Case Report, Clin Oncol Case Rep Vol: 6 Issue: 3
Prolonged Complete Response to Retreatment with Olaparib in a Patient with Resected Single Brain Metastasis of Recurrent BRCA2-Mutated Ovarian Cancer.
Gonzalo Lendinez Sanchez* , Tamara Diaz Redondo, Alfonso Sanchez Munoz
Department of Medical Oncology, Intercenter Unit. Regional and Virgen de la Victoria University Hospitals, IBIMA, Malaga, Spain.
*Corresponding Author: Gonzalo Lendinez Sanchez
Department of Medical Oncology
Intercenter Unit. Regional and Virgen de la Victoria University Hospitals, IBIMA, Malaga, Spain.
E-mail: gonlensa@hotmail.com
Received: February 25, 2023; Manuscript No: COCR-23-91511;
Editor Assigned: February 27, 2023; PreQC Id: COCR-23-91511 (PQ);
Reviewed: March 10, 2023; QC No: COCR-23-91511 (Q);
Revised: March 13, 2023; Manuscript No: COCR-23-91511 (R);
Published: March 17, 2023; DOI: 10.4172/cocr.6(3).281
Citation: Lendinez Sanchez G, Diaz Redondo T, Sanchez Munoz A (2023) Prolonged Complete Response to Retreatment with Olaparib in a Patient with Resected Single Brain Metastasis of Recurrent BRCA2-Mutated Ovarian Cancer. Clin Oncol Case Rep 6:3
Abstract
The incidence of brain metastases in ovarian cancer is quite rare, being approximately 1.34%. According to retrospective studies, patients with BRCA 1/2 mutations present a higher risk. The trimodal approach based on surgery, radiotherapy and chemotherapy presents greater benefits in terms of overall survival. In preclinical studies, inhibitors of Poly ADP ribose polymerase have been shown to cross the blood-brain barrier, being an alternative to control the disease at the systemic level and at the level of the central nervous system. The SOLO2 (olaparib), NOVA (niraparib) and ARIEL3 (rucaparib) clinical trials do not refer data on patients with brain metastases, the published evidence for iPARP in this population comes only from case reports.
We present the case of a 54-year-old woman with stage IV high-grade serous papillary carcinoma who, after 37 months of maintenance olaparib, presented a single brain lesion. After radical treatment restarted olaparib, remaining disease-free 13 months since Olaparib restart and 87 months since the diagnosis. This case suggests that, if we achieve a radical treatment of brain metastasis and the disease at a systemic level is controlled, a reasonable option is to maintain systemic maintenance treatment with iPARP.
Keywords: iPARP; Olaparib; Ovarian cancer; Brain metastases; BRCA; Case report; Retreatment; Surgery; Niraparib; Prolonged complete response
Introduction
The management of brain metastases is a challenge in cancer patients, the incidence of these in ovarian cancer is approximately 1.34% (0.49%-6.1%) [1]. Despite the variety of existing treatments (surgery, radiotherapy, and chemotherapy), there is no established guideline or protocol for the mangement of brain metastases from ovarian cancer.
Inhibitors of Poly ADP ribose polymerase (iPARP) have provided a new alternative for the management of metastatic disease, especially in patients with ovarian cancer with homologous recombination deficiency (HRD), however there is insufficient evidence on their efficacy for disease control at the CNS level.
We present the case of a 54-year-old woman diagnosed with high-grade papillary serous carcinoma of the ovary with a pathogenic germline mutation in BRCA2. During treatment with Olaparib, he presented a single brain lesion compatible with metastasis that, after radical surgical treatment, continued maintenance with Olaparib, remaining in complete radiological response. Given the impossibility of carrying out a randomized study in patients with these characteristics, we report our experience with the case of our patient, remaining free of disease after 13 months of treatment.
Case Presenatation
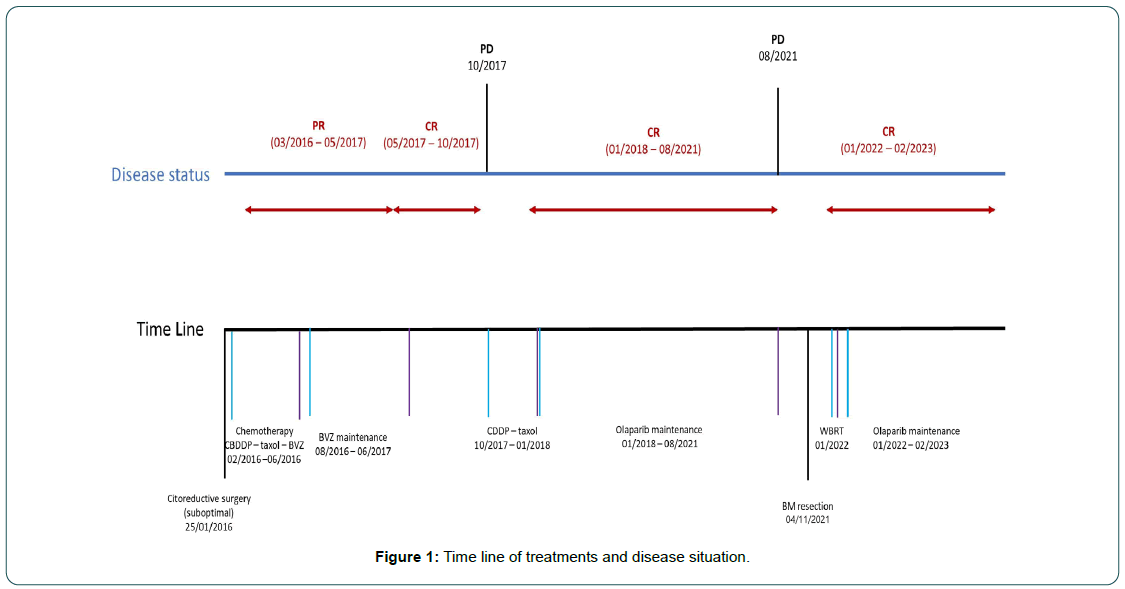
A 60-year-old woman, is diagnosed of ovarian neoplasia at the age of 54 after study of postmenopausal metrorrhagia. On 01/25/2016, suboptimal maximum effort cytoreductive surgery was performed with bilateral involvement of the ovary, uterus, and multiple peritoneal implants due to high-grade serous carcinoma and positive ascitic fluid cytology. The extension study shows thickening and pleural effusion that after thoracentesis confirms positive pleural fluid cytology for papillary adenocarcinoma. Initially IVA stage with post-surgical Ca 125 of 646 mg/dl. She received 7 cycles of chemotherapy with paclitaxel 175mg/m2-carboplatin AUC6-bevacizumab 7.5 mg/kg, obtaining Partial Response (PR) as the best radiological response and biochemical response with normalization of Ca 125. Subsequently, maintenance Bevacizumab for 16 cycles, radiological reaching Complete Response (CR) radiological. Four months after the end of bevacizumab, she presented peritoneal progression and was switched to cisplatin-paclitaxel (due to haematological toxicity with carboplatin), receiving 4 cycles obtaining radiological CR and normalization of tumour markers. BRCA2 germline mutation (c.2636_2637delCT (p.Ser879Terfs)) was identified starting maintenance with Olaparib 400 mg/12h. In August 2021 (37 months from Olaparib), she presented tonic-clonic seizures, detecting a 5cm x 4 cm right frontal cerebral SOL. Case was presented to a multidisciplinary committee, and in the absence of disease at another level, complete excision was decided confirming metastasis of high-grade serous papillary carcinoma positive for CK7+, WT1+, and RE+; CK20-.
She received adjuvant holocranial radiotherapy 30 Gy to 3 Gy daily and in the absence of systemic disease, olaparib was restarted at a dose of 250 mg/12h. She currently maintains a complete radiological response without brain disease more than 12 months after restarting olaparib. The periods of treatment and progression are summarized in Figure 1.
Discussion
We present for the first time a case of retreatment with olaparib after surgical excision of solitary brain metastasis given the absence of disease at another level, remaining in CR after more than 13 months of treatment. The incidence of brain metastases in ovarian cancer is approximately 1.34% (0.49%-6.1%) [1]. The Hazard Ratio (HR) for developing brain metastases in patients carrying BRCA mutations was 3.84 (95% CI: 1.60-9.22, p < 0.001) with no difference in terms of survival [2].
The best survival data are obtained with a trimodal approach (radiation therapy, surgery, and chemotherapy) while monotherapy treatment is associated with poorer survival (HR: 2.57, 95% CI: 1.64- 3.86) [3]. The rest of the published literature is in line with these findings, achieving an Overall Survival (OS) around 20 months-30 months [4]. The prognosis despite multidisciplinary treatment remains poor with median OS around one year [1].
Regarding systemic treatment, platinum derivatives remain the best therapeutic option in ovarian epithelial neoplasms and are capable of crossing the BBB, occasionally achieving adequate control of the disease, however, the results are poor with inferiority in regards to other treatment modalities [3]. iPARPs induce the formation of double-stranded DNA breaks by trapping PARP1 and blocking the single-stranded DNA break repair pathway. Tumor cells deficient in the Homologous Recombination Repair (HRD) pathway eventually die due to the inability to accurately repair DNA double-strand breaks, known as synthetic lethality [5, 6].
The SOLO2 (olaparib) NOVA (niraparib) and ARIEL3 (rucaparib) clinical trials demonstrated an increase in progression-free survival (PFS) in patients with BRCA mutations compared to placebo: 19.1 vs 5.5 (HR: 0.30); 21 vs 5.5 (HR: 0.27); 16.6 vs 5.4 (HR: 0.23) [7-9]. In addition, niraparib and rucaparib demonstrated increased PFS in the HRD subgroup and in the all-patients subgroup [8,9]. However, they do not make reference to data on patients with brain metastases. Post-hoc analyses on the efficacy of these drugs in this cohort have not been published. The only iPARP data published about this population come from clinical cases [5, 10-15]; whose main characteristics are presented in Table 1.
Table 1: Clinical cases of epithelial ovarian cancer with CNS disease treated with iPARPs published in PubMed (ADC = Adenocarcinoma; ATM = Ataxia Telangiectasia Mutated; BRCA = Breast Cancer Gene; Cht = Chemotherapy; CR = Complete Response; g = germline; HGSC= High Grade Serous Carcinoma; PFS= Progression Free Survival (since iPARP); PPC = Primary Peritoneal Cancer; PR = Partial response; s = somatic; SC = Serous Cystoadenocarcinoma; SD = Stable Disease; WBRT =Whole Brain Radiotherapy).
| Reference | Histology | Mutation | CNS disease | Management | iPARP | PFS | Best response |
|---|---|---|---|---|---|---|---|
| Tao M5 | HGSC | BRCA2s | Multiple | WBRT + Cht | Niraparib | 15m | PR |
| ATMg | |||||||
| Gray S10 | SC | BRCA1g | Multiple | WBRT + Cht* | Niraparib | 17m | SD |
| Zhang Z11 | HGSC | Wild type | Single | Surgery | Niraparib | 29m | CR |
| Gallego A12 | HGSC | BRCA1g | Multiple | WBRT + Cht | Olaparib | 42m | PR |
| Bangham M13 | ADC | BRCA2 | Lepto-meningeal | Cht | Olaparib | 12m | PR |
| Sakamoto I14 | PPC HGSC | BRCA1 | Multiple | WBRT + Cht | Olaparib | 22m | PR |
| Morales F15 | HGSC | BRCA1 | Multiple | WBRT + Cht | Olaparib | 9m | SD |
Niraparib has shown to be able to cross the BBB in animal models, being superior to other iPARPs. Despite the fact that the pharmacodynamic data of olaparib showed little activity at the brain level [16], cases such as the one presented in this work and other similar ones in the bibliography show good control of ovarian metastatic disease at the CNS level in patients with a BRCA 1/2 mutation, however, the current evidence is not enough to confirm this statement [12-15].
Conclusion
• The trimodal approach including surgery, radiotherapy and chemotherapy has shown higher survival.
• According to retrospective studies, the incidence of brain metastases in BRCA mutated ovarian cancer appears to be higher than in wild-type ovarian cancer.
• The activity that iPARPs may have in the prevention and treatment of brain metastases of ovarian origin is unknown. In this sense, if we achieve a radical treatment of brain metastasis (surgery and/or RT), and if the disease at a systemic level is controlled, a reasonable option is to maintain systemic maintenance treatment with iPARP.
References
- Borella F, Bertero L, Morrone A, Gambella A, Bovetti M, et al. (2020) Brain metastases from ovarian cancer: Current evidence in diagnosis, treatment, and prognosis. Can 12: 2156. [Google Scholar] [Cross Ref]
- Ratner E, Bala M, Louie-Gao M, Aydin E, Hazard S, et al. (2019) Increased risk of brain metastases in ovarian cancer patients with BRCA mutations. Gynecol Oncol 153: 568-573. [Google Scholar] [Cross Ref]
- Marchetti C, Ferrandina G, Cormio G, Gambino A, Cecere S, et al. (2016) Brain metastases in patients with EOC: Clinico-pathological and prognostic factors. A multicentric retrospective analysis from the MITO group (MITO 19). Gynecol Oncol 143: 532-538. [Google Scholar] [Cross Ref]
- Pakneshan S, Safarpour D, Tavassoli F, Jabbari B (2014) Brain metastasis from ovarian cancer: A systematic review. J Neuro-Oncol 119: 1-6. [Google Scholar] [Cross Ref]
- Tao M, Cheng J, Wu X (2020) Niraparib as maintenance therapy in germline ATM-mutated and somatic BRCA2-mutated ovarian cancer with brain metastases: A case report and literature review. Oncotargets Ther 13: 12979. [Google Scholar] [Cross Ref]
- Murai J, Huang SYN, Das BB, Renaud A, Zhang Y, et al. (2012) Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res 72: 5588-5599. [Google Scholar] [Cross Ref]
- Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, et al. (2017) Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 18: 1274-1284. [Google Scholar] [Cross Ref]
- Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, et al. (2016) Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. New Eng J Med 375: 2154-2164. [Google Scholar] [Cross Ref]
- Coleman RL, Oza AM, Lorusso D, Aghajanian C, Oaknin A, et al. (2017) Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390: 1949-1961. [Google Scholar] [Cross Ref]
- Gray S, Khor XY, Yiannakis D (2019) Niraparib as maintenance therapy in a patient with ovarian cancer and brain metastases. BMJ Case Rep 12: e230738. [Google Scholar] [Cross Ref]
- Xu M, Zhang Z, Du X, He H, He W, et al. (2022) Successful treatment of a patient with brain metastasis from ovarian cancer with BRCA wild type using niraparib: A case report and review of the literature. Front Oncol 1832. [Google Scholar] [Cross Ref]
- Gallego A, Garrido D, Yebenes L, Mendiola M, Castelo B, et al. (2021) Long-term response to olaparib in BRCA1-related ovarian cancer with brain metastases. Intern J Gynecol Can 31: 1292-1296. [Google Scholar] [Cross Ref]
- Bangham M, Goldstein R, Walton H, Ledermann JA (2016) Olaparib treatment for BRCA-mutant ovarian cancer with leptomeningeal disease. Gynecol Oncol Rep 18: 22. [Google Scholar] [Cross Ref]
- Sakamoto I, Hirotsu Y, Nakagomi H, Ikegami A, Teramoto K, et al. (2019) Durable response by olaparib for a Japanese patient with primary peritoneal cancer with multiple brain metastases: A case report. J Obstet Gynaecol Res 45: 743-747. [Google Scholar] [Cross Ref]
- Vasquez FM, Basave HNL, Herrera CM, Gonzalez RRP (2020) Clinically relevant response to treatment with olaparib in a patient with refractory multidrug-resistant ovarian cancer and central nervous system involvement: A case report. Am J Case Rep 21:e925990- e925991. [Google Scholar] [Cross Ref]
- Sun K, Mikule K, Wang Z, Poon G, Vaidyanathan A, et al. (2018) A comparative pharmacokinetic study of PARP inhibitors demonstrates favorable properties for niraparib efficacy in preclinical tumor models. Oncotarget 9: 37080. [Google Scholar] [Cross Ref]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi