is thought to be neuroendocrine, tumors can appear in extraskeletal sites, more often with increasing age.
Histology of these tumors reveals primitive round to oval tumor cells often with glycogen aggregates in the cytoplasm. CD99 is expressed in almost all cases of Ewing sarcoma in a characteristic membranous fashion, though not specific; membranous labeling by CD99 was detected in our case (Figure 1D). Vimentin stains most tumor cells and neural markers such as Neuron-Specific Enolase (NSE) are frequently expressed [9]. FLI1 is a sensitive marker for ES but is not specific. Other malignancies including lymphoblastic lymphomas, Desmoplastic Small Round Cell Tumor (DSRCT), Merkel cell carcinoma, and synovial carcinoma can show nuclear staining for this marker [10]. Some similar cases of peripheral Primitive Neuroectodermal Tumors (PNETs) of the pancreas exist in the medical literature. One large literature review by Komforti et al, 2018, documented 32 cases of histologically confirmed pancreatic Ewing sarcoma, with patients aged 2-60 with a median age of 20.5 years without significance in gender predominance [11]. Our patient, who was 58 years old at diagnosis, is among the oldest of reported patients to be diagnosed with EES of the pancreas, as the majority of reported cases occur in the second or third decade of life. Outcomes have varied among reported cases. Of 26 patients with documented follow up, two were reported alive with disease, 17 were alive without disease, and 6 died of disease with date of expiration ranging from 3 to 48 months.
Five-year survival rates in localized Ewing sarcoma have improved in recent decades due to advances in chemotherapy intensity or timing along with refinements in local control [12-14]. In patients with localized disease, those with extraskeletal sites have a greater overall survival when compared with patients with classic skeletal tumors [15-17].
Conclusion
Our case is important as it establishes the phenotypic range of this neuroendocrine tumor that typically arises in the bones of adolescents and young adults. While this clinical behavior in terms of location of metastaseswasnotconsistentwithatypicalrelapseofpancreaticcancer, it is admittedly also unusual for EES, which typically metastasizes to lung most often and less often to other bone and bone marrow sites. Additionally, the IHC and morphologic phenotype along with the entire pathologic workup may be informative regarding a differential diagnosis for pancreatic cancers with squamoid histomorphology and immunophenotype. Increasingly, emerging technologies such as next- generation sequencing are providing unexpected clarity to unusual diagnoses.
References
- May WA, Gishizky ML, Lessnick SL (1993) Ewing sarcoma 11;22 translocation produces a chimeric transcription factor that requires the DNA-binding domain encoded by FLI1 for transformation. Proc Natl Acad Sci 90: 5752-5756.
- Sorensen PH, Lessnick SL, Lopez-Terrada D, Liu XF, Triche TJ, et al. (1994) A second Ewing's sarcoma translocation, t(21;22), fuses the EWS gene to another ETS-family transcription factor, ERG. Nat Genet 6: 146-151.
- Brohl AS, Solomon DA, Chang W (2014) The genomic landscape of the Ewing Sarcoma family of tumors reveals recurrent STAG2 mutation. PLoS Genet 10: e1004475.
- Crompton BD, Stewart C, Taylor-Weiner A (2014) The genomic landscape of pediatric Ewing sarcoma. Cancer Discov 4: 1326-1341.
- Tirode F, Surdez D, Ma X (2014) Genomic landscape of Ewing sarcoma defines an aggressive subtype with co-association of STAG2 and TP53 mutations. Cancer Discov 4: 1342-1353.
- Reed DR, Hayashi M, Wagner L (2017) Treatment pathway of bone sarcoma in children, adolescents, and young adults. Cancer 123: 2206-2218.
- Kao YC, Owosho AA, Sung YS (2018) BCOR-CCNB3 Fusion positive sarcomas: A clinicopathologic and molecular analysis of 36 cases with comparison to morphologic spectrum and clinical behavior of other round cell sarcomas. Am J Surg Pathol 42: 604-615.
- Antonescu CR, Owosho AA, Zhang L (2017) Sarcomas with CIC-rearrangements are a distinct pathologic entity with aggressive outcome: A clinicopathologic and molecular study of 115 cases. Am J Surg Pathol 41: 941.
- Pathology and genetics of tumours of soft tissue and bone (Internet) 2018.
- Choi E, Gardner J, Lucas D (2014) Ewing sarcoma: Seminars in diagnostic pathology. 31: 39-47.
- Komforti M, Sokolovskaya E, D’Agostino C (2018) Extra-osseous Ewing sarcoma of the pancreas: case report with radiologic, pathologic, and molecular correlation, and brief review of the literature. Virchows Archiv.
- Esiashvili N, Goodman M, Marcus R (2008) Changes in incidence and survival of Ewing Sarcoma patients over the past 3 decades. J Pediatr Hematol/Oncol 30: 425-430.
- Womer RB, West DC, Krailo MD (2012) Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: A report from the Children's Oncology Group. J Clin Oncol 30: 4148-4154.
- Grier HE, Krailo MD, Tarbell NJ (2003) Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. The New England Journal of Medicine 348: 694-701.
- Applebaum MA, Goldsby R, Neuhaus J, DuBois SG (2012) Clinical features and outcomes in patients with Ewing sarcoma and regional lymph node involvement. Pediatr Blood Cancer 59: 617-620.
- Cash T, McIlvaine E, Krailo MD (2016) Comparison of clinical features and outcomes in patients with extraskeletal versus skeletal localized Ewing sarcoma: A report from the Children's Oncology Group. Pediatr Blood Cancer 63: 1771-1779.
- El Weshi A, Allam A, Ajarim D (2010) Extraskeletal Ewing's sarcoma family of tumours in adults: analysis of 57 patients from a single institution. Clin Oncol (R Coll Radiol) 22: 374-381.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi