Research Article, Clin Dermatol Res J Vol: 5 Issue: 3
Polidocanol Inhibits Voltage-Gated Sodium Currents
Steven Evans M1*, Benton Maglinger G2, Matthew Gayhart G3, Krishnakanth Madireddy N1 and Stephen Johnson R4
1Department of Neurology, Professor of Neurology, University of Louisville, United States
2Department of Neurology,University of Kentucky, College of Medicine, United States
3Department of Pathology, Virginia Commonwealth University School of Medicine, United Kingdom
4Department of Chemistry, Associate Professor of Chemistry, University of Illinois at Springfield, United States
*Corresponding Author: M Steven Evan MD
Department of Neurology, Professor of Neurology, University of Louisville, 401 E. Chestnut St., Suite 510 Louisville, KY 40202, United States
Tel: (502) 589-0802
Fax: (502) 589-0805
E-mail: steve.evans@louisville.edu
Received: July 30, 2020 Accepted: September 17, 2020 Published: September 23, 2020
Citation: Evans MS, Maglinger GB, Gayhart GM, Madireddy NK, Johnson RS (2020) Polidocanol Inhibits Voltage-Gated Sodium Currents. Clin Dermatol Res J 5:3.. doi: 10.37532/cdrj.2020.5(3).141
Abstract
Background: Polidocanol is used as a sclerosing agent for varicose and spider veins. Its presumed mechanism of action is endothelial cytotoxicity, but it was originally developed as an anesthetic, and also has antipruritic and antitussive effects. The mechanism of action of local anesthetics is inhibition of voltagegated sodium (Na+) channels. We asked whether polidocanol, like local anesthetics, inhibits voltage-gated sodium currents. Methods: We used whole cell voltage clamp recording to test polidocanol effects on Na+ currents, and trypan blue exclusion to assess cytotoxicity in catecholamine A differentiated (CAD) and N1E-115 cell lines. Results: Polidocanol at concentrations of 1 to 10 micromolar strongly inhibited voltage-gated Na+ currents, a potency similar to that of local anesthetics. Na+ current inhibition was concentrationdependent, with an IC50 of 1.59 micromolar and 90% inhibition at 10 micromolar. Both tonic (non-use-dependent) and phasic (usedependent) inhibition occurred. Another polyethylene glycol (PEG)- containing compound, benzonatate, was previously shown to strongly inhibit Na+ currents, but other PEG-containing compounds tested in this study, nonaethylene glycol monomethyl ether and PEG 400, had no effects. Polidocanol was cytotoxic to CAD cells, producing dose-dependent cell death with an LD50 of 159 micromolar and complete cell death at 1000 micromolar. Conclusions: We confirmed the cytoxicity of polidocanol in high concentrations, which may account for its usefulness as a vein sclerotic. We found that in much lower concentrations, similar to that of clinically-used local anesthetics, polidocanol inhibits voltagegated Na+ channels. Sodium channel inhibition may account for polidocanol’s anesthetic, antipruritic, and antitussive effects. Local anesthetic-like inhibition of Na+ currents of sensory nerve fibers in veins may account for its good tolerability as a vein sclerotic. Chemical compounds studied in this article: Polidocanol (PubChem CID: 656641); PEG-400 (PubChem CID: 4867); nonaethylene glycol monomethyl ether (PubChem CID: 11339376)
Keywords: Nav1.7, Voltage-gated ion channels, Local anesthetic, Patch clamp, Varicose veins
Abbreviations
CAD:Catecholamine-A Differentiated; TTX: Tetrodotoxin; PEG- 400: Polyethylene glycol 400; Na+: voltage-gated sodium; FDA: Food and Drug Administration; NGME:Nonaethylene Glycol Monomethyl ether; HPLC: High Performance Liquid Chromatography; MS:Mass Spectrometr
Introduction
Polidocanol (nonaethylene glycol monododecyl ether) was first developed in Germany in the 1920’s under the name Thesit®. In the 1950s, it was shown to have local anesthetic, antipruritic, and antitussive effects [1-3]. Polidocanol (Laureth-9) was FDA approved in 2010 for use as a non-ionic surfactant sclerosing agent to treat varicose veins under the proprietary names Asclera® and Varithena®. In addition to treating varicose veins and spider veins, polidocanol has many off-label uses. Reported uses include esophageal and gastric varices, tendinopathy, epicondylitis, vascular malformations, varicocele, hydrocele, spermatocele, aneurysmal bone cysts, itching, management of gastrointestinal bleeding, simple renal cysts, radiation-induced dermatitis, and hemorrhoids [4]. Polidocanol is thought to sclerose veins by endothelial toxicity and microvascular occlusion [5,6]. It is commonly used as a foam which allows for greater contact time with the vein wall and therefore a greater potential for endothelial cell death and vein wall obliteration [7]. Its effects seem not to be limited to the endothelium but also includes induction of apoptosis in the vein wall [8]. The drug is clinically well tolerated as a sclerosing agent, with side effect rates of 0.4% as a liquid and 1.1% as a foam [9]. The clinical use of polidocanol could be facilitated by a local anesthetic effect. Topical administration and intratendinous injections of polidocanol reduce pain associated with tendinopathies[10,11], suggesting that it may have a clinically relevant local anesthetic effect.
Clinically-used local anesthetics inhibit voltage-gated sodium channels, but effects of polidocanol on these channels have not previously been reported. Chemically, polidocanol consists of an alkyl chain of 12 carbons connected by an ether bond to an ethylene oxide chain having an average of 9 ethylene oxide units (Figure 1A). This N = 9 ethylene oxide chain is also present in another clinically used drug with local anesthetic effects, benzonatate (Tessalon®). The objective of this study is to assess polidocanol’s effects on voltage-gated sodium channels and cytotoxicity. We studied Na+ channels in two murine cell lines, CAD cells, which express primarily the Nav1.7 channel isoform [12,13] and N1E-115 neuroblastoma cells, which express primarily Nav1.3 [14].
Materials and Methods
Cell culture
Catecholamine A differentiated (CAD) cells were grown at 37°C in 21% O2, 5% CO2, in Ham’s DMEM/F12 medium (Gibco®; Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin and 0.2% Glutamax (all from Invitrogen/ThermoFisher Scientific, Waltham, MA). Cells were grown in 35 mm plastic culture dishes and passaged at a 1:4 dilution every 3 to 5 days after reaching 90% confluency. CAD cells can grow in a “differentiated” form when cultured without serum [15]. Our studies used the “undifferentiated” form of CAD cells [15].
N1E-115 cells were grown at 37°C in 21% O2, 5% CO2, in DMEM-high glucose with glutamine medium (Life Technologies) supplemented with 10% FBS and 1% penicillin/streptomycin. Cells were grown in 35 mm plastic culture dishes and passaged at a 1:2 dilution every 2 to 3 days after reaching 90% confluency.
For both lines, cells used for electrophysiological study were grown in 35 mm dishes containing 12 mm round glass coverslips.
Materials
Polidocanol (CAS 3055-99-0) was obtained from Sigma-Adrich (St. Louis, MO). Polyethylene glycol 400 (CAS 25322-68-3) was obtained from Alfa Aesar (Ward Hill, MA). Nonaethylene glycol monomethyl ether (CAS 6048-68-6) was obtained from Tokyo Chemical Industry Co. (Tokyo, Japan). Stock solutions of polidocanol, PEG-400, and nonaethylene glycol monomethyl ether (NGME) were made fresh daily and were all dissolved in the particular extracellular solution used for recording. Fresh extracellular solution was made at least every five days.
HPLC-MS
To examine the composition of polidocanol and NMGE, 1.0 ng of each material was injected into a Waters Acquity Ultra Performance Liquid Chromatograph equipped with a BEHTM C18 column, 1.7 μm, 130 Å, 50 x 2.1 mm at a flow rate of 0.150 mL/min; a 2.00 min hold at 90:10 A:B (Mobil Phase A = 0.01% Formic Acid; Mobil Phase B = acetonitrile:methanol 1:1) followed by a binary mobile phase gradient to 10:90 A:B in 30.00 minutes. The effluent stream from the chromatographic separation was coupled to a Waters Syanpt G2-Si high definition mass spectrometer with atmospheric pressure ionization and electrospray probe, StepwaveTM ion optics technology and TriWaveTM technology post acceleration conversion dynode and electron multiplier detection system (MassLynx 4.2). Full scan collection in a mass range from 250-1000 m/z through a quadrupole filter (Q1) was obtained with a capillary temperature of 310°C maintained at 3.1 kV. All mass spectra show the major molecular [M+H]+ ion.
Whole cell recording
Whole cell voltage-clamp recordings were done at room temperature using an Axopatch 200b amplifier (Molecular Devices, Sunnyvale CA). Electrodes were pulled from thin-walled borosilicate glass capillaries (1.5 OD, 1.0 ID; King Precision Glass, Inc., Claremont, CA) with a P-97 electrode puller (Sutter Instrument Co., Novato, CA) with electrode resistances of 1-2 MΩ when filled with internal solution. The internal solution for recording Na+ currents contained (in mM): 110 CsCl, 5 MgSO4, 10 EGTA (in CsOH), 4 ATP Na2-ATP, 25 HEPES (pH 7.2). The external solution contained (in mM): 100 NaCl, 10 tetraethylammonium chloride (TEA-Cl), 1 CaCl2, 1 CdCl2, 1 MgCl2, 10 D-glucose, 1 3,4 diaminopyridine, 0.1 NiCl2, 10 HEPES (pH 7.3). Recording began after 2-5 minutes to allow complete dialysis of the pipette solution with the intracellular contents and stabilize Na+ currents, which tended to increase after first establishing whole cell mode. Whole-cell capacitance and series resistance were compensated with the amplifier. Series resistance in whole cell mode was 2-3 MΩ and compensated by 50-80%. Linear leak currents were digitally subtracted using a P/4 protocol. Currents were filtered at 5 kHz using the low pass Bessel filter of the amplifier and sampled at 10 kHz using a Digidata 1322A interface and PClamp 10 software (Molecular Devices). Cells used for study had stable seal resistances of at least 1 gigaohm, input resistances of at least 500 megaohms, and Na+ peak currents of at least 500 pA.
The holding potential in all experiments was -80 mV unless stated otherwise. For study of tonic inhibition, currents were elicited at a rate of no greater than every 20 seconds, which eliminated phasic (use-dependent) effects of polidocanol on Na+ currents in CAD and NIE-115 cells. In some experiments, we specifically studied phasic inhibition by polidocanol, and currents were elicited at a rate of 20 Hz from a holding potential of -100 mV.
Cells were perfused with external solution at 0.5 ml per minute using a syringe pump through a chamber having a volume of 0.5 ml. Test solutions were applied through three-barrel square glass tubes and a perfusion system with computer-controlled valves (Warner VC-6MCS Perfusion Valve Control System; Warner Instruments, Hamden, CT).
Trypan blue assay
For this study, CAD cells were grown in T-25 flasks (Falcon; Becton Dickson Labware, Franklin Lakes, NJ). After growing to 70-90% confluency, cells were triturated in 1.5 mL growth medium and five 180 μL aliquots transferred to 24 well culture plates (Costar; Corning, Inc., Corning, NY) for trypan blue assays. Stock solutions of 10 mM polidocanol were made in growth medium. Stocks were serially diluted in growth medium, and the dilute polidocanol solutions were then added to CAD cells in growth medium for each concentration tested (1000, 100, 10, 1, 0.1 and 0 μM). Cells were incubated in polidocanol at room temperature for 60 minutes and then assessed for cytotoxicity using trypan blue staining (HyClone®, GE Healthcare Life Sciences, Little Chalfont, United Kingdom). A polidocanol-treated cell suspension was mixed 50:50 with 0.2 μm filtered trypan blue 0.4% solution in phosphate-buffered saline and then counted manually on a Bright-Line Hemacytometer (American Optical, Buffalo, NY). Total cells and dead, trypan blue-containing, cells were counted under 100X magnification.
Data analysis
Analysis and curve fitting was performed with Clampfit 10 (Molecular Devices), SigmaPlot 11 (Systat Software, Inc., San Jose, CA), and Excel (Microsoft Corporation, Redmond, WA). Data are shown as mean ± S.E.M. Statistical significance was determined using Student’s two tailed t tests.
Results
Polidocanol is a mixture
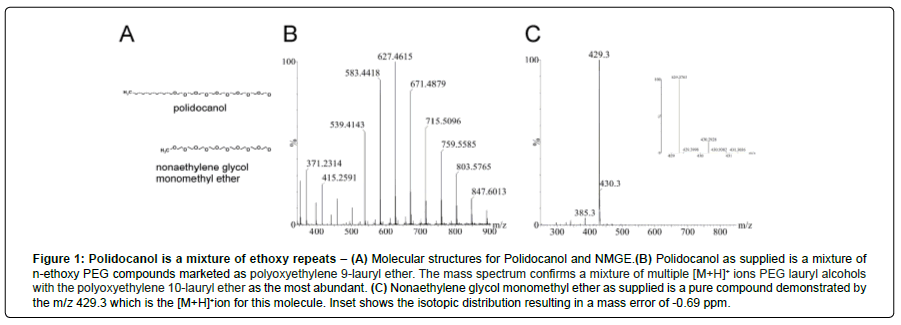
Drugs and chemicals containing polyethylene glycols are often supplied as mixtures because the various n-ethoxy derivatives are difficult to separate. This is true for the drug benzonatate, which is nominally a butylaminobenzoate oligomer with 9 ethoxy groups, but it is supplied as a mixture of oligomers with 3 to 17 ethoxy units [16]. For the present study, we measured the effects on Na+ currents of polidocanol (nonaethylene glycol monododecyl ether), nonaethylene glycol monomethyl ether (a monomethyl congener of polidocanol), and PEG400 (Figure 1A). Nonaethylene glycol monomethyl ether is the monomethyl congener of polidocanol. It is the major metabolite of the drug benzonatate, which is used clinically as an antitussive. PEG400 is a mixture of polyethylene glycols with an average molecular weight of 400 Da. The most abundant PEG in PEG400 is the 9-ethoxy congener, which has a molecular weight of 400 Da. To examine the chemical makeup of our test solutions provided by the supplier, we performed MS analysis of polidocanol and NGME. Polidocanol was found to be a mixture of ethoxy compounds that contains significant amounts of compounds with 8 to 14 ethoxy groups. The data showed 10 to be the most abundant, rather than the nominal 9 expected for nonaethylene glycol monododecyl ether (Figure 1B). In the analysis of nonaethylene glycol monomethyl ether from our supplier, we found that, in contrast, it is a pure nonaethylene compound (Figure 1C).
Figure 1: Polidocanol is a mixture of ethoxy repeats – (A) Molecular structures for Polidocanol and NMGE. (B) Polidocanol as supplied is a mixture of n-ethoxy PEG compounds marketed as polyoxyethylene 9-lauryl ether. The mass spectrum confirms a mixture of multiple [M+H]+ ions PEG lauryl alcohols with the polyoxyethylene 10-lauryl ether as the most abundant. (C) Nonaethylene glycol monomethyl ether as supplied is a pure compound demonstrated by the m/z 429.3 which is the [M+H]+ion for this molecule. Inset shows the isotopic distribution resulting in a mass error of -0.69 ppm.
Polidocanol produces slow onset Na+ channel inhibition that is not isoform-specific
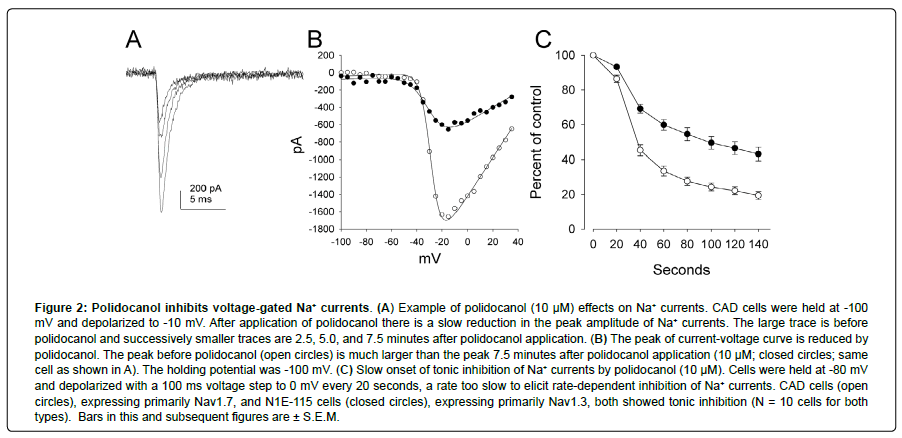
Lock of voltage-gated Na+ currents is the primary mechanism of local anesthetics, an action we find that polidocanol also has. Na+ channels have nine different isoforms, Nav1.1 through Nav1.9. Selective pain relief might be achieved with selective block of Nav1.7, which is highly expressed in sensory neurons mediating pain, but complete anesthesia is expected with nonspecific blockers. Polidocanol (10 μM) perfusion for 2 minutes produced marked inhibition of Na+ currents. With a gradual onset of action over 1-2 minutes, it inhibited both the Nav1.7 type channels found in CAD cells and the Nav1.3 type found in N1E-115 cells (Figure 2). These properties are like those of local anesthetics in that they indicate a non-isoform-specific effect, but polidocanol’s onset of action is slow by comparison.
Figure 2: Polidocanol inhibits voltage-gated Na+ currents. (A) Example of polidocanol (10 μM) effects on Na+ currents. CAD cells were held at -100 mV and depolarized to -10 mV. After application of polidocanol there is a slow reduction in the peak amplitude of Na+ currents. The large trace is before polidocanol and successively smaller traces are 2.5, 5.0, and 7.5 minutes after polidocanol application. (B) The peak of current-voltage curve is reduced by polidocanol. The peak before polidocanol (open circles) is much larger than the peak 7.5 minutes after polidocanol application (10 μM; closed circles; same cell as shown in A). The holding potential was ‑100 mV. (C) Slow onset of tonic inhibition of Na+ currents by polidocanol (10 μM). Cells were held at ‑80 mV and depolarized with a 100 ms voltage step to 0 mV every 20 seconds, a rate too slow to elicit rate-dependent inhibition of Na+ currents. CAD cells (open circles), expressing primarily Nav1.7, and N1E-115 cells (closed circles), expressing primarily Nav1.3, both showed tonic inhibition (N = 10 cells for both types). Bars in this and subsequent figures are ± S.E.M.
Polidocanol produces concentration-dependent inhibition of Na+ currents at low concentrations
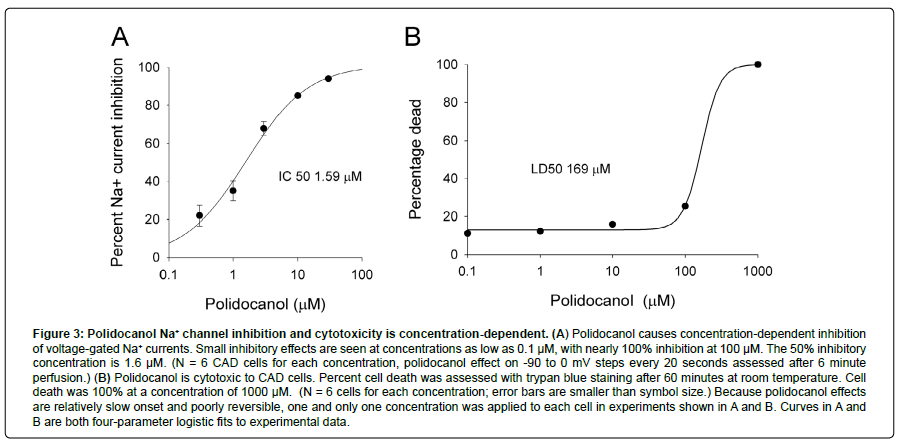
We studied the concentration-dependence of tonic inhibition in CAD cells. Polidocanol’s onset of action is slow compared to local anesthetics. We perfused polidocanol for 6 minutes and measured the inhibition produced at the end of that time. Polidocanol effectively, but slowly, inhibited Na+ currents with an IC50 of 1.59 μM, with essentially 100% inhibition at 100 μM (Figure 3A). This potency for Na+ channel inhibition is similar to that of local anesthetics. At concentrations of less than 100 μM, polidocanol did not change holding currents, indicating that it did not spontaneously open membrane channels. We could not test the effects of polidocanol on Na+ currents at a concentration of 100 μM because of very unstable recordings, possibly due to its cytotoxic effects. Na+ current inhibition by polidocanol was poorly reversible.
Polidocanol is cytotoxic at high concentrations
The most common use of polidocanol is to eliminate varicose and spider veins. Polidocanol is injected as a foam and effectively scleroses veins. This action is thought to be due to endothelial toxicity. We tested the cytotoxic effects of polidocanol on CAD cells using trypan blue staining (Figure 3B). At a concentration of 100 μM, polidocanol began to show measurable cytotoxicity in a trypan blue assay. Polidocanol at a concentration of 1000 μM caused 100% cell death. Cytotoxicity occurred at much higher concentrations than Na+ channel inhibition. Cytotoxic LD50 is estimated at 169 μM, which is over 100-fold greater than its IC50 for Na+ currents.
Figure 3: Polidocanol Na+ channel inhibition and cytotoxicity is concentration-dependent. (A) Polidocanol causes concentration-dependent inhibition of voltage-gated Na+ currents. Small inhibitory effects are seen at concentrations as low as 0.1 μM, with nearly 100% inhibition at 100 μM. The 50% inhibitory concentration is 1.6 μM. (N = 6 CAD cells for each concentration, polidocanol effect on -90 to 0 mV steps every 20 seconds assessed after 6 minute perfusion.) (B) Polidocanol is cytotoxic to CAD cells. Percent cell death was assessed with trypan blue staining after 60 minutes at room temperature. Cell death was 100% at a concentration of 1000 μM. (N = 6 cells for each concentration; error bars are smaller than symbol size.) Because polidocanol effects are relatively slow onset and poorly reversible, one and only one concentration was applied to each cell in experiments shown in A and B. Curves in A and B are both four-parameter logistic fits to experimental data.
Polidocanol produces phasic inhibition at lower concentrations than tonic inhibition, and inhibition is modestly state-dependent
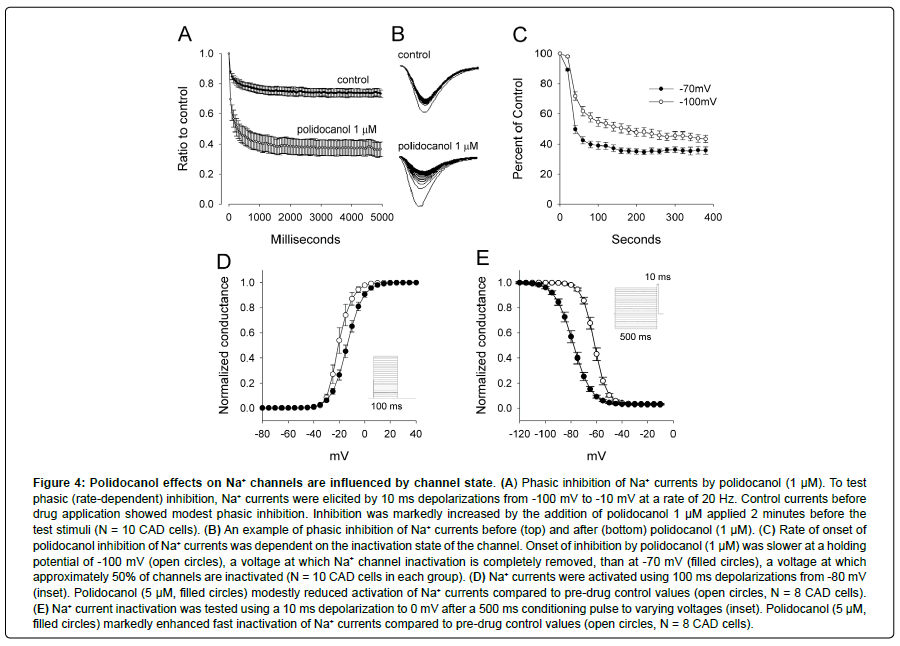
Local anesthetic action on Na+ channels is greater with rapid stimulation and is state-dependent. These effects are thought to be because anesthetics preferentially bind to the inactivated state of the Na+ channel. Like local anesthetics, polidocanol produced marked phasic inhibition, in which inhibition of Na+ channels was much more effective when stimuli were rapidly repeated (Figures 4A and 4B). Its effects were also modestly state-dependent, since polidocanol reduced sodium currents at -70 mV, a voltage at which approximately 50% of channels are inactivated, more rapidly than at -100 mV, at which inactivation is completely absent (Figure 4C). Although slower at hyperpolarized holding potentials, inhibition nevertheless reaches the same maximal value at both potentials.
Polidocanol effects on voltage-sensitive activation and inactivation
Na+ currents undergo sequential voltage-gated activation followed by voltage-gated inactivation. Local anesthetics reduce Na+ currents by enhancing voltage-gated inactivation. Activation of Na+ channels was studied by using 100 ms depolarizations from -80 mV to +40 mV in +5 mV steps. The voltage-sensitivity of activation was studied by fitting the current-conductance curve with a Boltzmann equation. Polidocanol produced a small positive shift in the curve in every one of eight CAD cells tested. The mean shift in the potential for 50% channel activation was +6.3 mV (Figure 4D, N = 8, P < 0.001, two-tailed t test).Inactivation of Na+ channels was studied using 500 ms conditioning pulses from -120 mV to 0 mV prior to a 10 ms test pulse to +10 mV. In every one of eight cells tested, polidocanol produced a marked shift of voltage-gated inactivation to more negative potentials. The mean shift in the potential for 50% channel inactivation was -15.9 mV (Figure 4E, N = 8, P <0.000001, two-tailed t test). This property is very similar to that of local anesthetics.
Figure 4: Polidocanol effects on Na+ channels are influenced by channel state. (A) Phasic inhibition of Na+ currents by polidocanol (1 μM). To test phasic (rate-dependent) inhibition, Na+ currents were elicited by 10 ms depolarizations from -100 mV to -10 mV at a rate of 20 Hz. Control currents before drug application showed modest phasic inhibition. Inhibition was markedly increased by the addition of polidocanol 1 μM applied 2 minutes before the test stimuli (N = 10 CAD cells). (B) An example of phasic inhibition of Na+ currents before (top) and after (bottom) polidocanol (1 μM). (C) Rate of onset of polidocanol inhibition of Na+ currents was dependent on the inactivation state of the channel. Onset of inhibition by polidocanol (1 μM) was slower at a holding potential of -100 mV (open circles), a voltage at which Na+ channel inactivation is completely removed, than at -70 mV (filled circles), a voltage at which approximately 50% of channels are inactivated (N = 10 CAD cells in each group). (D) Na+ currents were activated using 100 ms depolarizations from -80 mV (inset). Polidocanol (5 μM, filled circles) modestly reduced activation of Na+ currents compared to pre-drug control values (open circles, N = 8 CAD cells). (E) Na+ current inactivation was tested using a 10 ms depolarization to 0 mV after a 500 ms conditioning pulse to varying voltages (inset). Polidocanol (5 μM, filled circles) markedly enhanced fast inactivation of Na+ currents compared to pre-drug control values (open circles, N = 8 CAD cells).
Other PEG-containing compounds have little effect on Na+ currents
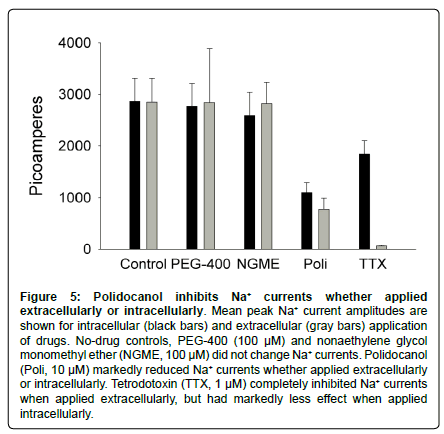
Another drug with local anesthetic properties, benzonatate, is also a polyethylene glycol-containing compound like polidocanol. We wondered whether the n-ethoxy chain of these drugs might itself have local anesthetic efficacy. We tested PEG-400, which is a mixture of n-ethoxy PEGs, with the nonaethoxy compound being the most abundant, and nonaethylene glycol monomethyl ether, which is a primary metabolite of benzonatate and is the monomethyl congener of polidocanol. The site of action of polidocanol on Na+ channels is unknown and could be intracellular. To ensure that drugs were accessible to an intracellular site for this study, we applied them both extracellularly (in the bath, not in the patch pipette) and intracellularly (in the patch pipette, not in the bath). We found that neither PEG- 400 nor nonaethylene glycol monomethyl ether, at concentrations of 100 μM, had significant effects on Na+ currents in CAD cells whether applied extracellularly or intracellularly (Figure 5). In contrast, polidocanol significantly reduced Na+ currents whether applied intracellularly or extracellularly. Tetrodotoxin (TTX), which binds to the extracellular surface of the channel pore, completely inhibited Na+ currents when applied extracellularly. It also had a partial effect when applied “intracellularly,” which may be due to leakage of TTX from the pipette prior to gigaohm seal formation.
Figure 5: Polidocanol inhibits Na+ currents whether applied extracellularly or intracellularly. Mean peak Na+ current amplitudes are shown for intracellular (black bars) and extracellular (gray bars) application of drugs. No-drug controls, PEG-400 (100 μM) and nonaethylene glycol monomethyl ether (NGME, 100 μM) did not change Na+ currents. Polidocanol (Poli, 10 μM) markedly reduced Na+ currents whether applied extracellularly or intracellularly. Tetrodotoxin (TTX, 1 μM) completely inhibited Na+ currents when applied extracellularly, but had markedly less effect when applied intracellularly.
Discussion
Polidocanol was developed in the early 1950s before the cellular mechanism of local anesthetic action was understood. We wondered if polidocanol’s mechanism of local anesthesia involved voltage-gated sodium channels, which is the cellular target of local anesthetics. We found that polidocanol inhibits voltage-gated Na+ currents in a manner similar to other local anesthetics and with similar potency. It inhibits both the Nav 1.7 channel type important in pain as well as the Nav1.3 type not involved in pain. Since polidocanol’s potency against Nav1.7 is only slightly greater, it is therefore expected to have broad anesthetic action rather than specificity for pain. Polidocanol’s inhibition of Na+ channels is similar to other local anesthetics, in that it is a) concentration-dependent, b) occurs with low micromolar concentrations, c) causes both tonic and phasic inhibition, d) affects Na+ channel voltage-dependent inactivation more than activation, and e) is not isoform-specific. However, it is unlike local anesthetics in being cytotoxic and lacking strong “state-dependent” inhibition [17].
Polidocanol is C12H25(OCH2-CH2-)nOH where n has an average value of 9. It was originally developed in the 1950s as a local anesthetic [2]with antitussive actions [3].It has never been used for those indications, although antipruritic actions may partially account for its popularity in over-the-counter skin preparations. It subsequently was found to have vein sclerotic actions, so its current most popular use, and its only FDA indication, is as a sclerotic for treatment of varicose and spider veins. Vein sclerosis likely occurs due to endothelial toxicity. As a vein sclerotic, it is highly tolerable, which could be because of its local anesthetic actions.
Clinically-used local anesthetics have three principal chemical components, a terminal amine, an intermediate linkage that is either an ester or an amide, and an aromatic ring. The first clinicallyuseful local anesthetic compound was cocaine, a naturally-occurring compound. Modern synthetic local anesthetics are structurally related to cocaine and are broadly divided into amino ester types, like cocaine, and amide types, like the popular drug, lidocaine. The mechanism of action of all of them is thought to be selective binding to the inactivated state of the Na+ channel. This property is the basis of their statedependent inhibition, having greater effects on phasic than on tonic inhibition and greater effects on voltage-dependent inactivation than on activation. Polidocanol shares these physiological properties but is dramatically different chemically. Polidocanol is an ether not an ester, is not an amide, and does not have an aromatic ring. It has a long PEG group, and therefore resembles the antitussive drug benzonatate, which also has a PEG group. Local anesthetics are supplied as a watersoluble salt, but it is thought that they bind intracellularly in their uncharged form which is lipid-soluble and can penetrate membranes. Polidocanol is uncharged. Its PEG group is amphipathic and its dodecyl group highly lipid-soluble. It is reasonable to speculate that the PEG group allows polidocanol to bind to the membrane outer leaf with the dodecyl group projecting intramembranously. It appears that the PEG group alone is insufficient to act on Na+ channels since PEG-400 has no effect on Na+ channels.
Although PEG alone has no effect on Na+ channels, other PEG-containing compounds can be quite potent. The antitussive benzonatate has a PEG group with an average ethoxy N of 9 and is a potent Na+ channel inhibitor [16,18]have shown that micelle-forming amphiphiles containing PEGs, such as Triton X-100 and Genapol X-100 (GX100) have potent local anesthetic-type effects on Na+ channels. They hypothesize that the local anesthetic actions of these micelle-forming amphiphiles depend on local membrane bending in the vicinity of the Na+ channel. GX100 resembles polidocanol in having an alkyl group attached by an ether linkage to a 9-ethoxy PEG group, but in GX100, the alkyl group has 13 carbons. Comparison between the two suggests that a PEG ether-linked to a straightcarbon chain can be a potent anesthetic. The observed lack of efficacy of nonaethylene glycol monomethyl ether on Na+ channels suggests that the monomethyl group may simply be too short to penetrate the membrane to have an effect. The number of ethoxy groups in the PEG most likely to affect Na+ channels is unknown, but past research suggests than N = 9 is most effective for pulmonary receptor effects and antitussive action [19].
Polidocanol acts as a local anesthetic at low concentrations by inhibiting voltage-gated sodium channels. At high concentrations, it is cytotoxic. These two attributes may work together to contribute to its tolerability and effectiveness as a vein sclerosing agent.
Author conflicts of interest
None
Acknowledgements
Funding: This work was supported by the University of Louisville (M.S. Evans) and the University of Illinois at Springfield (S.R. Johnson).
References
- Geimer R (1953) [Dermatological application and indications of thesit-containing external preparations]. Dermatol Wochenschr 128: 829-837.
- Soehring K, Frahm M, Miletzko K (1952) [Pharmacology of alkylpolyethylene oxide derivatives. IV. Local analgetic effects and observations on comparative evaluation of conduction inhibitors]. Arch Int Pharmacodyn Ther 91: 112-130.
- Zipf HF, Reichertz P (1957) [Production of total & reversible endoanesthesia of the pulmonary stretch receptors by means of an effect-time curve]. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol 231: 96-110.
- Ebrahimi SRS, Enamzadeh E, Babaei H (2017) An Evidence-Based Review of Off-Label Uses of Polidocanol. Curr Clin Pharmacol 12: 223-230.
- Eckmann DM, Kobayashi S, Li M (2005) Microvascular embolization following polidocanol microfoam sclerosant administration. Dermatol Surg 31: 636-643.
- Goldman PM (1989) Polidocanol (aethoxysklerol) for sclerotherapy of superficial venules and telangiectasias. J Dermatol Surg Oncol 15: 204-209.
- Bottaro E, Paterson JAJ, Quercia L, Zhang X, Hill M, et al. (2019) In vitro and ex vivo evaluation of the biological performance of sclerosing foams. Scientific Reports 9: 9880
- Whiteley MS, Dos Santos SJ, Fernandez-Hart TJ, Lee CT, Li JM (2016) Media Damage Following Detergent Sclerotherapy Appears to be Secondary to the Induction of Inflammation and Apoptosis: An Immunohistochemical Study Elucidating Previous Histological Observations. Eur J Vasc Endovasc Surg 51: 421-428.
- Guex JJ, Schliephake DE, Otto J, Mako S, Allaert FA, (2010) The French polidocanol study on long-term side effects: a survey covering 3,357 patient years. Dermatol Surg 2: 993-1003.
- Gatz M, Schrading S, Dirrichs T, Betsch M, Tingart M, et al. (2017) Topical polidocanol application in combination with static stretching in tendinopathies: a prospective pilot study. Muscles LigamentsTendons J 7: 88-97.
- Zeisig E, Fahlstrom M, Ohberg L, Alfredson H (2008) Pain relief after intratendinous injections in patients with tennis elbow: results of a randomised study. Br J Sports Med 42: 267-271.
- King AM, Yang XF, Wang Y, Dustrude ET, Barbosa C, et al. (2012) Identification of the benzyloxyphenyl pharmacophore: a structural unit that promotes sodium channel slow inactivation. ACS Chem Neurosci 3: 1037-1049.
- Wang Y, Park KD, Salome C, Wilson SM, Stables JP, et al. (2011) Development and characterization of novel derivatives of the antiepileptic drug lacosamide that exhibit far greater enhancement in slow inactivation of voltage-gated sodium channels. ACS Chem Neurosci 2: 90-106.
- Jo S, Bean BP (2011) Inhibition of neuronal voltage-gated sodium channels by brilliant blue G. Mol Pharmacol 80: 247-257.
- Wang H, Olsen RW (2000) Binding of the GABA(A) receptor-associated protein (GABARAP) to microtubules and microfilaments suggests involvement of the cytoskeleton in GABARAPGABA(A) receptor interaction. J Neurochem 75: 644-655.
- Evans MS, Maglinger GB, Fletcher AM, Johnson SR (2016) Benzonatate inhibition of voltage-gated sodium currents. Neuropharmacology 101: 179-187.
- Wang GK, Strichartz GR (2012) State-Dependent Inhibition of Sodium Channels by Local Anesthetics: A 40-Year Evolution. Biochem (Mosc) Suppl Ser A Membr Cell Biol 6: 120-127.
- Lundbaek JA, Birn P, Hansen AJ, Sogaard R, Nielsen C, et al.(2004) Regulation of sodium channel function by bilayer elasticity: the importance of hydrophobic coupling. Effects of Micelle-forming amphiphiles and cholesterol. J Gen Physiol 123: 599-621.
- Bucher K (1956) [New effect mechanism of the antitussive drug tessalon]. Schweiz Med Wochenschr 86: 94-96.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi