Review Article, J Immunol Tech Infect Dis Vol: 11 Issue: 4
Safety and Efficacy of Liposomal Amphotericin B as Empirical Therapy in Invasive Fungal Diseases: A Narrative Review
Methee Chayakulkeeree1*, Senthur Nambi 2, Rasmi Palassery 3and Biju George4
1Department of Medicine, Mahidol University, Bangkok, Thailand
2Department of Infectious Diseases, Apollo Hospitals, Chennai, India
3Department of Medical Oncology, MS Ramaiah Medical College, Karnataka, India
4Department of Hematology, Christian Medical College, Vellore, India
*Corresponding Author: Methee Chayakulkeeree, Department of Medicine, Mahidol University, Bangkok, Thailand, Tel: +662-419-9462; E-mail: methee.cha@mahidol.ac.th
Received date: 19 July, 2022, Manuscript No. JIDIT-22-69604; Editor assigned date: 22 July, 2022, PreQC No JIDIT-22-69604 (PQ); Reviewed date: 05 August, 2022, QC No. JIDIT-22-69604; Revised date: 03 October, 2022, Manuscript No. JIDIT-22-69604 (R); Published date: 10 October, 2022, DOI: 10.4172/2329-9541.1000332
Citation: Chayakulkeeree M, Nambi S, Palassery R, George B (2022) Safety and Efficacy of Liposomal Amphotericin B as Empirical Therapy in Invasive Fungal Diseases: A Narrative Review. J Immunol Tech Infect Dis 11:4.
Abstract
Invasive Fungal Diseases (IFDs) have been considered a critical cause of morbidity and mortality among patients with febrile neutropenia receiving hematopoietic stem cell transplant and/or chemotherapy for cancer treatment. Toxicity concerns and adverse events are significant challenges associated with administering conventional Amphotericin B (c-AmB). Therefore, lipid and liposomal formulations of amphotericin B are effective, well-tolerated and remain the standard of care in treating IFDs. These formulations are reported to exhibit a broad antifungal spectrum, low resistance and reduced toxicity. Empirical therapy with Liposomal Amphotericin B (L-AmB) has been found to enhance efficacy and lower toxicity and is recommended in neutropenic patients with different conditions like cancer and transplant. Additionally, L-AmB has demonstrated better efficacy and safety as compared to other antifungal agents like caspofungin, amphotericin B lipid complex and c-AmB, thus providing an added advantage over these agents.
Currently, studies published in the literature mostly focus on the efficacy of L-AmB for a single clinical condition. In most published studies, L-AmB has been administered in combination with other antifungal agents (either empirically or prophylactically). Moreover, studies exclusively focusing on the benefit of empirical therapy with L-AmB for treating IFD in patients with various conditions are not available. Therefore, this review collates and highlights the efficacy and safety of LAmB exclusively as empirical therapy in treating IFD in patients with conditions including cancer and transplant patients with neutropenia.
Keywords: Liposomal Amphotericin B, Empirical therapy, Invasive fungal disease, Febrile neutropenia, Hematological malignancy, Transplant, Safety, Efficacy
Introduction
Fungal diseases impact more than one billion people, with mortality in more than 1.5 million individuals globally [1]. Invasive Fungal Disease (IFD) has been regarded as one of the prevalent nosocomial infections that cause invasive diseases [2]. According to GAFFI, every year, over 300 million people across the globe and of all ages suffer from a serious fungal infection [3]. A 2 years prospective observational multicentric study conducted in 8 Asian countries reported the overall 30 days mortality due to IFDs to be 22.1% [4]. Furthermore, a Thailand based study reported the prevalence of serious fungal infection to be approximately 1.93% (1,254,562) of its population [5]. As per another prospective observational study conducted in India, the epidemiology of IFDs was reported to be 7.6% (253/3300) [6]. However, in India, data on the burden of IFD is found to be limited. The tropical climatic conditions in South East Asia are claimed to be favorable for the growth of various fungal infections such as candida, cryptococcus, rhizopus, mucor and aspergillus, which are regarded as opportunistic pathogens responsible for the manifestation of IFD [7,8].
The incidence of IFDs (e.g., invasive candidiasis) is observed in bloodstream infections or other parts of the body (including lungs, skin, sinuses, heart valves, liver, central nervous system, eyes, spleen or bone) [9]. The occurrence of IFD has been found to be frequent in the past few years, particularly among hospitalized patients. Moreover, in hospitals, immunocompromised patients (such as patients with acquired immunodeficiency syndrome, hematological diseases, malignant tumors, organ transplantation or burns) are reported to be highly susceptible to developing IFD. In addition, IFD is considered a critical cause of morbidity and mortality among patients with Febrile Neutropenia (FN), solid organ transplant, hematopoietic stem cell transplants and several other immunocompromised patients [10-14].
According to the infectious diseases society of American guidelines, the therapies for IFDs include polyenes (amphotericin B deoxycholate, lipid and liposomal formulations of amphotericin B), flucytosine, triazoles (isavuconazole, fluconazole, itraconazole, voriconazole and posaconazole) as well as the echinocandins (anidulafungin, caspofungin and micafungin) [15,16]. However, despite the availability of a wide range of antifungal treatments, high mortality rates have been reported with IFDs. Additionally, resistance has been observed among various fungal species, including some molds (Fusarium, Scedosporium , few Aspergillus species) against some antifungal agent [17].
Conventional amphotericin B (c-AmB; amphotericin B deoxycholate), a broad spectrum polyene antifungal, is generally preferred as an effective antifungal agent against several IFDs and has been used for decades. Additionally, amphotericin B deoxycholate is found to be associated with a low resistance rate as well as good clinical and pharmacological action [18]. However, concerns related to amphotericin B deoxycholate mediated toxicity (nephrotoxicity) have been considered one of the significant challenges. This further led to the development of lipid based amphotericin B. These lipid and liposomal formulations have exhibited a broad antifungal spectrum, low resistance and reduced nephrotoxicity. Liposomal Amphotericin B (L-AmB; AmBisome) is composed of a phospholipid layer structure and three major components (cholesterol, distearoyl phosphatidylglycerol and soy phosphatidylcholine) [19,20]. L-AmB possesses a favorable therapeutic index due to various physicochemical attributes that include the small size of liposomes (<100 nm; facilitates prolonged circulation, thereby aiding the distribution of the drug to various organs), composition releases amphotericin B from the liposome bilayer after coming in contact with the fungus; thus, reducing the adverse effects of amphotericin B on host tissues [21]. Besides, the formulation is reported to be clinically active against molds (such as Aspergillus spp. and Mucorales) and yeasts (such as Candida spp.) and is approved for the treatment of IFDs in many countries worldwide.
The primary antifungal prophylaxis has been beneficial in reducing IFDs; however, breakthroughs IFDs (manifestation of infection during antifungal drug exposure) were reported even after antifungal prophylaxis [22-24]. Manifestation of breakthrough fungal infections is evident when a prophylactic agent is inactive or resistant against the invading fungal pathogen and even in certain instances like intense immunosuppression or low prophylactic drug serum levels [25].
Limitations associated with prophylaxis therapy include cost, interference with diagnostic assays (such as the galactomannan assay, resulting in false-negative outcomes), drug toxicity, drug-drug interactions among heavily medicated patients and increased risk of resistance.
Empirical antifungal therapy is preferred as a treatment strategy in patients with various high-risk factors along with established IFDrelated clinical symptoms prior to any microbiological documentation, species identification and antibiotic susceptibility test result.
According to a study, the administration of empirical antifungal treatment in patients with a high risk of fungal infection should be considered as there is a close association between treatment initiation delay, clinical outcome and hospital mortality. It is found to be beneficial in decreasing the rate of mortality associated with fungal infections. Therefore, identifying risk factors during the initial stage is regarded as the basis of empirical treatment of fungal infections.
Empirical therapy with broad-spectrum antifungal agents is generally considered during the incubation and prodromal stage of the infection to combat treatment delay (as the generation of microbiological test reports requires around 24 to 72 hours). Studies have found empirical antifungal therapy as cost-effective compared to prophylactic treatment. In addition, empirical antifungal therapy with L-AmB (as a broad-spectrum antifungal agent) has been found to enhance efficacy and lower toxicity.
Currently, studies published in the literature mostly focus on the efficacy of L-AmB for a single clinical condition. In most published studies, L-AmB has been administered in combination with other antifungal agents (either empirically or prophylactically). Moreover, studies exclusively focusing on the benefit of empirical therapy with L-AmB for treating IFD in patients with various conditions are not available. Therefore, this review collates and highlights the efficacy and safety of L-AmB exclusively as empirical therapy in treating IFD in patients with conditions including cancer and transplant patients with neutropenia.
Literature Review
Efficacy of liposomal amphotericin B in treating IFD in patients with different conditions
IFD in cancer patients with febrile neutropenia: Invasive fungal diseases are considered a critical cause of morbidity and mortality among patients with FN receiving hematopoietic stem cell transplants and/or chemotherapy for cancer treatment. In addition, patients with hematological malignancies are usually susceptible to impaired defense mechanisms due to the disease or treatment, making them vulnerable to infection.
Studies depicting the efficacy of L-AmB as empirical therapy for treating IFDs in cancer patients with FN are listed below.
According to a Japan-based prospective study, L-AmB is the drug of choice, demonstrating high therapeutic effect in patients with FN (with baseline blood diseases like acute myeloid or lymphoid leukemia, myelodysplastic syndromes) with an increased risk of fungal infection. In another prospective study, empirical antifungal therapy with L-AmB showed a better response and reduced hospital stay than caspofungin in patients with a high risk of IFD and chemotherapy-induced FN compared to low-risk patients. Similarly, empirical therapy with L-AmB demonstrated broad-spectrum antifungal activity at a low dose (1 mg/kg/day) among high-risk patients (with prolonged FN, hematological malignancies and those undergoing intensive chemotherapy) (Table 1).
| Study design and patient population | Treatment regimen | Clinical response | |||
|---|---|---|---|---|---|
| One-arm, prospective, post-marketing observational study (n=399; children (≤ 15 years), adults (16-64 years) and elderly individuals (≥ 65 years)) | L-AmB (2.5 mg/kg/d; i.v. infusion) | Overall clinical response rate: 186/399; (46.6%) | |||
| Resolution of fever: 244/399; (61.2%) | |||||
| Survival for ≥ 7 days after completion of the therapy: 334/399; (83.7%) | |||||
| Absence of breakthrough fungal infections: 395/399 (99.0%) | |||||
| Successful treatment of baseline infection: 10/18 patients | |||||
| No discontinuation due to toxicity or lack of efficacy 243/399 (60.9%) | |||||
| A multicentric, open-labeled, single-armed phase II trials (n=80; 16-79 years) | Low dose L-AmB (1 mg/kg/day i.v.) | Treatment success rate: 53/80 (66.3%) | |||
| Treatment completion rate: 59/80 (73.8%) | |||||
| Defervescence prior to neutrophil recovery: 61/80 (76.3%) | |||||
| No mortality and IFD in the study period | |||||
| A prospective, randomized, controlled trial [n=104; children (≤ 18 years) | L-AmB | L-AmB | Caspofungin | ||
| (3 mg/kg/d i.v.; n=25) | Complete response rate | 0.88 | 0.839 | ||
| Caspofungin (50 mg/m2/d i.v., loading dose: 70 mg/m2; n=31) | Median hospital stay | 18 days | 28 days | ||
| A prospective, randomized, double-blind study (n=83; 2-17 years) | L-AmB | L-AmB | Caspofungin | ||
| (3 mg/kg/d i.v.; n=26) | Overall favorable response* | 8/18 (44.4%) | 17/41 (41.5%) | ||
| Caspofungin (70 mg/m2/d i.v.; n=56) | |||||
| A double-blind, randomized study (n=244; mean age: 42.0 ± 20.4 years) | L-AmB | L-AmB (3 mg/kg/d) | L-AmB (5 mg/kg/d) | ABLC | |
| (3 mg/kg/d; n=85 and 5 mg/kg/d; n=81) | A successful response to treatment | 34/85 (40%) | 34/81 (42%) | 26/78 (33.3%) | |
| ABLC (5 mg/kg/d; n=78) | Premature discontinuation of treatment | 11/85 (12.9%)a | 10/81 (12.3%)a | 25/78 (32.1%) | |
| An open, randomized, comparative, multicenter trial (n=106; 18-74 years) | (L-AmB) AmBisome (5 mg/kg/d; n=32, i.v.) | L-AmB | c-AmB | ||
| Complete response (p=0.03) | 14/32 patients | 6/34 patients | |||
| c-AmB (1 mg/kg/d; n=34, i.v.) | Failure | 11/32 patients | 15/34 patients | ||
Table 1: Efficacy outcomes of liposomal amphotericin B as empirical therapy in treating IFD in cancer patients with febrile neutropenia.
Comparative trials reported in the literature have also demonstrated the efficacy of L-AmB as empirical therapy over other antifungal drugs in treating IFD. A prospective study including children with persistent fever and neutropenia (receiving chemotherapy for cancer or had hematopoietic Stem Cell Transplantation (SCT)) showed an overall favorable treatment response within the L-AmB-treated group as compared to the caspofungin-treated group (Table 1).
According to another comparative trial, the incidence of a successful response to empirical therapy with L-AmB was higher compared to Amphotericin B Lipid Complex (ABLC) in treating suspected fungal infection in patients with FN (in both Bone Marrow Transplant (BMT) and non-BMT patients (solid tumor, acute leukemia, lymphoma, myelodysplasia, multiple myeloma)). In addition, a significantly high proportion of patients treated with ABLC prematurely discontinued the therapy compared to patients treated with L-AmB therapy.
In another comparative, multicenter trial, patients (neutropenic with documented or suspected IFDs) treated with L-AmB (AmBisome) demonstrated better responses than patients treated with c-AmB. In patients with documented infections (p=0.05) and pulmonary aspergillosis (p=0.096), a favorable trend in response to the therapy with L-AmB was observed on day 14. Also, the mortality rates were lower in patients treated with L-AmB (based on the malignancy status, p=0.03) compared to c-AmB.
IFD in transplant patients with neutropenia: Increased risk of fungal infections contributes to morbidity and mortality amongst transplant recipients. Immunosuppression, extended neutropenia and graft vs host disease in patients undergoing transplantation augment the incidence of fungal infections. Several studies have reported the efficacy of L-AmB as empirical therapy for fungal infection in transplant recipients.
A study conducted by Kruger, on 115 patients (with autologous or allogeneic bone marrow or peripheral blood SCT) reported L-AmB (empirical therapy) as an effective antimycotic therapy.
A Japan based retrospective study reported that low dose L-AmB (median daily dose of 1.2 mg/kg/day) could be effective as empirical therapy in patients with FN undergoing cord blood transplantation. Empirical antifungal therapy with L-AmB was also effective for treating persistent FN in patients undergoing allogeneic SCT. The study reported a higher overall success rate with fewer breakthrough infections and resolution of fever during neutropenia with empirical L-AmB treatment (Table 2).
| Study design and patient population | Treatment regimen | Clinical response | ||
|---|---|---|---|---|
| A single-center retrospective study (n=48; 17-68 years) | L-AmB (median daily dose 1.2 mg/kg/day i.v.; n=15) | Resolution of fever during neutropenia: 9/15 (60%) | ||
| n=134 | L-AmB (n=61) | L-AmB | Caspofungin | |
| Caspofungin (n=73) | Breakthrough infections observed | 4/61 (6%) | 11/73 (15%) | |
| Resolution of fever during neutropenia | 40/61 (66%) | 41/73 (56%) | ||
| Overall success rate | 28/61 (46%) | 24/73 (33%) | ||
Table 2: Efficacy outcomes of liposomal amphotericin B as empirical therapy in treating IFD in transplant patients.
Discussion
Safety and tolerability of Liposomal Amphotericin B in treating IFD in patients with different conditions
The safety and tolerability of L-AmB as empirical therapy in treating IFD among immunocompromised patients with neutropenia has been widely studied and reported in the literature.
A Japan based study was conducted on dose specific groups (<2.5 mg/kg/d, 2.5 mg/kg/d and >2.5 mg/kg/d) of patients with FN. The study outcome related to the safety information of L-AmB in the 3 groups is provided in Table 3.
| Patient population | Treatment regimen | Clinical outcome | ||
|---|---|---|---|---|
| n=399 | L-AmB (2.5 mg/kg/d; i.v. infusion) | No discontinuation due to toxicity or lack of efficacy in the dose specific groups are listed below: | ||
| Patients with FN (with baseline blood diseases like acute myeloid or lymphoid leukemia, myelodysplastic syndromes, etc.) | <2.5 mg/kg/d: 91/153 (59.5%) | |||
| 2.5 mg/kg/d: 43/61 (70.5%) | ||||
| >2.5 mg/kg/d: 109/184 (59.2%) | ||||
| n=30 | L-AmB | Infusion-related adverse drug events (mainly rigors/chills) | ||
| Patients with hematological malignancies and persistent FN | (Group A: high intermittent dose regimen: 10 mg/kg on day 1, 5 mg/kg on days 3 and 6) | High dose: 11/45 (24%) infusions | ||
| Standard dose: 12/201 (6%) infusions (p=0.002) | ||||
| (Group B: Standard dose: 3 mg/kg/d for 14 days) | Total episodes of hypokalemia | |||
| Group A: 57/143 (39% daily blood samples) | ||||
| Group B: 80/137 (58% daily blood samples) (p=0.21) | ||||
| n=687 | L-AmB | L-AmB | Conventional AmB | |
| Patients with persistent fever and neutropenia who had undergone bone marrow or peripheral-blood stem-cell transplantation or undergoing chemotherapy | (mean daily dose: 3.0 ± 0.9 mg/kg; n=343) | Serum creatinine during therapy (Nephrotoxic effects) | ||
| Conventional AmB (mean daily dose: 0.6 ± 0.2 mg/kg; n=344) | 1.5 times baseline value | 101/343 (29.4%) | 170/344 (49.4%) | |
| 2.0 times baseline value | 64/343 (18.7%) | 116/344 (33.7%) | ||
| 3.0 times baseline value | 28/343 (8.2%) | 57/344 (16.6%) | ||
| Hypokalemia | 23/343 (6.7%) | 40/344 (11.6%) | ||
| Infusion-related reactions | ||||
| Fever following infusion (increase of ≥ 1.0°C) | 58/343 (16.9%) | 150/344 (43.6%) | ||
| Chills or rigors | 63/343 (18.4%) | 187/344 (54.4%) | ||
| Other reactions | 57/343 (16.6%) | 82/344 (23.8%) | ||
Table 3: Safety outcomes of liposomal amphotericin B as empirical therapy in treating IFD in cancer and transplant patients with febrile neutropenia.
According to a randomized, double blind, multicenter trial conducted by Walsh, et al. L-AmB can be considered a suitable alternative to c-AmB as empirical treatment for neutropenic patients with bone marrow or SCT undergoing chemotherapy.
This study demonstrated reduced Infusion Related Reactions (IRRs), nephrotoxic effects and hypokalemia.
Intermittent therapy with high dose L-AmB (as empirical therapy) has been reported to be as safe and tolerable as the standard lower dose over an extended period in patients with persistent FN as per a pilot exploratory study. In addition, the study reported fewer episodes of hypokalemia in patients administered with intermittent high doses compared to the standard dose. Infusion-related adverse drug events exhibited a higher trend in patients administered with a high dose of L-AmB; however, no patient reported discontinuation of L-AmB therapy due to toxicity.
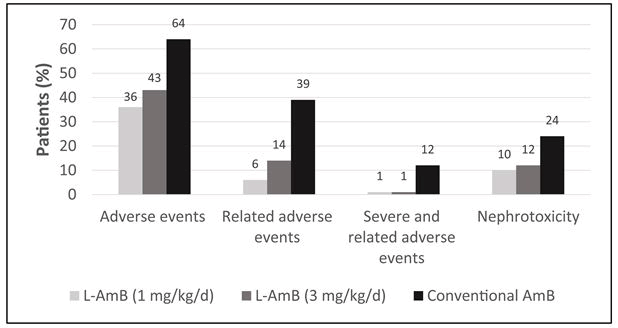
L-AmB with a dosage of 1 or 3 mg/kg/d has also been reported to be safer compared to the c-AmB in adults and children with pyrexia of unknown origin and neutropenia (few patients had hematological malignancies and fungal infection) in two open-label randomized multicenter trials. Patients treated with c-AmB experienced significantly more adverse events, nephrotoxicity and allergic reactions (c-AmB: 2% vs. L-AmB (1 mg/kg/d): 0.6% and L-AmB (3 mg/kg/d): 0.8% of the total number of doses) than patients treated empirically with L-AmB (p<0.01) (Figure 1).
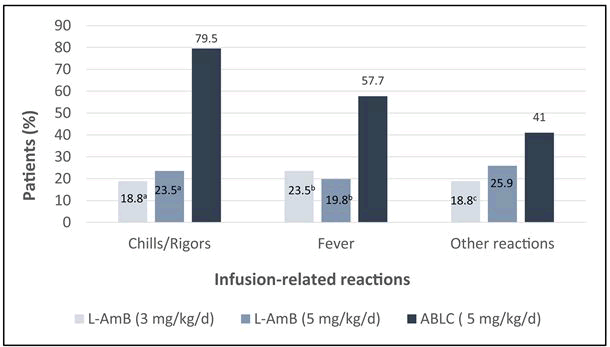
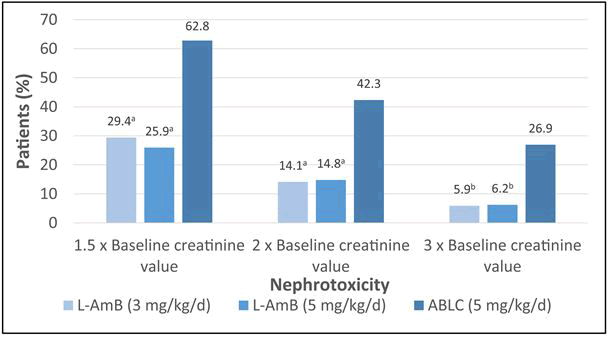
According to a randomized, double-blind comparative study, L-AmB as empirical therapy is reported to exhibit a superior safety profile and better tolerance than ABLC in patients with FN (including a few patients with both BMT and non-BMT) with suspected fungal infection. Further, the study reported a significantly lower frequency of IRRs (chills/rigors, fever and other reactions) on day 1 (Figure 2) and less nephrotoxicity (Figure 3) after the empirical treatment with L-AmB (3 mg/kg/d and 5 mg/kg/d) as compared to ABLC (5 mg/kg/d).
Conclusion
The Liposomal formulation of Amphotericin B (L-AmB) has represented a significant advancement in the drug delivery process. In view of the published literature, L-AmB is found to be safe and effective in treating IFDs empirically in patients with different underlying conditions (cancer and transplant). Patients successfully treated with the empirical treatment of L-AmB demonstrated high therapeutic effect, fewer breakthrough infections and resolution of fever. Furthermore, empirical therapy with L-AmB exhibited reduced adverse reactions, infusion related reactions, nephrotoxicity and hypokalemia. Therefore, based on this review article, L-AmB can be considered an effective and well tolerated empirical therapy in treating IFDs in febrile neutropenic patients with hematological malignancies and patients undergoing transplantation.
Acknowledgements
Writing assistance was provided by Turacoz healthcare solutions (www.turacoz.com). Gilead Sciences provided financial support for this narrative review to Turacoz healthcare solutions for medical writing and submission in a peer-reviewed journal.
Conflict of interest
There is no conflict of interest between the authors.
References
- Bongomin F, Gago S, Oladele RO, Denning DW (2017) Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi (Basel) 3:1-29.
- Li Y, Gao Y, Niu X, Wu Y, Du Y, et al. (2020) A 5-year review of invasive fungal infection at an academic medical center. Front Cell Infect Microbiol 10:553648.
- Rodrigues ML, Nosanchuk JD (2020) Fungal diseases as neglected pathogens: A wake-up call to public health officials. PLoS Negl Trop Dis 14:0007964.
- Hsu LY, Lee DG, Yeh SP, Bhurani D, Khanh BQ, et al. (2015) Epidemiology of invasive fungal diseases among patients with haematological disorders in the Asia-Pacific: a prospective observational study. Clin Microbiol Infect 21:594.
- Chayakulkeeree M, Denning DW (2017) Serious fungal infections in Thailand. Eur J Clin Microbiol Infect Dis 36:931-935.
[Crossref] [Googlescholar][Indexed]
- Dabas Y, Xess I, Pandey M, Ahmed J, Sachdev J, et al. (2021) Epidemiology and antifungal susceptibility patterns of invasive fungal infections (ifis) in india: A prospective observational study. J Fungi 8:33.
[Crossref] [Googlescholar][Indexed]
- Chakrabarti A, Chatterjee SS, MR S (2008) Overview of opportunistic fungal infections in India. Nippon Ishinkin gakkai zasshi 49:165-172.
- Pfaller MA, Diekema DJ (2007) Epidemiology of invasive candidiasis: A persistent public health problem. Clin Microbiol Rev 20:133-163.
- Arendrup MC (2010) Epidemiology of invasive candidiasis. Curr Opin in Crit Care 16:445-452.
[Crossref] [Googlescholar][Indexed]
- Chen K, Wang Q, Pleasants RA, Ge L, Liu W, et al. (2017) Empiric treatment against invasive fungal diseases in febrile neutropenic patients: A systematic review and network meta-analysis. BMC Infect Dis 17:159.
- Evans JD, Morris PJ, Knight SR (2014) Antifungal prophylaxis in liver transplantation: A systematic review and network meta-analysis. Am J Transplant 14:2765-2776.
- Sharifipour F, Rezaeetalab F, Naghibi M (2009) Pulmonary fungal infections in kidney transplant recipients: An 8-year study. Transplant Proc 41:1654-1656.
- Shoham S, Marr KA (2012) Invasive fungal infections in solid organ transplant recipients. Future Microbiol 7:639-55.
[Crossref] [Googlescholar][Indexed]
- Sole A, Morant P, Salavert M, Peman J, Morales P, et al. (2005) Aspergillus infections in lung transplant recipients: Risk factors and outcome. Clin Microbiol Infect 11:359-65.
- Patterson TF, Thompson GR, Denning DW, Fishman JA, Hadley S, et al. (2016) Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis.63: 1-60.
- Pound MW, Townsend ML, Dimondi V, Wilson D, Drew RH (2011) Overview of treatment options for invasive fungal infections. Med Mycol 49:561-80.
- Chu P, Sadullah S (2009) The current role of amphotericin B lipid complex in managing systemic fungal infections. Curr Med Res Opin 25:3011-3020.
- Cavassin FB, Bau-Carneiro JL, Vilas-Boas RR, Queiroz-Telles F (2021) Sixty years of amphotericin b: An overview of the main antifungal agent used to treat invasive fungal infections. Infect Dis Ther 10:115-147.
- Moen MD, Lyseng-Williamson KA, Scott LJ (2009) Liposomal amphotericin B: A review of its use as empirical therapy in febrile neutropenia and in the treatment of invasive fungal infections. Drugs 69:361-392.
- Steimbach LM, Tonin FS, Virtuoso S, Borba HH, Sanches AC, et al. (2017) Efficacy and safety of amphotericin B lipid-based formulations-A systematic review and meta-analysis. Mycoses 60:146-154.
- Adler-Moore JP, Gangneux J-P, Pappas PG (2016) Comparison between liposomal formulations of amphotericin B. Med Mycol 54:223-231.
- Cornely OA, Hoenigl M, Lass-Florl C, Chen SC, Kontoyiannis DP, et al. (2019) Defining breakthrough invasive fungal infection-Position paper of the mycoses study group education and research consortium and the European Confederation of Medical Mycology. Mycoses 62:716-729.
[Crossref] [Googlescholar][Indexed]
- Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, et al. (2007) Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 356:348-59.
- Jenks JD, Cornely OA, Chen SC, Thompson GR, Hoenigl M (2020) Breakthrough invasive fungal infections: Who is at risk?. Mycoses 63:1021-1032.
- Mercier T, Maertens J (2017) Clinical considerations in the early treatment of invasive mould infections and disease J Antimicrob Chemother 72: 29-38.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi