Research Article, Vector Biol J Vol: 2 Issue: 2
Profile of The Internal Transcribed Spacer 2 in Ribosomal DNA of Tabanus rubidus in Thailand and Myanmar; The Possibility of Using for Molecular Identification
Apiwat Tawatsin1, Payu Bhakdeenuan1, Yang Chao2, Tomoko Amano3, Hitoshi Sasaki2, Usavadee Thavara1, Somchai Sangkitporn1, Theeraphap Chareonviriyaphap4 and Padet Siriyasatien5*
1Department of Medical Sciences, Ministry of Public Health, Nonthaburi, Thailand
2Laboratory of Entomology, Department of Dairy Science, Rakuno Gakuen University, Hokkaido, Japan
3Laboratory of Animal genetics, Department of Animal genetics, Rakuno Gakuen University, Hokkaido, Japan
4Department of Entomology, Faculty of Agriculture, Kasetsart University, Bangkok, Thailand
5Department of Parasitology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand
*Corresponding Author : Padet Siriyasatien
Department of Parasitology, Faculty of Medicine, Chulalongkorn University, Bangkok 10330, Thailand
Tel: 66 (2) 256 4387
E-mail: padetcu@gmail.com
Received: September 23, 2017 Accepted: October 16, 2017 Published: October 23, 2017
Citation: Tawatsin A, Bhakdeenuan P, Chao Y, Amano T, Sasaki H, et al. (2017) Profile of The Internal Transcribed Spacer 2 in Ribosomal DNA of Tabanus rubidus in Thailand and Myanmar; The Possibility of Using for Molecular Identification. Vector Biol J 2:2. doi: 10.4172/2473-4810.1000121
Abstract
Tabanus rubidus is one of the most medically and veterinary important haematophagous insects. We demonstrated the profile of the Internal Transcribed Spacer 2 (ITS2) of ribosomal DNA in T. rubidus collected from various regions of Thailand and Myanmar. The ITS2 sequences in this study is high in A+T content (80.1%) and ranged from 589 to 622 bp. The highest intra and interspecific divergence among samples from same region was 1.2% and 11.2%, respectively. The sequences from this study were grouped by the same collecting region into individual clade using the Neighbor Joining with Kimura 2-Parameter tree. The data obtained in this study is useful for accurate species identification using ITS2 sequence which is the first step to provide the biological data for better integrated vector management programs for surra disease.
Keywords: Tabanus rubidus; Internal transcribed spacer 2; ITS2; Molecular identification
Introduction
Flies of the family Tabanidae are medically and economically important pests. These flies represent a serious nuisance in live stocks not only because of their painful biting and causing extreme annoyance, but also because sometimes they can be the mechanical vectors of Trypanosomiasis such as surra disease [1]. Surveillance method plays an important role for manage the integrated control strategies for Tabanids. However, this method required not only the biological information but also accurate species identification. Although traditional species identification in Tabanidae can particularly demonstrate in the genitalia and the external morphology [2], high experience and complete sample are required. At present, Molecular technique has been choosing for dissolved problems in taxonomic species discrimination because this technique is accurate, highly sensitive and can be generated even in damaged samples [3,4]. The Internal Transcribed Spacer 2 (ITS2) in ribosomal RNA transcribed region is one of the important DNA markers. This region is usually used as a tool for species identification due to it is highly reserved and well different sequence between spices [3-5].
This study generated the variation of the ITS2 in Tabanus rubidus which are known as an important species in Tabanidae and found worldwide especially in tropical areas [6,7]. We analyzed the ITS2 sequences in 29 samples collected from various regions in Thailand and Myanmar. Results from this study are valuable for manage the integrated control strategies of Tabanids files.
Material and Methods
Specimen collection
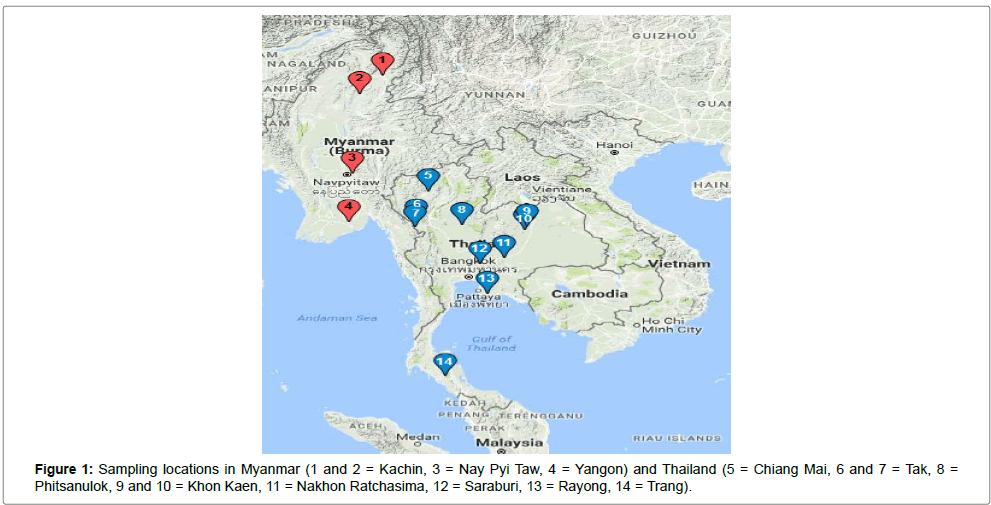
Tabanus rubidus specimens in this study (Table 1) were collected from various regions of Thailand and Myanmar during 2009–2015 by using the SASA99 trapping. Sampling locations in Thailand include of Chiang Mai, Tak, Phitsanulok, Khon Kaen, Nakhon Ratchasima, Saraburi, Rayong and Trang. Collecting sites in Myanmar include of Kachin, Nay Pyi Taw and Yangon. All specimens were identified to species level using a standard morphologically taxonomic key and preserved by air dried on pins until next step.
| Code | Sample sizes | Location | Locality | Date of collection | ITS2 length (bp) |
Latitude | Longitude | Accession number |
|---|---|---|---|---|---|---|---|---|
| R1 | 1 | Myanmar | Kachin 1 | 15/06/2010 | 592 | 25°24'31"N | 97°23'40"E | KY009684 |
| R2-R3 | 2 | Myanmar | Kachin 2 | 16/06/2010 | 591 | 24°22'20"N | 96°32'13"E | KY009682-3 |
| R4-R6 | 3 | Myanmar | Nay Pyi Taw | 12/01/2011 | 591/589/590 | 19°50'25"N | 96°15'59"E | KY009679-80 |
| R7 | 1 | Myanmar | Yangon | 20/06/2010 | 591 | 16°59'54"N | 96°08'53"E | KY009678 |
| R8-R10 | 3 | Thailand | Chiang Mai [Muang] | 29/09/2013 | 595 | 18°48'47"N | 99°04'15"E | KY009667-9 |
| R11 | 1 | Thailand | Tak [Mae Sot] | 20/09/2013 | 595 | 16°43'38"N | 98°33'42"E | KY009675 |
| R12-R13 | 2 | Thailand | Tak [Mae Ramat] | 20/09/2013 | 595 | 17°03'02"N | 98°36'46"E | KY009676-7 |
| R14-R16 | 3 | Thailand | Phitsanulok | 03/05/2011 | 598 | 16°49'51"N | 100°17'26"E | KY009664-6 |
| R17-R18 | 2 | Thailand | Khon Kaen [Ubonrat] | 05/05/2015 | 597 | 16°46'13"N | 102°38'20"E | KY009672-3 |
| R19 | 1 | Thailand | Khon Kaen [Ban fang] | 04/05/2015 | 597 | 16°33'53"N | 102°34'03"E | KX128914 |
| R20-R22 | 3 | Thailand | Nakhon Ratchasima | 23/11/2009 | 597 | 14°53'45"N | 101°50'07"E | KX128916-7, KY009674 |
| R23 | 1 | Thailand | Saraburi [Chaloem Phrakiat] | 03/03/2010 | 595 | 14°34'32"N | 100°55'22"E | KY009671 |
| R24-R26 | 3 | Thailand | Rayong [Nikhom Pattana] | 02/05/2012 | 596 | 12°46'42"N | 101°12'47"E | KX128912-3, KY009670 |
| R27-R29 | 3 | Thailand | Trang [Huay Yot] | 29/04/2014 | 622 | 7°46'53"N | 99°40'09"E | KU982612-4 |
Table 1: Details of T. rubidus samples that were collected from various regions of Thailand and Myanmar during 2009-2015.
DNA preparation
Genomic DNA was extracted from two legs of individual specimens by using the ISOHAIR® extraction kit (Nippon Gene, Japan), following the manufacturer’s manual. The extracted DNA concentration was quantified on NanoDrop ND-1000 spectrophotometer (Thermoscientific, DE, USA) and stored at 4ºC for further analysis.
Polymerase chain reaction
PCR amplification of ITS2 was conducted using the primer designed by Song [8]. The sequence of forward and reward primer are 5’TGCTTGGACTACATATGGTTGA3’ and 5’GTAGTCC CATATGAGTTGAGGTT3’, respectively.
PCR was performed using GoTaq Green Master Mix® (Promega, USA). The PCR reaction composed of 0.3 μl of template DNA solution (200 ng/ μl), 0.125 μl of 10 μM of each primer, 5.0 μl of GoTaq Green master mix and 5.0 μl of distilled water with the following cycling condition: 5 min at 94°C; followed by 30 cycles of 1 min at 94°C, 1 min at 47°C, 45 sec at 72°C and finally extension at 72°C for 10 min.
PCR amplicons were electrophoresed in a 1.0% agarose gel stained with the nucleic acid staining solution, RedSafeTM (iNtRON Biotechnology, Japan).
Cloning and sequencing
PCR products of each samples were cloned using the pGEM®-T Easy Vector (pGEM®-T Easy Vector System I; Promega, USA) according to the manufacturer’s manual. The recombinant plasmid DNA was extracted by using FavorPrepTM Plasmid DNA Extraction Mini kit (Favorgen Biotech Corp., Taiwan), following the manufacturer’s instruction. Plasmid DNA amount were quantified on a NanoDrop ND-1000 spectrophotometer (Thermo-scientific, USA). Two recombinant colonies from each specimens were chosen for DNA sequencing which was done by Applied Biosystems Corporation in Japan, using M13F (-20) primer (5’ GTAAAACGACGGCCAGT 3’). The consensus sequence was then used for further analysis.
Sequence and phylogenetic analysis
Sequence electropherograms from each individual samples were checked and manually edited with BioEdit Sequence Alignment Editor Program Version 7.2.5 [9]. The 5’-ends and 3’-ends of all ITS2 sequences were determined according to the sequences submitted by Tautz [10] and Song [8]. Further all completed sequences in this study were submitted to the GenBank database to be assigned the accession numbers.
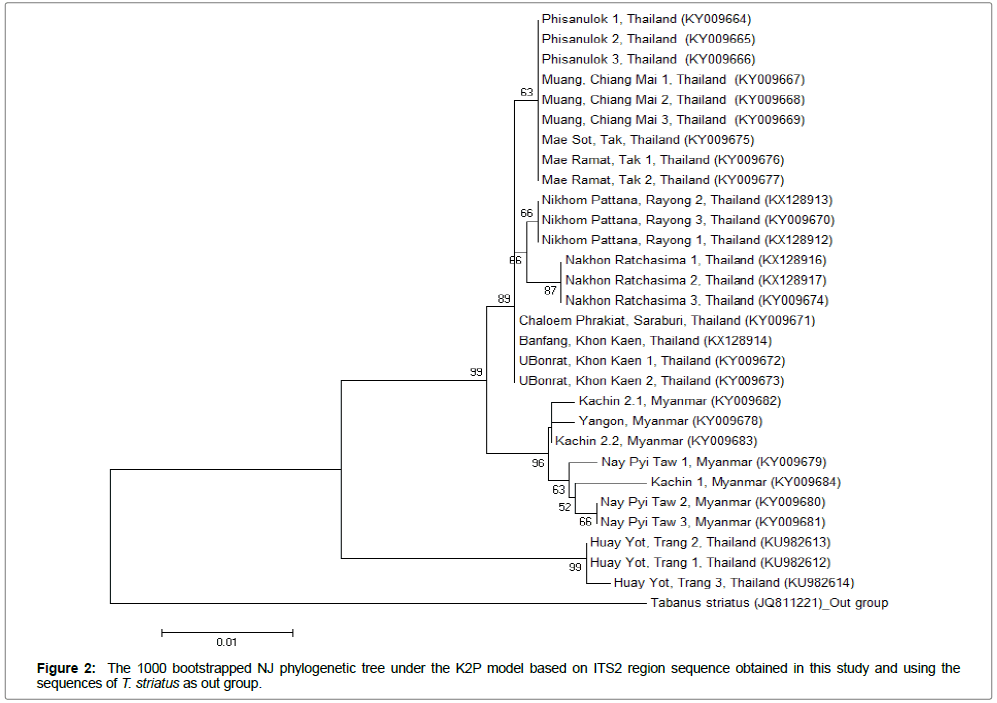
The Clustal W version 2.0 [11] belonged in the BioEdit Sequence Alignment Editor Program Version 7.2.5 (http://www.mbio.ncsu.edu/ BioEdit/bioedit.html ) was used to align and compute the percentage of intraspecific and interspecific divergences of the sequences. For evolutionary genetic divergence analysis, the 1000 bootstrapped Neighbor joining (NJ) tree using the Kimura 2-parmeter (K2P) model [12] was estimated using the MEGA version 6.06 (http://www. megasoftware.net/). Tabanus striatus was used as an out group.
Results
Twenty nine specimens of Tabanids flies collected from various places in Thailand and Myanmar were identified using a standard taxonomic key as Tabanus rubidus. The identification was confirmed by compared the ITS2 sequences of these specimens with database using BLASTN search tool. However, these sequences were matched with Dasysyrphus friuliensis’s sequence with 22-25% query cover and 72-80% identity because on reference of the completed ITS2 sequence of T. rubidus was submitted into NCBI database.
Sequence analysis
The amplified fragments of ITS2 from 26 samples of T. rubidus were various in lengths from 589 to 598 bp and 622 bp for 3 samples collected from Trang province, Thailand. The average A+T and G+C content of these sequences are 80.1% and 19.9%, respectively.
Intra and interspecific divergence pattern
The intraspecific divergence between specimens of the same places are found only in the samples collected from Trang province, Thailand (range 0.0-0.2%), Kachin and Nay Pyi Taw, Myanmar (both are range 0.2-1.2%).
The range of the average interspecific genetic divergence of each place when compared to the others were observed as 0.6-10.4% in Phitsanulok, 0.0 - 10.0% in Chiang Mai, 0.4-10.1% in Rayong, 9.8- 11.0% in Trang, 0.2-9.8% in Saraburi, 0.4-10.1% in Ban fang (Khon Kaen), 0.6-10.1% in Ubonrat (Khon Kaen), 1.1-10.5% in Nakhon Ratchasima, 0.0-10.0% in Mae Sot (Tak), 0.0-10.0% in Mae Ramat (Tak), 0.2-10.9% in Kachin, 0.9-11.0% in Nay Pyi Taw and 0.2-10.6% in Yangon, respectively (Table 2).
| R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 - R10 | R11 | R12 - R13 | R14 - R16 | R17 - R18 | R19 | R20 - R22 | R23 | R24 - R26 | R27 - R28 | R29 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R1 | - | |||||||||||||||||
| R2 | 1.2 | - | ||||||||||||||||
| R3 | 1.1 | 0.2 | - | |||||||||||||||
| R4 | 1.1 | 0.6 | 0.4 | - | ||||||||||||||
| R5 | 1.4 | 1.4 | 1.2 | 1.2 | - | |||||||||||||
| R6 | 1.2 | 1.2 | 1.1 | 1.1 | 0.2 | - | ||||||||||||
| R7 | 1.2 | 0.4 | 0.2 | 0.6 | 1.4 | 1.2 | - | |||||||||||
| R8 - R10 | 2.1 | 1.7 | 1.6 | 1.9 | 2.4 | 2.2 | 1.7 | - | ||||||||||
| R11 | 2.1 | 1.7 | 1.6 | 1.9 | 2.4 | 2.2 | 1.7 | 0 | - | |||||||||
| R12 - R13 | 2.1 | 1.7 | 1.6 | 1.9 | 2.4 | 2.2 | 1.7 | 0 | 0 | - | ||||||||
| R14 - R16 | 2.6 | 2.2 | 2.1 | 2.4 | 2.9 | 2.7 | 2.2 | 0.6 | 0.6 | 0.6 | - | |||||||
| R17 - R18 | 2.2 | 1.9 | 1.7 | 2.1 | 2.6 | 2.4 | 1.9 | 0.6 | 0.6 | 0.6 | 0.7 | - | ||||||
| R19 | 2.2 | 1.9 | 1.7 | 2.1 | 2.6 | 2.4 | 1.9 | 0.6 | 0.6 | 0.6 | 1.0 | 0.4 | - | |||||
| R20 - R22 | 2.7 | 2.4 | 2.2 | 2.6 | 3.1 | 2.9 | 2.4 | 1.1 | 1.1 | 1.1 | 1.5 | 0.9 | 0.6 | - | ||||
| R23 | 1.9 | 1.6 | 1.4 | 1.7 | 2.2 | 2.1 | 1.6 | 0.2 | 0.2 | 0.2 | 0.7 | 0.4 | 0.4 | 0.9 | - | |||
| R24 - R26 | 2.2 | 1.9 | 1.7 | 2.1 | 2.6 | 2.4 | 1.9 | 0.6 | 0.6 | 0.6 | 1.1 | 0.4 | 0.4 | 0.7 | 0.4 | - | ||
| R27 - R28 | 10.9 | 10.5 | 10.4 | 10.7 | 11.0 | 11.0 | 10.5 | 9.9 | 9.9 | 9.9 | 10.3 | 10.0 | 10.0 | 10.4 | 9.7 | 10.0 | - | |
| R29 | 11.0 | 10.7 | 10.5 | 10.9 | 11.2 | 11.2 | 10.7 | 10.1 | 10.1 | 10.1 | 10.5 | 10.2 | 10.2 | 10.5 | 9.9 | 10.2 | 0.2 | - |
Table 2: Intra and interspecific divergences of ITS2 sequence of T. rubidus collected from various region of Thailand and Myanmar (number are percentage) Codes are corresponding to Table 1 where R1-R7 = T. rubidus collected from Myanmar, R8-R29 = T. rubidus collected from Thailand.
Phylogenetic tree
The Neighbor Joining phylogenetic tree, constructed basing on the ITS2 sequences obtained in this study and using the sequences of T. striatus as out group, demonstrated that samples from Thailand and Myanmar were clearly separated into 2 clades with high bootstrap support value (99%). However, samples collected from Trang (Thailand) were grouped out from clade of both Thailand and Myanmar. Although paraphyly was found in clade of samples collected from Khon Kaen (Thailand) and Nay Pyi Taw (Myanmar), there is no overlap of sequences between Thailand and Myanmar clade (Figures 1 and 2).
Discussion
This is the survey study of the variation in the ITS2 of ribosomal DNA in T. rubidus collected from several regions of Thailand and Myanmar. The total length of the ITS2 sequences in this study ranged from 589 to 622 bp (average 598 bp). The longest was found in sequences of samples collected from Trang province, Thailand. DNA barcode data in this study had a high average of A+T content (80.1%) which seem to be found in class insect. Highly A+T content was also supported with earlier study in Simuliidae (71-83.8%) [13] and in Hydrotaea, Musca and Stomoxys (average 76.6%) [14].
Although the sequence variation between each clones from same sample was not found in this study, Intraspecific divergence among samples from same region was observed in the group of samples collected from Trang province, Thailand (range 0.0-0.2%), Kachin, Myanmar (range 0.2-1.2%) and Nay Pyi Taw, Myanmar (range 0.2- 1.2%).
The interspecific divergence among samples from various geographic areas showed no difference was detected among the sequences from Chiang Mai and Tak (Thailand). However, the slightly different was found among the others sequences with range from 0.2–11.2%. The higher different due to the sequences of samples collected from Trang, Thailand (the interspecific divergence compared to the others samples range from 9.9-11.2%). When samples from Trang province were cut off, the interspecific divergence drops down to 0.0-3.1% where are slightly higher than the limit of intraspecific divergence (3.0%) for species discrimination in insects [15]. The significant difference of the samples from Trang province was due to a 4 bp (TAAG) and 29 bp (CACATATATTATTGGAAA CCGCAAAACAT) insertion in the sequence which was slightly similar to the early report in sequences of Musca domestica [8,14]. However, the high different among specimens had been observed in Tabanids flies (6.34%) [16] and Musca domestica (6.9%) [14]. Furthermore, the results from theses previous research showed that each were cladded into individual group separated from sister group by phylogenetic tree as well.
The NJ with K2P tree based on ITS2 sequences able to group samples from the same region into individual clade except the samples collected from Kachin (Myanmar) and Nay Pyi Taw (Myanmar) are paraphyletic clade. Almost all samples from Thailand showed the differentiation between geographical regions where samples from northern (Chiang Mai, Tak and Phitsanulok), north eastern (Nakhon Ratchasima) and eastern (Rayong) were individually grouped with 63-87% bootstrap supported. Although samples from Khon Kaen (north eastern) were grouped in the same clade of samples from Saraburi (central), this clade was separated out with high bootstrap support value (89%). Furthermore, samples from Thailand and Myanmar were clearly separated from each other with 99% bootstrap value. However, the group of samples from Trang (southern) was cladded out from the others may be due to Trang province is far away from other regions and the T. rubidus in this zone may be distributed from Malaysia which is neighbor with southern of Thailand.
Conclusion
In conclusion, we studied the profile of ITS2 of ribosomal DNA in 29 specimens of T. rubidus collected from various regions of Thailand and Myanmar. ITS2 sequences showed the significant differences among nearly all samples from various geographic areas, although there was a slightly different among the sequences. The data generated in this study is useful for accurate species identification using ITS2 sequence which is the first step to provide the biological data for better vector control management for surra disease. However, further extensive survey for more samples and closely related species need to be investigated.
Acknowledgement
This study was supported by National Science and Technology Development Agency (Thailand) for the Research Chair Grant.
References
- Foil LD, Hogsette JA (1994) Biology and control of tabanids, stable flies and horn flies. Rev Sci Tech 13: 1125-1158.
- Service M (2008) Medical Entomology for Students. Cambridge University Press. United Kingdom
- Hajibabaei M, Singer GAC, Hebert PDN, Hickey DA (2007) DNA barcoding: how it complements taxonomy, molecular phylogenetics and population genetics. Trends Genet 23: 167-172.
- Kress WJ, Erickson DL (2008) DNA barcodes: genes, genomics, and bioinformatics. PNAS (USA) 105: 2
- Chen S, Yao H, Han J, Liu C, Song J, et al. (2010) Validation of the ITS2 Region as a Novel DNA Barcode for Identifying Medicinal Plant Species. PLoS ONE 5: 8.
- Desquesnes M, Holzmuller P, Lai DH, Dargentes A, Lun ZR, et al. (2013) Trypanosoma evansi and surra: a review and perspectives on origin, history, distribution, taxonomy, morphology, hosts, and pathogenic effects. Biomed Res Int: 194176.
- Tumrasvin W (1989) Tabanus species and their distribution in Thailand (Diptera: Tabanidae). Southeast Asian J Trop Med Public Health 20: 319-323.
- Song Z, Wang X, Liang G (2008) Species identification of some common necrophagous flies in Guangdong province, southern China based on the rDNA internal transcribed spacer 2 (ITS2). Forensic Sci Int 175: 17-22.
- Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic acids symposium series 41: 95-98.
- Tautz D, Hancock JM, Webb DA, Tautz C, Dover GA (1988) Complete sequences of the rRNA genes of Drosophila melanogaster. Mol Biol Evol 5: 366-376.
- Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22: 4673-4680.
- Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24: 1596-1599
- Thanwisai A, Kuvangkadilok C, Baimai V (2006) Molecular phylogeny of black flies (Diptera: Simuliidae) from Thailand, using ITS2 rDNA. Genetica 128: 177-204
- Bhakdeenuan P, Siriyasatien P, Payungporn S, Preativatanyou K, Thavara U, et al (2012) Molecular Analysis of Medically and Veterinary Important Muscid Flies (Diptera: Muscidae) in Thailand. Thai Veter Med 42: 10.
- Hebert PD, Cywinska A, Ball SL, de Waard JR (2003) Biological identifications through DNA barcodes. Proc Biol Sci 270: 313-321
- Banerjee D, Kumar V, Maity A, Ghosh B, Tyagi K, et al. (2015) Identification through DNA barcoding of Tabanidae (Diptera) vectors of surra disease in India. Acta Tropica 150: 52-58.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi