Research Article, Vector Biol J Vol: 3 Issue: 1
Potential of Nardostachys jatamansi Extracts to Manage Indian Strain of Aedes aegypti: A Novel Approach for Vector Control
Sarita Kumar*, Aarti Sharma and Radhika Warikoo
Department of Zoology, Acharya Narendra Dev College, University of Delhi, New Delhi, India
*Corresponding Author : Sarita Kumar
Department of Zoology, Acharya Narendra Dev College, University of Delhi, Kalkaji, New Delhi 110019, India
Tel: 91 11 2629 4542
E-mail: saritakumar@andc.du.ac.in
Received: March 20, 2018 Accepted: July 10, 2018 Published: July 25, 2018
Citation: Kumar S, Sharma A, Warikoo R (2018) Potential of Nardostachys jatamansi Extracts to Manage Indian Strain of Aedes aegypti: A Novel Approach for Vector Control. Vector Biol J 3:1. doi: 10.4172/2473-4810.1000129
Abstract
The antagonistic impacts of chemical toxicants-based intrusion measures for the control of mosquitoes have received extensive public apprehension because of numerous problems; including insecticide resistance, revival of pests, environmental contamination, lethal effects on humans and other non-target organisms. These complications have reasoned the requirement to explore and formulate the alternative plant-based strategies possessing eco-safety, bio-degradability and non-toxicity to nontarget organisms. Spikenard plant, Nardostachys jatamansi also known as the healing oil is grown in Northern India and China. Though earlier used as perfume and for healing purposes, it is not widely used today. In the current study, the efficacy of the roots of spikenard plant, Nardostachys jatamansi were explored against larvae of dengue vector, Aedes aegypti. The roots were separately extracted in two solvents; hexane and petroleum ether; which were then assessed against early fourth instars of Aedes aegypti as per procedure recommended by WHO. The larvae dead and in the moribund state were scored after 24 h of exposure and data was analysed statistically to calculate LC values. Both extracts proved to possess excellent larvicidal potential. The bioassays with hexane roots extracts resulted in LC50 and LC90 value of 140.64 and 302.54 ppm, respectively after 24 h of exposure, while the extracts prepared from petroleum ether showed LC50 and LC90 value of 84.50 and 214.12 ppm, respectively. The petroleum ether root extract exhibited 1.7–fold more larvicidal potential as compared to hexane root extract. The treated larvae that remained alive formed larvalpupal intermediates establishing the delayed toxic effects of the extracts. Further investigations are required to assess the impact of extracts on non-target organisms and explore the suitability of its use in the fields.
Keywords: Nardostachys jatamansi; Aedes; Larvicidal; Anal papillae; Demelanization; Intermediates
Introduction
With the potential to transmit several diseases and affect more than a million populations across the globe, mosquitoes remain “public enemy number one” as has been asserted by the World Health Organisation [1]. The Dengue fever mosquito, Aedes aegypti, is one of the most widespread and serious disease vectors causing severe health hazards especially Zika fever, dengue fever, dengue hemorrhagic fever, and dengue shock syndrome [2]. The control of dengue fever has become an imperative concern from the public health perspective as the figures of reported cases continue to rise year after year [3].
Since long, mosquito control program is principally dependent on the application of synthetic insecticides in the fields. Nevertheless, continued application of these chemicals has disturbed native biotic systems and has resulted in the recovery of mosquito populations. Moreover, the discerning pressure of these toxicants has increased the rate of resistance development in mosquito populations at an alarming rate [4] which has then amplified the need of new products that are environmentally safe, degradable and target-specific.
Co-evolution has fortified plants with overabundance of chemical defenses against insect predators. This benefit has been exploited by mankind to use various plant parts or their extracts to control insects since ancient times. Phytocomponents are being explored as alternate agents of mosquito managements because of the occurrence of abundant biodegradable bioactive compounds in them [5-7]. They are known to interrupt transmission of mosquito-borne diseases at both individual and community level.
Nardostachys jatamansi is a persistent herb that grows in West and Northwest China, Bhutan, India and Nepal. In traditional Chinese medicine, the roots and rhizomes of N. jatamansi are used for their sedative and tonic effects. It is also used as a stimulant, antiseptic, insect repellent and for the treatment of epilepsy, hysteria, convulsive affections, stomachache, constipation and cholera in Ayurvedic and Unani systems of medicine [8]. Previous phytochemical investigations of N. jatamansi revealed the presence of phenolic compounds, caffeoylquinic acid derivatives, lignans, neolignans, monoterpenoids, sesquiterpenoids, diterpenoids, and iridoids [9-11].
The available literature, however, reveals that the larvicidal and growth-inhibiting effects induced by this plant have not been explored against mosquito vectors. Hence, this study was undertaken to assess the potential of this herb as an effective mosquito control agent to manage Ae. aegypti populations. The results of the present study would be valuable in encouraging research directing at the formulation of novel agent for mosquito control based on bioactive compounds isolated from native plant source.
Materials and Methods
Mosquito rearing
Present studies are conducted on the early fourth instars of Ae. aegypti collected from Delhi, India and neighbouring areas. The colony of dengue vector was maintained in an insect culture laboratory at controlled conditions of 80% ± 5% RH, 28ºC ± 1ºC and 14L/10D photoperiod [12]. Adults were fed on soaked raisins while female adults were given blood meals on alternate days for egg maturation. The enamel bowl lined with Whatman filter paper were placed in the cages for collection of eggs which were then transferred to de-chlorinated water-filled enamel trays for hatching. A mixture of grinded dog biscuits and yeast powder (1:3) was added to the water to provide nutrition for the larvae. The larvae were reared and pupae collected were transferred to the cages for emergence.
Plant collection and preparation of plant extract
Roots of the N. jatamansi plant were collected from areas in and around New Delhi, India. The roots were cleaned, washed with distilled water and kept under shade at room temperature (27ºC ± 2ºC) for about 3 weeks. The dried roots were scrutinized for any infection and crushed with the help of mortar and pestle. The powdered roots were sieved methodically to obtain fine powder.
The powdered roots were weighed and two parts of 200 g each were separated. These were extracted with one litre of two different solvents separately; viz. petroleum ether (PE) and hexane. The solvents were selected on the basis of their polarity. The extraction was performed in a soxhlet apparatus at a temperature not beyond the solvent boiling temperature. The extraction was carried out for 3 days, eight hours per day. The extracts were concentrated at 45ºC through a Buchi vacuum evaporator under low pressure which were then stored in a refrigerator in the form of stock solution of 1000 ppm.
Larval bioassay of Nardostachys jatamansi root extracts against Ae. aegypti
The extracts formed were assessed for their anti-mosquito potential against Ae. aegypti. The dilutions of both the hexane and PE root extracts were prepared with ethanol as the solvent. For each assay, 1 mL of each root extract was mixed with 100 mL of distilled water and shaken vigorously to ensure a consistent test solution. The early fourth instars of Ae. aegypti, in batches of 20, were taken out in 99 mL of distilled water and transferred to distilled water-extract mixture. For each extract, a total of four replicates were carried out concurrently along with controls where larvae were exposed to the ethanol-distilled water mixture. The dead and moribund larvae were scored after 24 h. The tests were repeated with other extract to measure the larvicidal effectiveness of N. jatamansi against Ae. aegypti.
Statistical analysis of data
Larvicidal assays resulting in control mortality and pupae higher than 20% were rejected and conducted again. The control mortality ranged below 20% was adjusted using Abbott’s formula [13]. The data were statistically analysed by SPSS 19.0 Programme. The lethal concentrations causing 50% and 90% larval mortality were calculated with 95% confidence limits in respective bioassay in order to estimate variations between the larval samples.
Morphological studies in extract-treated Ae. aegypti larvae
During each assay, the exposed larvae were examined for morphological modifications under light microscope. All body segments; and other organs such as antennae, eyes, mouth brushes, setae and anal gills were observed and compared with those of the controls. The exposed larvae were also observed prudently for any deviations in pigmentation.
Delayed toxicity effects on the survived larvae of ‘treated’ Ae. aegypti
The larvae that survived were studied till the next generation to observe any developmental abnormalities; such as formation of larval-pupal intermediates or pupal-adult intermediates; and delayed toxicity effects.
Results and Discussion
The present studies explored the bio-efficacy of hexane and petroleum ether extracts; prepared from the roots of N. jatamansi; as larvicidal agents against early fourth instars of Ae. aegypti (Table 1). The assays demonstrated 1.7-fold higher cidal potential of petroleum ether (PE) root extracts as compared to the hexane root extracts. Larval exposure to PE root extract resulted in LC50 and LC90 values of 84.50 ppm and 214.12 ppm, respectively against early fourth instars of Ae. aegypti, while treatment with hexane root extract revealed respective lethal values of 140.64 ppm and 302.54 ppm
| Extract | Larvicidal activity | S.E. | χ2 (df) | Regression Coefficient | |
|---|---|---|---|---|---|
| LC 50 (ppm) | LC 90 (ppm) | ||||
| Hexane Root Extract |
140.64 (89.807-207.408) | 302.54 (191.136-469.695) | 0.52 | 3.192 (4) | 3.668 |
| Petroleum Ether Root Extract |
84.50 (79.987-106.554) | 214.12 (128.987-324.152) | 0.96 | 2.809 (4) | 5.467 |
Table 1: Larvicidal activity of the root extracts of Nardostachys jatamansi against early fourth instars of Ae.aegypti. Values in parentheses indicate the lower and upper 95% fiducial limits. No mortality was observed in the control. LC50 - lethal concentration that kills 50 % of the exposed larvae, LC90 - lethal concentration that kills 90% of the exposed larvae; S.E.=Standard error, χ2=chi-square, df=degree of freedom, Test samples were transformed into log covariant (log10), P>0.05, level of significance is greater than 0.05, no heterogeneity factor is used in the calculation of confidence limits, Values are mean of three replicates;
Jang et al. [14] testified more than 90% larval mortality in Ae. aegypti and Cx. pipiens when exposed to 200 ppm of Cassia obtusifolia, C. tora and Vicia tetrassperma methanol extracts. Leaf extracts of Cassia fistula in different solvents viz.methanol, benzene and acetone have also been found highly effective against Ae. aegypti larvae resulting in LC50 values of 10.69 mg/L, 18.27 mg/L and 23.95 mg/L, respectively [15]. Another study conducted by Govindarajan [16] evaluated the efficacy of ‘king of bitters’ Andrographis paniculata leaf extracts against third instars of Ae. aegypti and Cx. quinquefasciatus and found it to be more effective against Ae. aegypti. The LC50 values of benzene, hexane, ethyl acetate, methanol and chloroform extracts of A. paniculata leaves reported were 112.19, 137.48, 118.67, 102.05, 91.92 ppm against early third instars of Cx. quinquefasciatus and 119.58, 146.34, 124.24, 110.12, 99.54 ppm against Ae. aegypti, respectively revealing the chloroform extracts being most effective.
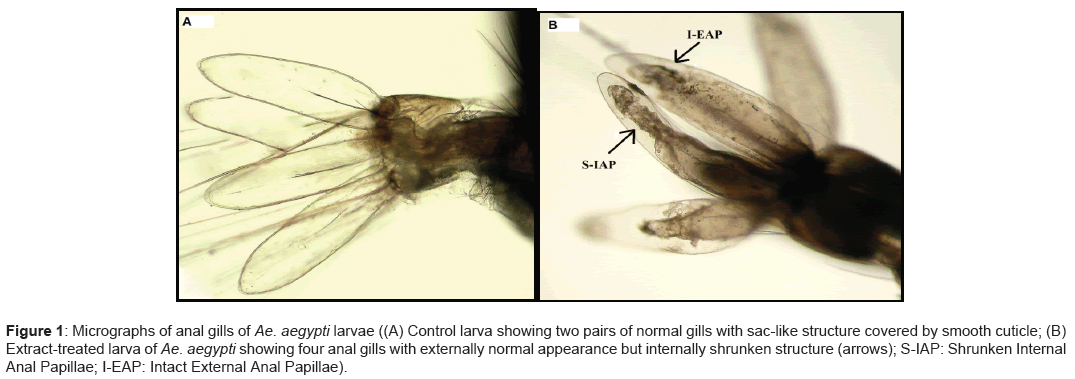
Studies on the larval morphological modifications discovered a conspicuous variation in the anal papillae of the larvae exposed to N. jatamansi root extract as compared to the control larvae. The anal gills of the exposed larvae established destruction in the form of internal cuticular contraction whereas the external membrane was integral and undamaged (Figures 1 A and B). Furthermore, the remaining morphological architecture and cuticular sculpturing of the body segments and other organs; such as antennae, eyes, setae, siphon, and, mouth and ventral brushes did not show any noteworthy alteration. Similarly shrunken anal papillae have been found in the Ae. aegypti larvae after exposure with ethanolic extracts of Piper nigrum, while remaining organs had a typical appearance [5,17]. Chaithong et al. [17] also recommended disruption of the osmotic and ionic regulation caused by structural distortion of the anal papillae which may have resulted in larval dysfunction leading to the mortality of dengue vector larvae. Another notable observation was the reduced cuticular pigmentation in the exposed larvae suggesting de-melanization caused by extract (Figure 2).
Figure 1: Micrographs of anal gills of Ae. aegypti larvae ((A) Control larva showing two pairs of normal gills with sac-like structure covered by smooth cuticle; (B) Extract-treated larva of Ae. aegypti showing four anal gills with externally normal appearance but internally shrunken structure (arrows); S-IAP: Shrunken Internal Anal Papillae; I-EAP: Intact External Anal Papillae).
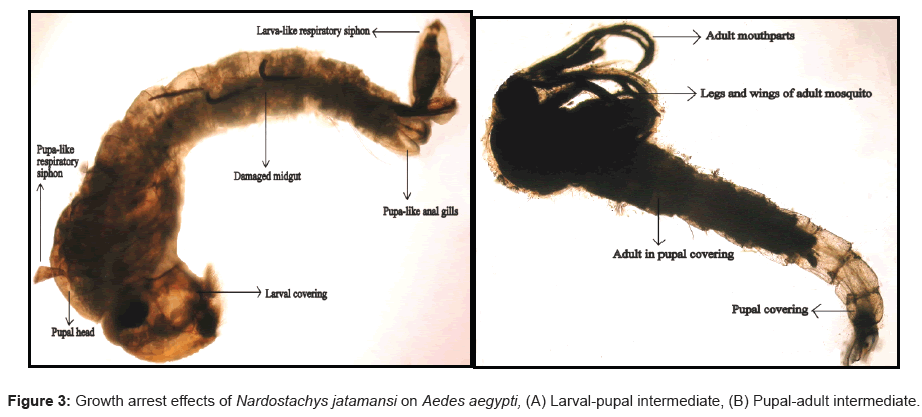
Both the petroleum ether and hexane extracts showed significant effect on the growth and development of dengue larvae. Our investigations exhibited hexane root extract as the more efficient extract in terms of growth-arrest potency resulting in the formation of as high as 8.93% of larval-pupal intermediates and 1.79% of pupal-adult intermediates (Figure 3). These studies were in concordance with the studies conducted by Sakthivadivel and Thilagavathy [18] who reported 57% larval mortality, a prolonged larval growth duration of 11 days, development of 9% larval-pupal intermediates and 9% pupal mortality, formation of 5% pupal–adult intermediates and 2% adult mortality in Ae. aegypti. Morphogenetic abnormalities among the developmental stages of Ae. aegypti, Anopheles stephensi and Cx. quinquefasciatus have been described on treatment with lower concentrations of certain plant extracts viz., Madhuca longifolia, Ageratum conyzoides and Acorus calumus [19]. Such prolonged larval and pupal periods, and development of intermediates designates the intrusion of the bioactive compounds present in the extracts with the regular hormonal synchronization of the metabolic processes of the developing mosquito stages.
Gas chromatography-mass spectrometry (GC-MS) analysis of essential oil water-distilled from N. jatamansi roots resulted in the identification of twenty-nine compounds which accounted for 98.1% of the total oil [20]. The prime components recognized in their order of decreasing amounts were calerene (25.9%)>patchouol (10.6%)>α-gurjunene (7.5%)>aristolone (7.1%)>β-maaliene (6.5%)>spathulenol (4.3%). The analysis also showed the presence of much higher sesquiternoids content (85.4%) in the essential oil than monoterpenoids (8.5%). The component, patchoulol has been established to display pupicidal and repellent properties against mosquitoes; Ae. aegypti, An. stephensi and Cx. quinquefasciatus [21].
An attempt has been made to assess the potential role of N. jatamansi root extracts to control mosquitoes. Due to their nonbiodegradability and less harmful effects on non-target organisms, natural plant products are being preferred over the conventional chemical insecticides which have now developed resistance amongst the mosquito population. Thus, it is worthwhile to identify new bioactive compounds from natural products against mosquitoes. Further studies of the active principles involved and their mode of action, formulated preparations for enhancing potency and stability, effects on non-target organisms and the environment are needed along with the field trials to recommend N. jatamasni as an anti-mosquito product to combat and protect from mosquitoes in a control program.
References
- World Health Organization (1996) Report of the WHO informal consultation on the evaluation on the testing of insecticides, CTD/WHO PES/IC/96.1. WHO, Geneva.
- Pancharoen C, Kulwichit W, Tantawichien T, Thisyakorn U, Thisyakorn C (2002) Dengue infection: a global concern. J Med Assoc Thai 85: 25-33.
- National Vector Borne Disease Control Programme (NVBDCP) (2018) Dengue Cases and Deaths in the Country since 2007.
- Mohan DR, Ramaswamy M (2007) Evaluation of larvicidal activity of the leaf extract of a weed plant, Ageratina adenophora, against two important species of mosquitoes, Aedes aegypti and Culex quinquefasciatus. Afr J Biotechnol 6: 631-638.
- Kumar S, Warikoo R, Wahab N (2010) Larvicidal potential of ethanolic extracts of dried fruits of three species of peppercorns against different instars of an Indian strain of dengue fever mosquito, Aedes aegypti L. (Diptera: Culicidae). Parasitol Res 107: 901-907.
- Kumar S, Singh AP, Nair G, Batra S, Seth A, et al. (2011a) Impact of Parthenium hysterophorus leaf extracts on the fecundity, fertility and behavioural response of Aedes aegypti L. Parasitol Res 108: 853-859.
- Kumar S, Warikoo R, Misha M, Seth A, Wahab N (2011b) Larvicidal efficacy of the Citrus limetta peel extracts against Indian strains of Anopheles stephensi Liston and Aedes aegypti L. Parasitol Res 111: 173-178.
- Parveen Z, Siddique S, Shafique M, Khan SJ, Khanum R (2011) Volatile constituents, antibacterial and antioxidant activities of essential oil from Nardostachys jatamansi DC. Roots. Pharmacol 3: 329-337.
- Chatterjee A, Basak B, Saha M, Dutta U, Mukhopadhyay C, et al. (2000) Structure and stereochemistry of nardostachysin, a new terpenoid ester constituent of the rhizomes of Nardostachys jatamansi. J Nat Prod 63: 1531-1533.
- Rao GV, Annamalai T, Mukhopadhyay T (2008) A new sesquiterpene aldehyde from the plant, Nardostachys jatamansi DC. Indian J Chem 47B: 163-165.
- Liu ML, Duan YH, Zhang JB, Yu Y, Dai Y, et al. (2013) Novel sesquiterpenes from Nardostachys chinensis Batal. Tetrahedron 69: 6574-6578.
- Warikoo R, Wahab N, Kumar S (2011) Larvicidal potential of commercially available pine (Pinus longifolia) and cinnamon (Cinnamomum zeylanicum) oils against an Indian strain of dengue fever mosquito, Aedes aegypti L. (Diptera: Culicidae). Acta Entomol Sinica 54: 793-798.
- Abbott WB (1925) A method for computing the effectiveness of an insecticide. J Econ Entomol 18: 265-267.
- Jang YS, Jeon JH, Lee HS (2005) Mosquito larvicidal activity of active constituent derived from Chamaecyparis obtusa leaves against 3 mosquito species. J Am Mosq Control Assoc 21: 400-403.
- Govindarajan M (2009) Bioefficacy of Cassia fistula Linn. (Leguminosae) leaf extract against Chikungunya vector, Aedes aegypti (Diptera: Culicidae). Eur Rev Med Pharmacol Sci 13: 99-103.
- Govindarajan M (2011) Evaluation of Andrographis paniculata Burm.f. (Family: Acanthaceae) extracts against Culex quinquefasciatus (Say.) and Aedes aegypti (Linn.) (Diptera: Culicidae). Asian Pac J Trop Med 3: 176-181.
- Chaithong U, Choochote W, Kamsuk K, Jitpakdi A, Tippawangkosol P, et al. (2006) Larvicidal effect of pepper plants on Aedes aegypti (L.) (Diptera: Culicidae). J Vector Ecol 31: 138-143.
- Sakthivadivel M, Thilagavathy D (2003) Larvicidal and chemosterilant activity of the acetone fraction of petroleum ether extract from Argemone mexicana L. seed. Biores Tech 89: 213-216.
- Sujatha CH, Vasuki V, Mariappan T, Kalyanasundaaram M, Das PK (1988) Evaluation of plant extracts for biological activity against mosquitoes. Inter Pest Contr 30: 122-124.
- Liu XC, Liu ZL (2014) Evaluation of insecticidal activity of Nardostachys jatamansi essential oil against some grain storage insects. J Entomol Zool Studies 2: 335-340.
- Gokulakrishnan J, Kuppusamy E, Shanmugam D, Appavu A, Kaliyamoorthi K (2013) Pupicidal and repellent activities of Pogostemon cablin essential oil chemical compounds against medically important human vector mosquitoes. Asian Pac J Trop Dis 3: 26-31.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi