Research Article, J Immunol Tech Infect Dis Vol: 6 Issue: 1
Progression of Common Variable Immunodeficiency in Romanian Patients
Malina Oana Sava1* and Diana Deleanu2
1Department of Otorhinolaryngology, County Clinical Emergency Hospital, Cluj- Napoca, Romania
2Department of Allergy and Immunology, Iuliu Hatieganu University of Medicine and Pharmacy, Cluj-Napoca, Romania
*Corresponding Author : Malina Oana Sava
Department of Otorhinolaryngology, County Clinical Emergency Hospital, Cluj-Napoca, Romania
Tel: 0264432629
E-mail: malina_102@yahoo.com
Received: June 05, 2017 Accepted: August 21, 2017 Published: August 26, 2017
Citation: Sava MO, Deleanu D (2017) Progression of Common Variable Immunodeficiency in Romanian Patients. J Immunol Tech Infect Dis 6:1. doi: 10.4172/2329-9541.1000156
Abstract
Common variable immunodeficiency (CVID) is the most prevalent symptomatic primary immunodeficiency and belongs to the class of predominantly antibody deficiencies. For the positive diagnosis of CVID the following criteria must be fulfilled: a) serum levels of Ig G and at least one of the classes IgA and IgM at least 2 standard deviations below the average for age; b) patient age ≥ 4 years at diagnosis and c) any other defined causes of hypogammaglobulimenia (primary or secondary) have been excluded. Recent sets of diagnostic criteria additionally include clinical, serum immunology, immunophenotype, and histological characteristics that support the diagnosis of CVID (i.e. they increase the diagnostic probability).
Keywords: Common variable immunodeficiency; Immunoglobulin; Infectious disease; Autoimmune disease; Serum immunology; Immunophenotype
Abbreviations
CVID: Common Variable Immunodeficiency; Ig: Immunoglobulin; OFGI: ‘Octavian Fodor’ Gastrenterology Institute (The Clinic Where This Research Was Conducted); ESID: European Society for Immunodeficiencies; PAGID: Pan American Group for Immune Deficiency; IVIG: Intravenous Immunoglobulins; AO: Age of the Patient at the Onset of CVID; AD: Age of the Patient When Being Diagnosed With CVID; DD: Diagnostic Delay-Time from Onset of CVID to Diagnosis; M-W: Mann-Whitney Test; K-S: Kolmogorov-Smirnov Test; RTI: Respiratory Tract Infections; UTI: Urinary Tract Infections; HSV: Herpes Simplex Virus; BLP: Benign Lymphoproliferation; LAP: Lymphadenopathy; NHL: Non-Hodgkin Lymphoma; LIP: Lymphoid Interstitial Pneumonia; ILD: Interstitial Lung Disease; GLILD: Granulomatous Lymphocytic Interstitial Lung Disease; COPD: Chronic Obstructive Pulmonary Disease
Introduction
Common variable immunodeficiency (CVID) is the most prevalent symptomatic primary immunodeficiency and belongs to the class of predominantly antibody deficiencies. For the positive diagnosis of CVID the following criteria must be fulfilled: a) serum levels of Ig G and at least one of the classes IgA and IgM at least 2 standard deviations below the average for age; b) patient age ≥ 4 years at diagnosis and c) any other defined causes of hypogammaglobulimenia (primary or secondary) have been excluded. Recent sets of diagnostic criteria additionally include clinical, serum immunology, immunophenotype, and histological characteristics that support the diagnosis of CVID (i.e. they increase the diagnostic probability). Primary antibody deficiencies with a known causative genetic defect are no longer included in CVID [1-4].
CVID has infectious and noninfectious complications. Severe, recurrent and sometimes chronical bacterial infections are present in approximately 95% of CVID patients [5]. Infection location is predominantly respiratory or digestive and their majority is caused by common pathogens [2]. Noninfectious manifestations of CVID are present in approximately 70% of patients. These include: autoimmune diseases (esp. autoimmune cytopenias), enteropathy, hepatomegaly, chronic granulomatous disease, lymph proliferative diseases, cancer, splenomegaly and bronchiectasis. Of these, the autoimmune, inflammatory and lymphoproliferative diseases are considered intrinsically disease related complications (i.e. they are not possibly caused by other infectious or noninfectious disease manifestations or by their treatment) [6]. For other disease complications, possibly causative associations have been found with other infectious or noninfectious disease manifestations or with the treatments for these (e.g.: bronchiectasis- severe infections, lymphoid neoplasia- polyclonal lymphocytic infiltration, and splenomegalyseveral CVID complications, cancer- immunosuppressive treatment). Numerous genetic and immunological defects have been identified so far in patients with CVID and their prevalence varies among the analyzed cohorts. A classification in subtypes of CVID based on the immunophenotype defects (EUROclass) has been proposed [7]. For some immunophenotype and serum immunography defects correlation with certain clinical disease manifestations has been confirmed (low class-switched memory B-cells with splenomegaly, high serum IgM levels with polyclonal lymphocytic infiltration and lymphoma, etc)[7,8]. Reduced count of class-switched memory B-cells is the most constant immunophenotype defect (found in 80- 90% of CVID patients) [2] and is a diagnostic criteria for CVID in the ESID Registry- Working Definitions for Clinical Diagnosis of PID, 2016 (2016 Revised ESID criteria) [1].
It must be said that: only some associations between the various CVID manifestations (clinical-clinical or immunologic-clinical) have been statistically significant, as in some cohorts the number of cases of complications was low. Additionally, the associations found by some studies could not be confirmed by the subsequent studies. [5,7-9]
In Europe there are high differences in CVID prevalence in the general population between the states and in the prevalence of disease complications between the centers. Some of these differences are statistically significant [7]. What is more, these prevalence parameters differ between the cohorts in Europe and those in the USA [10,11]. This indicates the low relevance of the statistic results obtained in a uni- or multicentric group of patients for other groups of patients.
Scientific medical literature presently includes no characterization of a group of Romanian CVID patients. Only 2 Romanian centers for diagnosis and treatment of CVID have reported their primary immunodeficiency patients to the former ESID registry (working from 2004 to 2014; the data of the new ESID registry are available to ESID members only): Timișoara- 7 patients and Târgu Mureș- 4 patients. Because of the low reporting rate, European studies carried out so far have not analyzed the features of Romanian patients separately by country or by center. Moreover, none of the Romanian centers has published the features of the CVID patients in its database. Knowledge of the progression of CVID in Romanian patients is necessary because, as previously shown, the features of the already studied cohorts differ significantly and have limited relevance for other groups of patients with this disease.
The main objective of this study is the demographic, immunological and clinical characterization of the CVID patients included in the Immunodeficiencies Registry of the “Octavian Fodor” Gastroenterology Institute (OFGI), Cluj-Napoca, Romania. Additional objectives are testing for the association of these features with the mode of disease progression and with survival and comparing the features of patients in this cohort with those of the reference cohorts studied previously [7,9,12].
Materials and Method
Regarding the study format, this study has two parts:
(1) Descriptive and (2) analytical: observational retrospective cohort type.
In the study were included patients treated for CVID at the OFGI at any time since the start of the National Program for Substitution Therapy in Humoral Immunodeficiencies in Adults- August 8, 2011 – until June 2016.
The criteria for inclusion in the study were: patient age over 18 at the time of inclusion in the study or at death (the condition was applied to patients deceased before inclusion in the study); confirmed diagnosis of CVID based on current diagnostic criteria: ESID/PAGID Criteria in 2011-2015, Revised ESID Criteria (the 2015 version was applied to these patients; for CVID this version is identical to the 2016 Revised ESID Criteria) [1,4].
The exclusion criteria were: primary immunodeficiency other than CVID in a patient included in the National Program for Substitution Therapy in Humoral Immunodeficiencies in Adults at the OFGI; reclassification according to the 2015 Revised ESID Criteria of a patient previously diagnosed with CVID; the identification of a secondary cause for the clinical and/or immunological (serum immunogram, immunophentype) phenotype of CVID; insufficient data for validating the CVID diagnosis according to the criteria in use at the time of data collection for the study; patient’s refusal to participate in the study.
Data required for the study were collected from October 2015 to July 2016. The data sources used were: the patient’s medical records (hospital discharge papers, medical letters, results of imaging/ laboratory investigations) found in the Immunodeficiencies Registry of OFGI or explicitly requested from patients; patient medical records from their admissions during the data collection period for this study; the electronic patient registry of the OFGI; patient medical history taken from the patients or their families.
For data collection we used a semi-structured questionnaire, which included: personal data; pedigree for confirmed immunodeficiencies, history of infections, non-infectious complications of CVID, neoplasia, allergies; AO, AD, mode of onset of disease; diagnostic criteria fulfilled; disease manifestations: infections (location, frequency, severity, other information), autoimmune diseases (disease, timing, other remarks), lymphocytic infiltration (disease, timing, other remarks), neoplasia (disease, timing, other remarks), enteropathy (disease, timing, other remarks), others diseases (disease, timing, other remarks); immunophentype; comorbidities (immunological, others); treatment (of CVID- initial and average maintenance dose of intravenous immunoglobulins (IVIG), body weight, side effects, treatment history; specific treatment for disease complications); disease progression: disease duration (from onset to present / to death), survival.
A database was created in a Microsoft Excel 2013 file, in which the data from the questionnaires were entered. Conversion into binary data and calculations of secondary parameters were made based on these primary data.
We anticipated the existence of missing data. We established that statistical parameters dependent on variables with missing data be calculated based only on the obtained data, regardless of whether the missing data will be random or non-random. The following categories had missing data: pedigree, AO, DD, mode of disease onset, infections (incomplete history of infections), comorbidities, immunophenotype, initial and maintenance doses of IVIG. The data of patients having missing data were reassessed completely. The missing data were considered random absent data, because no differences in disease progression or mortality were observed in these patients compared to the rest of the cohort. The exception from this convention was the immunophentype parameters. Immunophenotype sheets of different patients contained different parameters; each parameter was determined for 4-13 patients. Consequently, we set a 20% significance threshold for the prevalence of changes of an immunophenotype parameter and we did not test for correlations between clinical and immunophenotypical disease manifestations.
For classification and processing of data, the following were also established:
- Severe infections were defined as in [7] and [13].
- in assessing the progression of infections under substitution therapy we considered the following improvement criteria: absence of severe infections that had occurred before treatment (2 points), a ≥ 50% decrease in the frequency/ the absence of an infection type present before treatment (1 point) and worsening criteria: occurrence/ increased frequency of severe infections (2 points), increased frequency of an infection type (1 point), appearance of a new type of infection (1 point)
- Patients with simultaneous lymphoma were not included in the ‘splenomegaly’ category because in this clinical context splenomegaly cannot be considered an intrinsic manifestation of CVID.
- The cases of intestinal lymphoproliferation were not included in the category of ‘benign lymphoproliferation’ (BLP), but in a separate category: ‘enteropathy’. A previous reference study proposed these two entities as distinct clinical phenotypes of disease because no association between them was found for the chosen statistical significance threshold (p<.001) [7].
Descriptive statistical processing was performed with the Microsoft Excel 2013 program and consisted of:
- distribution by gender, area of origin and mode of disease onset; frequency distributions for: age, AO, AD (age groups), DD (5-year intervals); median for age, AO, AD and DD (the median was used as a parameter for centrality because these data had non-normal distribution);
- prevalence for clinical manifestations, subtypes of clinical manifestations, relevant family history, relevant comorbidities, the belonging to one or more clinical phenotypes of CVID proposed by Chapel et al [2];
- average serum immunoglobulin levels before initiation of IVIG treatment, prevalence of low serum levels for IgA, IgM, IgA and IgM, respectively; prevalence of immunophenotype changes;
- average maintenance dose of IVIG, specific treatments for non-infectious manifestations, prevalence and type of response to treatments, prevalence of and causes for interruption of treatment;
- survival rate at 5 and 10 years, median of survival, AO and DD in deceased patients, causes of death.
Analytical statistical processing was performed with the GraphPad Prism 7.01 program and it consisted of:
- verifying the normality of distribution of age values, AO, AD, DD and serum immunoglobulin levels at diagnosis with the D’Agostino&Pearson and Shapiro-Wilk tests;
- testing for difference between genders and between areas of origin for age, AO, AD, DD with the Mann-Whitney (Wilcoxon rank-sum) and Kolmogorov-Smirnov tests;
- Comparison to the gender distribution in the European cohort (binomial test) [10].
- Testing for correlations between clinical manifestations (Fisher’s exact test), between clinical manifestations and AO/ AD/ DD, between immunological and clinical manifestations (Fisher exact test), between immunological manifestations and AO/ AD/ DD (Spearman test for correlation), between clinical manifestations and family history / comorbidities (Fisher exact test). The tested correlations were chosen based on a possible pathophysiological causality and on the results obtained in larger cohorts in previous studies, in order to delineate distinct clinical phenotypes of CVID and to identify the risk factors for a particular phenotype.
In all statistical tests applied differences were considered significant if p <0.05 (marked ‘*’ in the Results section).
Results
Demographic characteristics
32 patients with CVID were included in the study. The prevalence of the disease was 1: 29,000 in Cluj County and 1: 22,000 in Cluj- Napoca. Table 1 shows the distribution by gender and by place of origin. The gender distribution in this cohort was significantly different from that at European level (p=.02*) [12].
| Number | % | ||
|---|---|---|---|
| Gender | Male | 9 | 28 |
| Female | 23 | 72 | |
| Place of origin | Urban | 25 | 78 |
| Rural | 7 | 22 |
Table 1: Distribution by gender and by place of origin.
Table 2 indicates the distribution of patient age, AO, AD, DD and time of disease evolution. All five parameters had a non-normal distribution to the Shapiro-Wilk and D’Agostino&Pearson tests. Between genders the difference was statistically insignificant for AO, DD and time of disease evolution and significant for AD (MW: p=.03*, K-S: p=.02*). Between places of origin the difference was statistically insignificant for the AO, AD, DD and time of disease evolution.
| Minimum | Maximum | Median (µ) | Gender | Place of origin | Patients % | |||
|---|---|---|---|---|---|---|---|---|
| µ male | µ female | µ urban | µ rural |
|||||
| Age | 18 | 74 | 52.5 | |||||
| Age at onset | 0.5 | 72 | 25 | 25 | 27 | 24.5 | 25 | |
| < 18 years | 34% | |||||||
| Age at diagnosis | 5 | 72 | 48 | 37 | 52 | 50 | 40 | |
| < 18 years | 6% | |||||||
| Diagnostic delay | 0 | 51 | 6 | 4 | 5 | 5 | 5 | |
| < 5 years | 50% | |||||||
| > 15 years | 35% | |||||||
| Disease evolution | 1 | 58 | 10 | 11 | 10 | 9 | 13 | |
| deceased | 8 | 9% | ||||||
| undeceased | 10.5 | 91% | ||||||
Table 2: Distribution parameters for age, AO, AD, DD and time of disease evolution- general/ by gender/ by place of origin.
Diagnosis
The positive diagnosis was initially established based on the ESID/PAGID-1999 criteria in 30 patients (94%) and on the 2015 Revised ESID Criteria in 2 patients (6%). 2 patients did not fulfill the 2015 Revised ESID Criteria. A patient had at the time of diagnosis an inflammatory disease that is not considered specific to CVID (interstitial cystitis). During the treatment with IVIG she presented exclusively with cystitis, but only some of the episodes were bacteriologically differentiated from interstitial cystitis. In another patient the diagnosis was incidental and prior to diagnosis she had no history of infectious or non-infectious manifestations possibly due to CVID. During the treatment with IVIG she experienced lower urinary tract infections (2 episodes/ year) and an episode of cellulitis of the calf; the patient had type II diabetes mellitus concomitantly. Both patients discontinued the treatment for more than 6 months for reasons unrelated to the disease.
Immunological manifestations
Parameters based on serum immunoglobulins levels at diagnosis are shown in Table 3 (serum immunoglobulin levels had a non-normal distribution). IgA and IgM levels were undetectable at diagnosis in 1 patient.
| Average | Median | % patients^ | |
|---|---|---|---|
| IgG | 337 | 380 | |
| very low (<150 mg/dl) | 31 % | ||
| IgA | 64 | 38 | 21% |
| IgM | 34 | 19 | 18% |
| high (>500 mg/dl) | 0 | ||
| IgA & IgM | 61% |
Table 3: Serum immunoglobulin levels at diagnosis.
Immunophenotyping of peripheral blood lymphocytes was performed in 12 patients (37%); the changes observed are shown in Table 4.
| Low levels | High levels | No. of patients assessed | |
|---|---|---|---|
| T lymphocytes % | 42% | 12 (38%) | |
| CD4+% | 25% | 12 | |
| CD8+% | 42% | 12 | |
| B lymphocytes % | 67% | 6 (19%) | |
| NK cells % | 50% | 6 | |
| Lymphocytes | 20% | 10 (31%) | |
| CD4+ | 27% | 11 (34%) | |
| CD4+/CD8+ | 46% | 13 (41%) | |
| B lymphocytes | 80% | 5 (16%) | |
| B lymphocytes <1% | 20% | 5 |
Table 4: Immunophenotype changes in patients with CVID.
Clinical manifestations
Table 5 shows the mode of clinical onset of CVID. One patient (3%) was asymptomatic before the time of diagnosis.
| infections | 19 (60%) |
|---|---|
| Hp-pozitive gastritis | 1(3%) |
| (Herpes zoster | 1 |
| Systemic HSV1 infection | 1 |
| lower RTI | 5 (16%) |
| upper RTI | 8 (25%) |
| upper and lower RTI | 3 (9%) |
| non-Hodgkin lymphoma | 4 (12%) |
| uncertain | 4 (12%) |
| autoimmune disease | 3 (9%) |
| dermatomyositis | 1(3%) |
| thyroiditis | 2(6%) |
Table 5: Mode of onset of CVID (no. of patients).
The prevalence of CVID manifestations was determined, considering the period from disease onset to present or to death (Table 6).
| Category | No. of pts. | Prevalence |
|---|---|---|
| Infections | 100% | |
| Lower respiratory tract | 1 | 63% |
| Bronchitis | 18 | 56% |
| Pneumonia | 20 | 63% |
| Upper respiratory tract | 13 | 41% |
| Lower urinary tract | 14 | 44% |
| Digestive | 10 | 31% |
| Chronic/ acute helicobacter pylori gastritis | 3 | 9% |
| Acute diarrhea | 8 | 25% |
| Parasitic infections | 3 | 9% |
| Sinusitis | 1 | 25% |
| Otitis media | 1 | 12% |
| Sepsis | 1 | 9% |
| Septic arthritis | 1 | 6% |
| Bacterial skin/ soft tissue infections | 1 | 16% |
| Specific viral infections | 1 | 19% |
| Herpes simplex virus infections | 4 | 12% |
| Herpes zoster | 3 | 9% |
| Viral hepatitis (B or A) | 3 | 9% |
| Candida stomatitis | 1 | 9% |
| Tuberculosis | 1 | 9% |
| Others | 1 | 3% each |
| Autoimmune diseases | 9 | 28% |
| Cytopenias | 1 | 3% |
| Immune thrombocytopenic purpura | 1 | |
| solid organ | 6 | 19% |
| Crohn's disease Hyperthyroidism/ hypothyroidism Hashimoto's thyroiditis Graves’ disease Autoimmune alopecia Atopic dermatitis Psoriasis vulgaris Ovarian failure |
1 1 1 1 1 1 1 1 |
|
| Systemic | 5 | 16% |
| Cryoglobulinemia Dermatomyositis Behcet's disease Undifferentiated systemic vasculitis Antisynthetase syndrome Antiphospholipid syndrome |
1 1 1 1 1 4 |
|
| Polyclonal lymphocytic infiltration | 5 | 16% |
| Lymphadenopathy Splenomegaly Hepatomegaly Enteropathy Lymphoid interstitial pneumonitis, (Pulmonary granulomasf) |
5 6a 4 b 1c |
|
| Tumorsg | 11 | 34% |
| Lymphomas | 7 | |
| NHL Hodgkin |
6 1 |
19% |
| Other cancers | 4 | 12% |
| Benign | 1 | |
| Chronic digestive diseases | 5 | 16% |
| Atrophic gastritis Crohn's disease Enteropathy |
2 d 3 |
9% |
| Chronic pulmonary diseases | 10 | 31% |
| Bronchiectasis Fibrosing interstitial lung disease |
7 3 |
22% 9% |
| Inflammatory diseases | 3 | 9% |
| Recurrent aphthous ulcers Recurrent interstitial cystitis Pyoderma gangrenosum, Panniculitis |
1 1 1 |
|
| Iron deficiency anemia | 3 | 9% |
| Others | 3 | 9% |
| No non-infectious complications | 6 | 19% |
b enteropathy belongs to chronic digestive diseases,
c both manifestations were present in the same patient and belong to chronic pulmonary diseases,
d Crohn's disease belongs to autoimmune diseases,
e these manifestations were present in the same patient,
f histology did not confirm imaging findings,
g see Table X
Table 6: Non-infectious complications in patients with CVID.
100% of patients had infections, 66% of which had at least one severe infection. The severe infections found were: lung infections (41% of patients), sepsis (9%), severe acute diarrhea (9%), septic arthritis (6%), cellulitis (12%) and complicated Herpes zoster (6%). The severe lung infections were mainly pneumonia or bronchopneumonia which required hospitalization, intravenous antibiotic treatment and/or oxygen therapy. Additionally, there were two cases of secondary pulmonary tuberculosis, which responded to specific treatment. There was also a case of extensive superinfected bronchiectasis, with chronic lung abscesses and secondary pulmonary tuberculosis, for which the patient died due to the tuberculosis. In the last case the following microorganisms were identified in successive cultures from sputum or bronchial aspiration: Pseudomonas aeruginosa (most frequent result), Staphylococcus aureus, Streptococcus pneumoniae, Corynebacteria, Streptococcus viridans, Staphylococcus epidermidis and Candida albicans. Of the 3 cases of sepsis, 2 occurred before the beginning of the IVIG treatment and were caused by Pseudomonas aeruginosa and HSV type 1, respectively. These cases were cured by specific antimicrobial and supportive treatment. Pseudomonas aeruginosa caused 2 episodes of sepsis in the same patient, at an interval of approximately 2 years; the second episode associated septic myocarditis with subsequent dilative cardiomyopathy. The second patient had a chronic systemic infection with HSV type 1, worsened by repeated episodes of sepsis in infancy and an episode of herpetic encephalitis in adulthood; the sepsis episodes associated acute viral hepatitis and diffuse and severe bacterial super infection of the generalized cutaneous rash. The third case of sepsis occurred after the first 2 doses of substitution therapy and the etiology remained unknown. Concomitantly, the patient underwent chemotherapy for early recurrence of NHL; this patient deceased after about 2 months. All 3 patients with sepsis were female. Two of the cases of acute severe diarrhea were caused by Clostridium difficile; both patients were concomitantly undergoing chemotherapy for NHL and IVIG therapy; the third was caused by Salmonella species and Campylobacter jejuni before initiation of IVIG therapy. In the first case of septic arthritis the right elbow was affected, the result of bacterial examination of synovial fluid was not available to the investigators and surgical treatment was required. In the other case, the elbow and knee joints were successively affected and the microbiological examination of the synovial fluid was negative. Arthritis of the elbow responded to treatment with Teicoplanin; arthritis of the knee did not respond to treatment with Ciprofloxacin, Teicoplanin and Imipenem-Cilastatin, required surgical intervention and resulted in ankylosis. Both cases of arthritis occurred before initiation of IVIG therapy.
Table 7 shows the progression of satient infection status after initiation of IVIG treatment. In 30% of patients the risk of infection during IVIG treatment could not be assessed correctly because there was at least one confusion factor: chemotherapeutic or immunosuppressive treatment, type 2 diabetes mellitus or advanced cancer.
| Infections (%) | Total | Under IVIG treatment | |
|---|---|---|---|
| All | 100 | 93 | |
| Severe | 66 | 30 | |
| Infection Status |
improvement | 43 | |
| worsening | 14 | ||
Table 7: Infection progression after initiation of IVIG substitution.
The prevalence of infections by location for all disease duration is shown in Table 6. Of the patients with tuberculosis, two had concomitant bronchiectasis and the third had concomitant NHL. All patients with oral candidiasis were under systemic or inhaler corticosteroid treatment.
For infections with prevalence >10% in this cohort, the following parameters were determined: prevalence before treatment, prevalence under treatment, prevalence difference, percentage of cases of improvement (out of all the existing cases before treatment), percentage of newly emerged cases during treatment (out of the total number of cases under treatment). After initiation of IVIG treatment in some patients were observed the absence or decreased frequency or severity of previous infections with a given location and the emergence of infections in a new location, respectively. Thus, the prevalence of pneumonia, bronchitis and acute diarrhea decreased, the prevalence of sinusitis increased and the prevalence of otitis media and lower UTI remained constant (Table 8).
| pretreatment cases (total) | remission/improvement | new location/worsening | cases under treatment (total) | Evolution under treatment | |
|---|---|---|---|---|---|
| Pneumonia | 14 | 7 | 1 | 8 | ↓ 43% |
| Acute bronchitis | 9 | 6 | 3 | 6 | ↓ 33% |
| Sinusitis | 5 | 2 | 4 | 6 | ↑ 20% |
| Otitis media | 3 | 1 | 1 | 3 | stationary |
| UTI | 8 | 4 | 5 | 8 | stationary |
| Acute diarrhea | 8 | 6 | 3 | 5 | ↓ 37% |
Table 8: The progression of infections by location after initiation of IVIG treatment.
Microbiological examinations available for this study (59% of patients) indicated the following: lower RTI with Haemophilus influenzae, Streptococcus pneumoniae, Staphylococcus aureus and Pseudomonas aeruginosa and, in one case, Moraxella catarrhalis and Klebsiella pneumoniae; enterocolitis with: Clostridium difficile, Campylobacter spp, Salmonella spp. and, in one case, Pseudomonas aeruginosa and Serratia marcescens; UTI with Escherichia coli; colonization of the lower respiratory tract in patients with bronchiectasis or chronic obstructive pulmonary disease with Haemophilus influenzae (50%), Candida albicans, Streptococcus pneumoniae and Pseudomonas aeruginosa; colonization of the lower respiratory tract in patients without bronchiectasis or chronic obstructive pulmonary disease with Staphylococcus aureus, Candida spp. (predominantly C. albicans) and, in one case: Haemophilus influenzae, Gram-negative bacilli (Escherichia coli, Acinetobacter baumannii, Citrobacter braakii, Enterobacter cloacae, Stenotrophomonas maltophila, Pseudomonas putida) or commensal oropharyngeal bacteria (Rothia mucilaginosa). In 2 of the 5 patients with documented opportunistic infections these were present only during chemotherapy for lymphoma; the other patients did not have lymphoma and were not undergoing chemotherapy or immunosuppressive therapy.
The available data on antibiotic treatments and prophylaxis received by patients in this cohort were insufficient for statistical processing.
28% of patients had autoimmune diseases (Table 6) systemic, solid organ or autoimmune cytopenias. 5 patients had each two autoimmune diseases. In 4 cases there was a coexistence of a systemic autoimmune disease with secondary antiphospholipid syndrome; one patient had Hashimoto’s thyroiditis and systemic vasculitis and another patient had autoimmune processes at more than three solid organs with no systemic autoimmune disease. No coexistence of autoimmune cytopenias with autoimmune diseases of solid organ or systemic was observed in any patient. The gender rate of patients with autoimmune diseases is 2: 7 (male: female). The association of autoimmune diseases with female gender was tested but was not statistically significant (p = 1). 2 cases (29%) of solid organ autoimmune disease completely resolved after specific treatment (with immunosuppressive and / or corticosteroid or non-steroidal anti-inflammatory medications): Graves’ disease and Crohn’s disease. It must be said that 5 patients had transient or persistent cytopenias, but the immunological diagnosis was only positive for the patient mentioned above.
16% of patients had BLP. In Table 9, the cases of BLP identified in this cohort are presented separately because of their heterogeneity. All of these patients had lymphadenopathy at multiple locations and each patient had additionally: only splenomegaly, only hepatomegaly, hepatosplenomegaly, intestinal lymphoid hyperplasia or LIP (this patient had concomitant hepatosplenomegaly and pulmonary granulomas were detected at high-resolution computed tomography; pulmonary biopsy confirmed the lymphoid infiltration of the lung histologically, but no granulomas were found). Biopsies were performed to 2 patients (40%): from lymph nodes and lymph nodes and lungs, respectively; the histological result was atypical lymphoid hyperplasia in both cases. The gender rate of patients with BLP is 2: 3 (male: female). None of the patients with BLP had been diagnosed with lymphoma by the end of the study. In the two histologically confirmed cases of atypical lymphocytic hyperplasia, corticosteroid treatment was indicated and partial remission was achieved. In the patient with celiac disease specific treatment for enteropathy was indicated; under treatment the infiltrative formations were stationary. No specific treatment was indicated to the other patients.
| LAP | splenomegaly | hepatomegaly | LIP | Histological type | |
|---|---|---|---|---|---|
| 1. | + | + | + | + | atypical lymphoid hyperplasia |
| 2. | + | + | + | - | unknown |
| 3. | + | + | - | - | atypical lymphoid hyperplasia |
| 4. | + | + | + | - | Unknown |
| 5. | + | - | + | - | unknown |
Table 9: Benign lymphoproliferation in patients with CVID.
34% of patients had tumors (Table 6). A patient had a benign tumor (neurinoma of cranial nerve VII) and a patient successively had Hodgkin’s lymphoma and colorectal carcinoma. The specific types of malignancies identified in this cohort are shown in Table 10. The gender rate of patients with malignant tumors was 3: 7 (male: female). Complete remission without recurrence by the end of the study was achieved in 66% (4 patients) of cases of NHL and healing was achieved in 3 of the solid tumors. There were two cases of relapse: large B-cell NHL and angioimmunoblastic T-cell lymphoma and two deaths caused by malignant tumors: metastatic colorectal carcinoma and angioimmunoblastic T-cell lymphoma with early relapse.
| Lymphoma | No. of pts. | Other cancers | No. of pts. |
|---|---|---|---|
| Hodgkin's lymphoma | 1 | Colorectal carcinoma | 1 |
| Non-Hodgkin's lymphoma | 6 | Basal cell carcinoma | 1 |
| Large B-cell | 3 | Thyroid papillary carcinoma | 1 |
| Burkitt type | 2 | Cervical carcinoma | 1 |
| Angioimmunoblastic T-cell | 1 |
Table 10: Malignant tumors in patients with CVID.
16% of patients had non-infectious digestive diseases. In Table 11, the digestive diseases identified in this cohort are presented descriptively for each patient, because of concomitant presence of several types of digestive disorders in the same patient. There were 3 cases (60%) of coexistence with BLP in other organs and 1 case of coexistence with Helicobacter pylori- positive chronic gastritis; there were no cases of coexistence with other autoimmune diseases or lymphoma. All cases had a histological diagnosis. Specific treatment was indicated for celiac disease (immunosuppressant and corticosteroid) and Crohn’s disease (anti-inflammatory, corticosteroid and immunosuppressant) and partial remission was obtained in both cases.
| No. of pacient | Digestive diseases | Helicobacter pylori- positive gastritis | BLP in other organs |
|---|---|---|---|
| 1 | Atrophic gastritis | Unknown* | Lymph nodes, spleen |
| 2 | Celiac disease unresponsive to gluten, erythematous antral gastritis, esophagitis | Lymph nodes, liver, spleen | |
| 3 | Nodular lymphoid hyperplasia of duodenum, esophagitis | Acute erosive, repetitive | |
| 4 | Nodular lymphoid hyperplasia of terminal ileum | Lymph nodes, liver, spleen | |
| 5 | Crohn's disease | - | - |
Table 11: Non-infectious digestive diseases in patients with CVID
25% of patients had bronchiectasis. 12% of patients had ILD. High resolution computed tomography was performed in all 4 cases of ILD. 3 of them were diagnosed as definite fibrosing ILD based on imaging findings only. In the remaining case imaging findings raised the suspicion of sarcoidosis and transbronchial lung biopsy was performed, which indicated lymphocytic interstitial pneumonitis (atypical lymphoid hyperplasia of the lung with no granulomas). Frequent coexistence of fibrosing ILD with systemic autoimmune diseases and bronchiectasis was observed.
12% of patients had splenomegaly. 2 patients had hypersplenism. In 2 patients splenectomy was indicated for immune thrombocytopenic purpura non-responsive to medication, and for an unknown indication, respectively. 9% of patients had hepatomegaly of unknown cause. Coexistence with lymphoid infiltration of other organs (in all hepatomegaly cases) and with enteropathy was observed. 9% of the patients were diagnosed with iron deficiency anemia, in association with the following manifestations: Crohn’s disease, Hunner ulcers and esophagitis, Helicobacter pylori- positive acute erosive gastritis, nodular lymphoid hyperplasia of the duodenum. In other patients with low hemoglobin levels, the differential diagnosis with anemia of chronic disease could not be made because serum iron and serum ferritin levels were not available.
9% of patients had specific inflammatory diseases. All affected patients were female. Two cases (aphthous ulcers and interstitial cystitis) were not associated with other non-infectious manifestations of CVID. The third case (pyoderma gangrenosum and paniculitis) occurred in a patient with concomitant colorectal cancer. No significant improvement was seen under IVIG treatment in any of the cases.
Apart from the classical manifestations described in CVID patients, the following were identified in patients in this cohort: idiopathic meningitis (2 patients), subcortical leukoencephalopathy (1 patient), ischemic optic neuropathy (1 patient), retinal necrosis (1 patient). For these diseases, etiology could not be established. Their appearance in these patients determined the immunological investigation and the diagnosis of CVID. Some appeared successively in the same patient: idiopathic meningitis and subcortical leukoencephalopathy, idiopathic meningitis and retinal necrosis, respectively.
Correlations between CVID manifestations-Clinical phenotypes
Table 12 presents the associations found between the noninfectious clinical manifestations of CVID.
| Infl/Ai diseases | LAP/BLP | Enteropathy | Spleno-megaly | Hepato-megaly | Bronchi-ectasis | ILD | Fibrosing ILD | ||
|---|---|---|---|---|---|---|---|---|---|
| Ai/ Infl diseases | 1/5 (p>0.99) | 0 | 0 | 0 | 3/8 † (p>0.05) |
2/4 (p=0.25) |
2/3 (p=0.18) |
||
| LAP/BLP^ | 2/3 (p=0.05) |
4/6 * (p=0.002) | 3/4 * (p=0.0001) |
2/8 (p=0.57) |
2/4 (p=0.10) |
1/3 (p=0.41) |
|||
| Enteropathy | 2/6 (p=0.08) |
2/4 (p=0.10) |
1/8 † | 1/4 (p=0.51) |
1/3 (p=0.41) | ||||
| Spleno- megaly | 3/4 * (p=0.01) |
4/8 * (p=0.02) |
1/4 (p=0.15) |
1/3 (p=0.47) | |||||
| Hepato- megaly | 2/8 † (p=0.57) |
2/4 (p=0.06) |
1/3 (p=0.26) | ||||||
| Bronchi-ectasis | 2/4* (p=0.03) |
2/3 (p=0.14) | |||||||
| ILD | |||||||||
Table 12: Correlations between non-infectious complications of CVID.
Severe infections (pneumonia/sepsis) did not correlate with bronchiectasis (p=0.08). Diagnostic delay did not correlate with low serum of IgG levels at diagnosis (p =0.83). Low serum IgG levels at diagnosis (IgG <150 mg/dL) correlated with severe infections (p=0.01*, OR=13). No correlation between IgG and IgM values at diagnosis was found. Correlations were weak between IgG and IgA (p<0.001*; r=0,62) and between IgA and IgM (p=0.004*; r=0,51) levels at diagnosis, respectively.
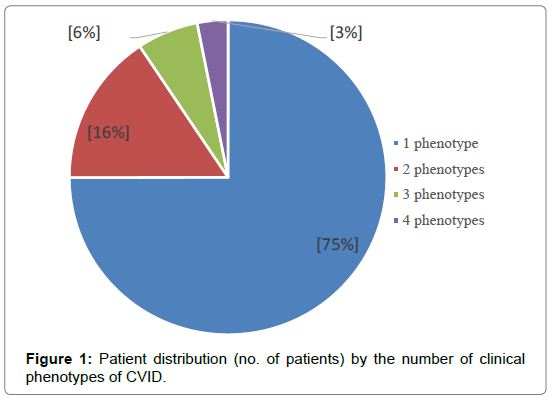
The number of clinical phenotypes of CVID was determined in every patient. Patient having only infections constituted a distinct phenotype. Based on the non-infectious complications, the following phenotypes were defined: autoimmune disease, BLP, tumors, enteropathy, inflammatory diseases, bronchiectasis, splenomegaly and ILD. The complications between which a correlation was found were considered to constitute a single disease phenotype (BLP/ lymphadenopathy - splenomegaly - hepatomegaly, bronchiectasis and splenomegaly, bronchiectasis and severe infections, bronchiectasis and ILD). The enteropathy and BLP of other organs present at the same patient were considered two distinct phenotypes. Thus, only one phenotype was present in 75% of patients, 2 phenotypes 16%, 3 phenotypes in 6% and 4 phenotypes in 3% (Figure 1).
Treatment
The initial dose of IVIG in adult patients ranged from 400 to 600 mg/kg. The average maintenance dose of IVIG used in adult patients was 375 mg/kg. 21.8% of patients had adverse effects to IGIV treatment. The adverse effects identified were: edema at the site of administration, fever, chills, malaise, exanthema, vertigo, arthralgia, vomiting, hematochezia, hair loss, phlogistic reactions. 3 patients (9%) experienced phlogistic reactions. 3 patients (9%) discontinued the treatment because of the adverse effects. 5 patients discontinued the treatment for medical or non-medical reasons unrelated to treatment, and a patient interrupted treatment due to normalization of serum Ig levels.
The specific treatments for CVID manifestations were mentioned in the description of these diseases. 84% of patients were treated by physicians of specialties other than immunology for infectious or non-infectious complications of CVID. 9 patients (28%) required surgery for malignant (12%) or non-malignant (16%) complications of CVID (Table 13). The only ineffective operation was the repair of the oroantral communication of the maxillary sinus (maxillary sinusitis recurred after the repair). All patients were re-examined clinically, biochemically, by complete blood counts and levels of serum antibodies at each presentation for IVIG administration. For the newly discovered complications at a presentation specific investigations or examination by other specialties were indicated. 78% of the patients were monitored by doctors from other specialties for infectious or non-infectious complications of CVID. The disease manifestations found were monitored periodically, as indicated by the treating physician (hematologist, rheumatologist, etc.).
| Disease | Intervention | No. pat. | |
|---|---|---|---|
| Non-malignant | Bronchiectasis of right lung upper lobe | Upper right pulmonary lobectomy | 1 |
| Oroantral communication → Persistent acute maxillary sinusitis | Closure of oroantral communication | 1 | |
| Immune thrombocytopenic purpura | Splenectomy | 1 | |
| Septic arthritis | Surgical drainage | 2 | |
| Malignant | Gastric B-cell NHL | Gastrectomy | 1 |
| Thyroid papillary carcinoma | Thyroidectomy | 1 | |
| Right thoracic basal cell carcinoma | Excision of carcinoma | 1 | |
| Cervical carcinoma | Hysterectomy + bilateral ovarectomy | 1 |
Table 13: Surgical treatments for complications of CVID.
Family history, comorbidities
A patient had 1st and 2nd degree relatives with confirmed antibody deficiencies: a brother with selective IgG deficiency and a nephew with CVID. In the other 1st and 2nd degree relatives of the patient antibody deficiencies were ruled out. The patient was diagnosed with selective IgA deficiency 21 years before establishing the diagnosis of CVID. Another patient had a daughter with selective IgG deficiency. 50% of patients had 1st and 2nd degree relatives with cancer, but presence of cancer at relatives did not correlate with presence at patients (p=0.67). 2 patients (6%) had 1st degree relatives with autoimmune disease and both had autoimmune disease; presence of autoimmune diseases at relatives did not correlate with presence at patients (p=0.06). 3 patients (10%) had relatives with increased susceptibility to infections, without being investigated for immunodeficiencies. Based on patient family history no cases of other possible CVID manifestations were found in 1st and 2nd degree relatives of patients in this cohort.
47% of patients had allergies. 22% of patients had type II diabetes mellitus. 6% of the patients had COPD. Of the patients in which the comorbidities are known, all of those who had phlogistic reactions to IGIV had allergies. Diabetes mellitus did not correlate with either severe infections (p=0.68) or lower UTI (p=1). One of the two COPD patients experienced severe and frequent lung infections.
Survival
At the time of completion of data collection 3 patients were deceased, which represents a mortality rate of 10%. The survival rate was 96% at 5 years from onset of disease and 90% at 10 years. The median of survival from onset of disease was 8 years. The median DD in deceased patients was 5 years. The median AO was 16 years. Immunophenotype was not performed in any of the deceased patients. All deceased patients were female. 2 of the deceased patients were from urban area and one patient was from rural area. The association of lower survival with female gender and urban area, respectively, was not significant (psex=0.53; parea=1). All deceased patients had noninfectious complications of CVID. Table 14 represents the cases of death. By the end of data collection, 3 patients were lost to followup; it is unknown whether they died, but based on previous clinical course of disease there is no suspicion of death because of a CVIDrelated cause.
| Cause of death | AO | Sv | Other relevant disease manifestations | |
|---|---|---|---|---|
| 1. | Secondary pulmonary tuberculosis | 10 | 8 | bronchiectsis, lung abscesses, splenomegaly |
| 2. | Metastatic colorectal carcinoma | 16 | 8 | remitted Hodgkin's lymphoma |
| 3. | Angioimmunoblastic T-cell lymphoma | 57 | 2 | - |
Table 14: Deceased patients.
Discussions
The studies carried out previously on cohorts of CVID patients have shown variability of clinical and immunological features among patients and prevalence differences of these features between diagnostic centers/ countries. Correlations that were statistically significant in some studies could not be reproduced in other studies, and this fact questions the previously proposed causative associations [7,9]. Consequently, knowledge of the features of some cohorts of CVID patients is of limited use for the medical management of another group of patients. In turn, an ethnically new group of patients requires characterization. This may improve the knowledge about the possible manifestations of CVID and their determinism, which primarily benefits the patients from that ethnic group. This is the first study that characterizes and analyses a group of Romanian CVID patients.
In the first part of this study demographic, immunological and clinical features of the participant patients were determined. In the second part of the study we searched for correlations between these features.
The prevalence for Cluj county and Cluj Napoca, respectively, fitted into the European prevalence range (1:10000- 1:50000) [14]. We decided to determine the prevalence for these territories because there are patients in our database that come from geographical regions that are officially attended by other centres of Immunoglobulin substitution treatment (București, Iași, Zalău, Baia Mare, Deva, Târgu Jiu). Prevalence was higher in Cluj Napoca compared to Cluj county, which is consistent with the higher number of diagnosed patients from urban compared to rural areas. In turn, DD did not differ between the urban and rural areas. In the main previous studies there are no data on the distribution by area of origin, thus no comparison with other cohorts could be made [7,9,12]. These determinations were performed to identify a possible lower rate of diagnosing in rural areas and small towns, given that this disease is little known. The differences found could be confirmed/ ruled out by a study at national level.
There were more female than male patients and the AD was higher in women than in men. The hypothesis of a milder course of disease in women was discordant with the AO and the DD being similar in the two genders. We subsequently checked for differences in disease progression between the genders.
The majority of participant patients (94%) had been diagnosed using the ESID/PAGID-1999 diagnostic criteria, which are considered less strict than the 2015 Revised ESID Criteria [15]. In the two patients not fulfilling the present criteria the need to maintain this diagnosis and to continue treatment with IVIG is debatable. All available cohort studies were published until 2014 and thus contain no references to patients in this situation. Our patients in particular, who interrupted IVIG substitution, may be reassessed by the 2015 Revised ESID Criteria. In patients on IVIG substitution, the immune defect can only be proven if they have a low number of class-switched memory B-cells [3].
The major drawback at the OFGI in assessing patients suspected of CVID is the fact that specific antibody response (to vaccines and/ or isohaemagglutinins) is not being measured. This is a compulsory criterion in diagnosing an antibody deficiency [16]. Omission of this standard at the OFGI is due to administrative reasons, yet this approach renders the diagnosis of CVID inaccurate. The diagnosis of CVID in these patients is maintained because they all have clinical criteria for antibody deficiency (frequent and severe infections with typical microorganisms, against which immune defense is mainly by antibody production); have low levels of at least 2 classes of immunoglobulins; and cellular immunodeficiency and secondary causes for antibody deficiency were not found. Testing for specific antibody responses needs to be included in the diagnostic algorithm used at the OFGI; in the new sets of diagnostic criteria [1,3] these tests are listed as alternative to counting class-switched memory B-cells (at least one of the two must have abnormal values within the same subset of criteria), so that for highest sensitivity of the diagnostic algorithm, both tests need to be performed.
One particular feature of the patients in this cohort is the absence of cases with high serum IgM at diagnosis. This fact delineates CVID from hyper-IgM syndromes and may represent a positive prognostic factor.[5,7]
Absence of immunophenotype determinations in the majority of participant patients raises two important problems: (1) immunophenotype exclusion of concomitant cellular immunodeficiency is lacking, this being established on clinical criteria only; (2) the class-switched memory B-cell status of the patients is unknown. These are both diagnostic criteria in the 2015 Revised ESID Criteria [1].
Patient history regarding infections was divided in: all disease duration, before treatment, under treatment. Both globally (the categories: ‘all infections’ and ‘severe infections’) and for each infection location the following patterns were observed: improvement/ stationary frequency and severity/ worsening. This fact suggests that only some infections respond favorably to immunoglobulin substitution. The present study indicates that IVIG treatment is effective for prevention of lower RTI and acute diarrhea. These are the most frequent type of infections in CVID patients [2,12], which is consistent with the global decrease of infection prevalence after treatment initiation. In turn, IVIG treatment was ineffective in decreasing the prevalence of otitis media, lower UTI (stationary prevalence) and sinusitis (increasing prevalence). A longitudinal cohort study [12] obtained both consistent (decrease in prevalence of all infections and pneumonia; increase in prevalence of sinusitis; stationary prevalence of UTI) and inconsistent (increase in prevalence of acute bronchitis and decrease in prevalence of otitis media) results with the present study. The prevalence of digestive infections is designated by the term ‘chronic diarrhea’ in the aforementioned study and by ‘acute diarrhea’ and ‘digestive parasitic infections’ in our study. Given that these terms are not overlapping, the two results were not compared. We believe that the two terms chosen for the present study ensure a better delineation between the cases of infectious and noninfectious diarrhea, respectively, which is necessary for the accuracy of a retrospective study. No risk factors have been identified that could explain the high and stationary prevalence of UTI under treatment in patients in this cohort. The number of patients with otitis media in this group was low (4 patients); this decreases the significance of this result. The aforementioned study explains its results by the fact that immunoglobulin preparations predominantly replace IgG and only to an insignificant degree IgA and IgM. IgG protect against acute and invasive infections (pneumonia, acute bronchitis, otitis media, acute diarrhea, septicemia, meningitis), whereas IgA and IgM- the major secretory immunoglobulins on the mucosae- protect against chronic mucosal infections (chronic rhinosinusitis, chronic bronchitis, chronic diarrhea).[12] Based on these remarks it has been suggested that immunoglobulin substitution may be unjustified in patients having exclusively CVID manifestations of which it is known that they usually do not respond to this treatment (e.g. chronic rhinosinusitis, other noninfectious complications than autoimmune diseases). This way, for instance, for chronic rhinosinusitis it has been proposed that IVIG treatment only be initiated if antibiotic prophylaxis is ineffective.[17] Nevertheless, it is currently recommended to adjust the IVIG doses so as to concomittantly:
1) Maintain through IgG levels at least near the middle of the normal range and
2) reduce the incidence of significant infections [18]. Indeed, recent studies have shown that high doses of immunoglobulins provide a significantly better control of significant infections, including those with lower responsiveness to substitution therapy (e.g. chronic rhinosinusitis).[19,20]
Oligoarthritis with negative bacterial cultures corresponds to the description of oligoarthritis due to Mycoplasma spp, which is considered a typical infection in hypogammaglobulinemias.[2] In the management of patients with CVID it is important to know that both bacterial culture and serological determinations are useless in diagnosing this infection (antibody response is usually inadequate and altered by the substitution immunoglobulins). The diagnostic method of choice is the multipathogen PCR analysis in synovial fluid [2]. The high prevalence of infections with Mycobacteruim tuberculosis and Candida albicans compared to other cohorts [2] is explicable by the high prevalence of tuberculosis in Romania [21] and by the presence of many additional risk factors (immunosuppression caused by lymphoma/ glucocorticoid treatment, chronic pulmonary disease).
The bacteria causing infections in our cohort are predominantly those previously reported [2,5,10]. Sporadic opportunistic infections were found both in patients undergoing chemotherapy and in patients with no other identifiable risk factors. Bacterial and/ or fungal colonization of the lower respiratory tract was found in patient with and without chronic pulmonary disease. Previous studies did not investigate the rate and type or colonization of the lower respiratory tract, thus no comparison with the pattern of colonization in other CVID cohorts could be made and the cause of colonization in patients without chronic pulmonary disease remains unknown. As previously mentioned, IgA and IgM deficit decreases the microorganism neutralization capacity at mucosal level. The statistically significant association between severe infections and bronchiectasis found by Chapel et al. [7] could not be reproduced in either a larger cohort [9] or the present study. Colonization is possibly an inflammatory trigger even in the absence of acute infections and a precursory phenomenon, with causal role, in the appearance of bronchiectasis and other chronic pulmonary diseases found in CVID. A longitudinal study is needed for testing this hypothesis.
All the previously reported categories of noninfectious CVID complications [2,9,10] were found in the present group of patients. We found prevalence above 20% for: tumors of any type, lymphoma, autoimmune diseases, chronic pulmonary disease of any type and bronchiectasis; prevalence between 10-20% for: BLP, chronic digestive disease of any type, splenomegaly and hepatomegaly; and prevalence under 10% for: enteropathy, fibrosing ILD and LIP. These results are consistent with those obtained for the general European cohort with the exception of a higher prevalence of tumors (34% vs 8%) and a lower prevalence of granulomatous disease (3% vs 9%) in the present study [9]. We found no correlation between the patient and family history of cancer that would explain the additional risk for neoplasia. We found no predominance of any of the noninfectious CVID complications in any of the genders.
A single case of autoimmune cytopenia was confirmed, although cytopenias are considered the most frequent autoimmune diseases within CVID [2,10]. We found frequent association of antiphospholipid syndrome with systemic autoimmune diseases (80% of cases) and concomitant family history of autoimmune disease in some patients with autoimmune diseases, but none of these was statistically significant. The category ‘inflammatory diseases’ had not been used in the reference studies about CVID. It is uncertain whether inflammatory diseases found in patients in this cohort make an homogenous and well-delineated group and whether their appearance is linked to CVID. They are being mentioned because there were 3 affected patients (approximately 10%) and because in 2 of these the inflammatory disease led to investigating the patient immune status and the diagnosing CVID. In the case where inflammatory diseases appeared concomitantly with colorectal cancer it is possible that these are paraneoplastic manifestations.
BLP was located in lymph nodes, spleen, liver, lungs and/or bowel. Intestinal lymphocytic infiltration was not included in this category for the statistic determinations. This choice is justified by the lack of a statistically significant association between the lymphocytic infiltration of other organs and that of the intestine in patients in this cohort. The histological diagnosis of atypical lymphoid hyperplasia in both patients in which a biopsy was performed is most likely a selection error, given that biopsy was only indicated to patients in which lymphoma was suspected. A succession from BLP to NHL was not found in any patient, but it must be said that in 4 (66%) of the patients with NHL this was the first diagnosed manifestation of CVID.
The most numerous group of malignant tumors was the B-cell NHL, in consistency with previous studies. Conversely, no case of gastric carcinoma was found, this being the second most frequent malignant tumor in CVID patients [2].
Interestingly, we found significant correlation of ILD, but not of the subgroup fibrosing ILD, with bronchiectasis. These associations have not been tested by previous studies [7,12] and the small size of this cohort makes the interpretation of this results difficult. Testing for these correlations on a larger cohort and prospectively would aid in explaining the progression of chronic lung disease in CVID patients. The radiological aspect of sarcoidosis in a patient with CVID is suggestive for granulomatous lymphocytic interstitial lung disease (the most common cause of ILD in CVID [19]. Although in this patient this diagnosis was not confirmed by transbronchial lung biopsy [2], there are suggestions that open lung biopsy is the diagnostic method of choice [22,23]. All patients with hepatomegaly had infiltration of lymphoid organs (lymph nodes, spleen) on imaging studies and it was assumed hepatomegaly is caused by benign lymphoproliferation. While hepatomegaly did not progress in these patients, admittedly the follow-up period was short (up to 5.5 years) and in the absence of liver biopsy, concomitant presence of liver nodular regenerative hyperplasia (the main liver disease in CVID [24] could not be ruled out.
We tested for correlations between the clinical manifestations of CVID. Combinations of manifestations were selected based on frequent coexistence or on the known possibility of a pathogenic link between them. We found correlations between lymphadenopathies, splenomegaly and hepatomegaly, which supports their inclusion in the common clinical phenotype ‘BLP’, concordantly with previous proposal [7]. Additionally, we found a correlation between bronchiectasis and splenomegaly, this result being discordant with that from the general European cohort [9]. No pathogenic explanation was found for this association. Proving specific correlations between the clinical manifestations of CVID allow delineation of independent clinical phenotypes. The most important consequence of confirming these phenotypes is the possibility to identify the patients at risk of developing certain disease manifestations based on those already present. Consequently, these patients can be monitored for the appearance of the manifestations specific to their phenotype.
The average maintenance dose of IVIG used in these patients was in accordance with current recommendations [21,25]. 25% of patients interrupted treatment for medical or nonmedical reasons. This fact may negatively influence the prognosis of these patients, given that since introduction of substitution therapy in CVID patients the frequency of infections has decreased, quality of life has improved and patient survival has increased by almost 40 years [6]. Especially in patients not undergoing substitution therapy prompt and correct antibiotic treatment is necessary in order to prevent the appearance of certain chronic diseases or their progression (esp. bronchiectasis) [6,21]. Worsening of infection status under substitution therapy in some patients is an indicator of insufficient immunoglobulin substitution. Increase of IVIG doses in spite of adequate IgG trough levels would benefit patients with persistent infections [18,19].
A case of progression of selective IgA deficit to CVID and two cases of familial aggregation of antibody deficiencies were identified in this cohort. For familial cases of antibody deficiency genetic diagnosis is recommended. [10]
Almost 50% of patients had allergies. The high rate of diagnosing is probably partly due to the fact that physicians monitoring and treating CVID at our center are also allergologists. Local glucocorticoid treatment for allergies may decrease the defense capacity at mucosal level, thus increasing the risk for infections.
The low number of deceased patients is consistent with the short follow-up time since diagnosis. In turn, this did not allow for a statistical analysis of potential mortality risk factors and for a relevant comparison with the groups of deceased patients from other studies. The cases of death found confirm the fact that malignant tumors are a frequent cause of death in CVID patients [5] and stresses the high susceptibility for a severe infection uncharacteristic of CVID (tuberculosis) in a patient debilitated by the severe course in a typical CVID manifestation (bronchiectasis).
The main limits of this study are the low number of patients included, the retrospective format and the unavailability of specific antibody response measurements. The first issue could not be corrected, as in the study were included all patients form the IDR of the OFGI who fulfilled the inclusion criteria. The retrospective study format had several disadvantages. In some patients we could not reconstruct the time succession of the various disease manifestations/ of these in relation to possible confounding factors. Investigations relevant for the study (specific antibody response testings, immunophenotypings, biopsies, bacterial cultures, etc) had not been performed previously or their results could not be obtained. There are approximate data for: AO, number of infections, time of onset of a disease, adverse effects to IVIG treatment and there are missing data. Only adult patients were included in the study and this did not allow for an adequate description of the age distribution of disease manifestations. The low statistical significance threshold is justified by the small size of the patient group, but this could be the cause of some differences (distribution and AD in the two genders) and correlations (bronchiectasis- splenomegaly, bronchiectasis- ILD) that could not be explained subsequently.
Conclusion
The results obtained in this study allow conclusions to be drawn on the possible ways of clinical progression of CVID and demographic, clinical, immunological and therapeutic factors that may influence the progression of this disease.
• Gender distribution showed that most patients (72%) diagnosed with CVID were women. The age at diagnosis was higher in women than in men. No other differences in disease progression between the two genders were found.
• Distribution by place of origin showed that most patients (78%) diagnosed with CVID were from urban area. There were no differences in chronological parameters (age at onset of disease, age at diagnosis, diagnostic delay, the average time of the disease evolution, survival time) between the places of origin.
• The average age at onset of disease was 25 years. The average age at diagnosis was 48 years. The average diagnosis delay was 6 years. The diagnostic delay was < 5 years at 50% of the patients and > 15 years at 35% of the patients. The average time of the disease evolution was 10 years.
• At diagnosis 61% of patients had low levels of IgA and IgM, 21% only of IgA, 18% only of IgM. 31 of the patients had very low IgG levels (<150 mg/dL) at diagnosis. There were no patients with high IgM levels.
• The clinical onset of the disease was by: infections in 59% of patients, non-Hodgkin’s lymphoma in 13%, autoimmune disease in 9%, inflammatory disease in 6% and uncertain in 13%.
• All patients had infections. 66% of patients had severe infections. The most prevalent infections were: upper (41%) and lower (63%) respiratory tract infections, lower urinary tract infections (44%), digestive infections, sinusitis, otitis media, skin and soft tissue infections, specific viral infections.
• After initiation of IVIG treatment: prevalence of infections of any type (by 7%), severe infections (by 36%), upper respiratory tract infections and acute diarrhea decreased; prevalence of sinusitis increased; infection status improved in 43% of patients and worsened in 14%.
• 81% of patients had non-infectious manifestations of CVID. In the order of frequency, the non-infectious diseases were: neoplasia (predominantly non-Hodgkin’s lymphomas), autoimmune diseases (predominantly systemic and solid organs), bronchiectasis, splenomegaly, benign lymphoproliferation, chronic digestive diseases (predominantly enteropathy), hepatomegaly, interstitial lung disease, iron deficiency anemia, specific inflammatory diseases, lymphoid interstitial pneumonitis.
• Statistically significant correlations were found between the following disease manifestations: benign lymphoproliferation / lymphadenopathy - splenomegaly - hepatomegaly, bronchiectasis and splenomegaly, interstitial lung disease and bronchiectasis, low IgG levels at diagnosis and severe infections.
• 75% of patients had only one clinical phenotype of the disease.
• Besides IVIG treatment patient management consisted mainly of: monitoring, medication (mainly antibiotic, immunosuppressive and chemotherapeutic) and surgery.
• Disease manifestations did not correlate with family history and patient comorbidities, respectively.
• The cases of death had infectious and non-infectious causes. The average survival time since disease onset in deceased patients was 8 years. All deceased patients were female.
References
- ESID Registry (2016) Working Definitions for Clinical Diagnosis of PID. European Society for Immunodeficiencies.
- Salzer U, Warnatz K, Peter HH (2012) Common variable immunodeficiency- an update. Arthritis Res Ther 14:223.
- Ameratunga R, Woon ST, Gillis D, Koopmans W, Steele R (2013) New diagnostic criteria for common variable immunodeficiency (CVID), which may assist with decision to treat with intravenous or subcutaneous immunoglobulin. Clin Exp Immuno 174: 203-211.
- Conley ME (1999) Diagnostic guidelines - An International Consensus document. Clin Immunol 93:189.
- Resnick ES, Moshier EL, Godbold JH, Cunningham-Rundles C (2012) Morbidity and mortality in common variable immune deficiency over 4 decades. Blood 119: 1650-1657.
- Chapel H, Cunningham-Rundles C (2009) Update in understanding Common Variable Immunodeficiency Disorders (CVIDs) and the management of patients with these conditions. Br J Haematol 145: 709-727.
- Chapel H, Lucas M, Lee M, Bjorkander J, Webster D, et al. (2008) Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood 112: 277-286.
- Wehr C, Kivioja T, Schmitt C, Ferry B, Witte T, et al. (2008) The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood 111: 77-85.
- Quinti I, Soresina A, Spadaro G, Martino S, Donnanno S, et al. (2007) Long-term follow-up and outcome of a large cohort of patients with common variable immunodeficiency. J Clin Immunol 27: 308-316.
- Ahn S, Cunningham-Rundles C, Feldweg A (2017) Clinical manifestations, epidemiology, and diagnosis of common variable immunodeficiency in adults.
- Cunningham-Rundles C (2012) The many faces of common variable immunodeficiency. Hematology Am Soc Hematol Educ Program 2012: 301-305.
- Gathmann B, Mahlaoui N, Ceredih, Gérard L, Oksenhendler E, Warnatz K et al (2014) Clinical picture and treatment of 2212 patients with common variable immunodeficiency. J Allergy Clin Immunol 134: 116-126.
- Definitions of infection severity (2016) Bone and Marrow Transplant Clinical Trial Network.
- Rosen FS, Eibl A, Roifam C, Fischer A, Volanakis J (1999) Primary immunodeficiency diseases. Report of an IUIS Scientific Committee. Clin Exp Immunol 118: 1-28.
- Ameratunga R, Brewerton M, Slade C, Jordan A, Gillis D, et al. (2014) Comparison of diagnostic criteria for common variable immunodeficiency disorder. Front Immunol 5:415.
- Bonilla FA, Khan DA, Ballas ZK, Chinen J, Frank MM, et al. (2015) Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol 136:1186-1205e1-78.
- Ocampo CJ, Peters AT (2013) Antibody deficiency in chronic rhinosinusitis: epidemiology and burden of illness. Am J Rhinol Allergy 27: 34-38.
- Ahn S, Cunningham-Rundles C (2016) Treatment and prognosis of common variable immunodeficiency.
- Walsh JE, Gurrola JG 2nd, Graham SM, Mott SL, Ballas ZK (2017) Immunoglobulin replacement therapy reduces chronic rhinosinusitis in patients with antibody deficiency. Int Forum Allergy Rhinol 7: 30-36.
- Orange JS, Grossman WJ, Navickis RJ, Wilkes MM (2010) Impact of trough IgG on pneumonia incidence in primary immunodeficiency: A meta-analysis of clinical studies. Clin Immunol 137: 21-30.
- de Colombani P, Hollo V, Jansen N, Kremer K, Labelle S, et al. (2014) Review of the tuberculosis programme in Romania. WHO Regional Office for Europe.UN City, Copenhagen, Denmark.
- Routes J (2017) Granulomatous-Lymphocytic interstitial lung disease in common variable immunodeficiency.Cleveland Clinic-Center for continuing education.
- Rao N, Mackinnon AC, Routes JM (2015) Granulomatous and Lymphocytic Interstitial Lung Disease (GLILD): A Spectrum of Pulmonary Histopathological Lesions in Common Variable Immunodeficiency (CVID) - Histological and Immunohistochemical Analysis of 16 cases. Hum Pathol 46: 1306-1314.
- Malamut G, Ziol M, Suarez F, Beaugrand M, Viallard JF, et al. (2008) Nodular regenerative hyperplasia: the main liver disease in patients with primary hypogammaglobulinemia and hepatic abnormalities. J Hepatol 48: 74-82.
- CSL Behring GmbH Privigen: EPAR- Product Information. European Medicines Agency Website.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi