Case Report, Clin Oncol Case Rep Vol: 3 Issue: 5
Sequential Decitabine and Carboplatin Induced Stabilisation of Tumour Burden in a Patient with Immunotherapy-Resistant Metastatic Melanoma
Andre Van der Westhuizen1,2, Naomi Knoblauch1, Moira C Graves2, Richard Levy1,2, Luke Hesson3, Ricardo E Vilain2,4 and Nikola A Bowden2*
1Calvary Mater Hospital, Newcastle, Australia
2School of Medicine and Public Health, University of Newcastle and Hunter Medical Research Institute, Australia
3Kinghorn Centre for Clinical Genomics Core Facility, Garvan Institute of Medical Research, Australia
4Department of Anatomical Pathology, NSW Health Pathology, John Hunter Hospital, Australia
*Corresponding Author: Nikola A Bowden, School of Medicine and Public Health Faculty of Health and Medicine, University of Newcastle and Hunter Medical Research Institute, NSW, Australia, E-mail: Nikola.bowden@newcastle. edu.ac
Received: September 02, 2020 Accepted: October 01, 2020 Published: October 20, 2020
Citation: Westhuizen AVd, Knoblauch N, Graves MC, Levy R, Hesson L, et al. (2020) Sequential Decitabine and Carboplatin Induced Stabilisation of Tumour Burden in a Patient with Immunotherapy-Resistant Metastatic Melanoma. Clin Oncol Case Rep 3:5. DOI: 10.37532/cocr.2020.3(5).147
Abstract
Despite significant progress in the treatment of metastatic melanoma in recent years with targeted and immune checkpoint inhibitor therapy, there is no effective treatment option once resistance to all of these treatments occurs. Here, we report stabilisation of disease in a patient with anti-PD1 immunotherapy resistance after sequential low-dose decitabine and carboplatin. Stable disease allowed for re-challenge with anti-PD1 immunotherapy.
Stable disease with no disease progression was seen in a 47-year old Caucasian male diagnosed with metastatic melanoma with unknown primary who had progressed on previous BRAF/MEK inhibitors (dabrafenib and trametinib) and anti-PD1 immunotherapy (pembrolizumab) after 9 weeks on the novel Phase 2 study (PRIME001: Pilot early phase II study of decitabine and carboplatin in patients with advanced melanoma ACTRN12616000440426). The patient received two 4-week cycles of decitabine 7 mg/ m2 intravenous infusion (IVI)/day for 5 days (D1-D5) followed by Carboplatin AUC 5 IVI on day 8 (D8) and Week 3 and Week 4 no treatment. There were no toxicities >grade 2. Stable disease (RECIST 1.1) was recorded at week 9, the patient re-commenced Pembrolizumab and continued to have stable disease for a further 9 months. Tumour biopsies at baseline and at disease progression 9 months post re-commencing Pembrolizumab both showed high PD-L1 expression and moderate tumour infiltrating lymphocytes, predominately restricted to the tumour periphery. Genomic analysis identified high tumour mutation load and 4 mutations in common to the 2 biopsies. Peripheral immune cell profiles displayed an increase and stabilisation of CD4+ and CD8+ T-cells, particularly with the expression of CXCR6.
Sequential decitabine and carboplatin is a feasible priming regime, with a tolerable safety profile for patients with advanced melanoma who are resistant to prior immunotherapy. The priming treatment limits the burden of disease to allow effective ongoing treatment with immune checkpoint inhibitors.
Keywords: Melanoma; Immunotherapy; Resistance; Anti-PD1; Decitabine; Carboplatin; Priming
Background
Despite significant progress in the treatment of metastatic melanoma in recent years with targeted and immunotherapy, there is no effective treatment option once resistance to all of these treatments occurs. Melanoma patients with disease progression especially after targeted therapy, do not respond as well to immune checkpoint inhibitor immunotherapy because of high disease burden and the development of immunotherapy resistance. To overcome this problem, there needs to be a decrease in burden of disease as well as re-establishing of immune sensitivity. We aimed to test a novel way of sequencing and combining agents to decrease the disease burden and to re-establish sensitivity to immune checkpoint inhibitors.
DNA methylation is an epigenetic DNA modification involving the addition of a methyl group to cytosine by DNA methyltransferases. When present in the promoter region, methylation can block expression of genes. In melanoma, the mutation load is higher in methylated promoter regions [1,2]. DNA methylation can be drastically altered in cancer genomes which led to the development of DNA methyltransferase inhibitors 5-azacytosine (azacitidine) and 2’-deoxy-5-azacytidine (decitabine). Both inhibitors are used in the treatment of myelodysplastic syndrome (MDS), but have proven disappointing in single agent clinincal trials for solid tumours [3]. Low dose decitabine leads to hypomethylation of replicating cells, whereas at high doses it induces toxicity and cell death. Therefore, low-dose decitabine leads to re-instated expression of silenced genes. Preclinical studies have strongly supported decitabine and azacitidine in combination treatment [4,5], as it allows expression of immune response pathways, DNA repair and cancer-specific neoantigens previously silenced by methylation, which is hypothesised to increase response to immunotherapy [6,7]. This has recently led to clinical trials testing the efficacy of azacitidine and immunotherapy in combination [8].
Platinum chemotherapy is used to treat most solid tumours, with germ-cell tumours obtaining cure rates of over 90% [9]. Despite this, it has limited efficacy in melanoma (~10%) and as a consequence is rarely used in treatment [10]. The mechanism of action for cisplatin and carboplatin is insertion of platinum into DNA to form crosslinks. When left unrepaired the damage can lead to C>T mutations, similar to UV. Recognition of the DNA cross-links by the DNA repair process called Nucleotide Excision Repair (NER) triggers apoptosis rather than attempting to repair the damage [11]. It is well known that platinum chemotherapy also elicits a tumour-specific immune response [12,13], but once resistance occurs this response is lost. Therefore, once platinum resistance occurs, reduced levels of NER result in accumulation of DNA damage within the cells, limited apoptosis and a reduction in tumour-specific immune response.
We hypothesised that sequential treatment with decitabine and carboplatin would
• Decrease methylation of the melanoma genome, inducing neoantigen expression and increase the tumour-specific immune response
• Increase the mutation load and
• Prime for re-challenge with anti-PD-1 immunotherapy
In pre-clinical assessment of the novel treatment combination, melanoma cell lines were treated with 0.26 μM decitabine for 72 hours followed by 8 μg/mL carboplatin for 48 hours. The doses used were the lowest clinically relevant doses to induce a response. The majority of melanoma cell lines showed an induction of XPC (range=1.1-3.2 fold increase) and increased apoptotic cell death after the decitabine/ carboplatin sequential treatment compared to carboplatin alone (range=1.6-2.2 fold increase). Only one melanoma cell line showed no increase in XPC or apoptosis after the sequential combination treatment, but did express markers of senescence. All cells lines displayed a significant decrease in cell proliferation after the sequential combination [4]. Here, we report the first case of a clinical response to ‘priming’ melanoma with decitabine and carboplatin followed by re-challenge with pembrolizumab.
Case Presentation
A 47-year old Caucasian male was diagnosed with Stage III metastatic melanoma with unknown primary confirmed by lymphadenopathy in the right posterior triangle of the neck. Right level I-IV neck dissection identified 10/30 lymph nodes were involved and extracapsular extension was present. Radiotherapy was administered to the neck region of 48 Gy in 20 fractions.
13 months later the patient proresssed to Stage IV melanoma when a new mass was detected in the right supraclavicular area and was PET scan FDG avid. A segment 3 liver lesion was also detected, and it was not PET scan FDG avid but was confirmed to be present on abdominal ultrasound. A right neck dissection was performed and 6/11 lymph nodes were involved with metastatic melanoma.
A further 10 months later, a subcutaneous mass was detected in the right gluteal area and a right inguinal lymphadenectomy was performed. One out of 16 lymph nodes were involved with no extracapsular extension. PET scan following surgery demonstrated a FDG avid segment 3 liver metastasis and CT scan at the time demonstrated 2 liver lesions. A left hepatectomy was performed for the liver metastasis and histology confirmed 2 well circumscribed melanoma deposits with clear surgical margins.
A 3-month follow up CT scan demonstrated a 3 cm × 3 cm right external iliac metastasis. Right pelvic lymph node dissection confirmed 6/9 lymph nodes were involved with 4/6 showed complete replacement with metastatic melanoma with extracapsular extension. Radiotherapy was administered to the right pelvic lymph node area of 48 Gy in 20 fractions.
Eight months later, recurrent disease in a single large node in the left external iliac station as well as disease in at least 3 nodes in the right external iliac station were identified by PET. The disease extent was unfavourable to offer surgery again as it was bilateral and he has had several previous surgeries with shorter intervals of recurrence. BRAF V600E was confirmed by molecular testing and targeted therapy with dabrafenib and trametinib was commenced. Ten months after commencement of treatment progressive disease was confirmed with size increase noted in the left and right external lymphadenopathy of more than 20% according to Response Evaluation Criteria in Solid Tumors (RECIST 1.1). The patient commenced on checkpoint inhibitor pembrolizumab (anti-PD1 antibody). Partial response was recorded in the left external iliac nodes and stable disease in the right external iliac nodes with no evidence of new metastasis after 3 months of immunotherapy.
Clinical disease control occurred for nearly 2 years, 30 cycles of Pembrolizumab were received until disease progression was confirmed. The patient developed auto-immune thyroiditis on pembrolizumab and eventually developed hypothyroidism and was started on thyroid hormone replacement therapy. Apart from grade 1 rash that responded to oral and topical steroid therapy, no other significant immune related adverse events were noted to immunotherapy.
A PET scan confirmed increase in right external iliac nodal disease and increase in the left external iliac nodes as well as new disease nodes in the midline mesentery anterior to S1. LDH was normal at the time of disease progression. The patient declined Ipilimumab treatment and consented to enrol into the early phase investigator-initiated clinical trial, PRIME001 pilot early phase II study of decitabine and carboplatin in patients with advanced melanoma (ACTRN12616000440426).
A mesenteric lymph node was removed at the commencement of the trial to confirm metastatic disease. There was a modest tumour lymphocyte infiltrate restricted to the periphery of the tumour and high expression of PD-L1 on the border of the tumour tissue (Figure 1A and 1B).
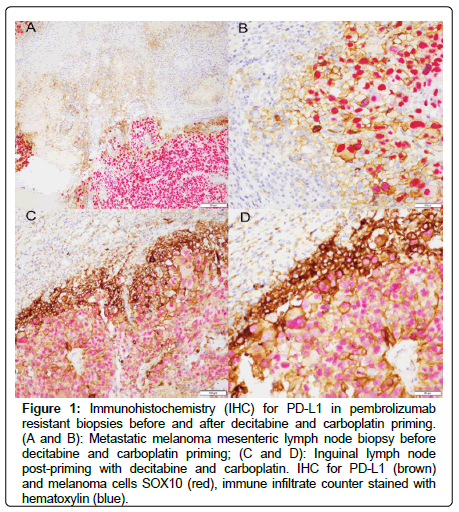
Figure 1: Immunohistochemistry (IHC) for PD-L1 in pembrolizumab resistant biopsies before and after decitabine and carboplatin priming. (A and B): Metastatic melanoma mesenteric lymph node biopsy before decitabine and carboplatin priming; (C and D): Inguinal lymph node post-priming with decitabine and carboplatin. IHC for PD-L1 (brown) and melanoma cells SOX10 (red), immune infiltrate counter stained with hematoxylin (blue).
Two 4-week cycles were administered of decitabine 7 mg/ m2 intravenous infusion (IVI)/day for 5 days (D1-D5) followed by Carboplatin AUC 5 IVI on day 8 (D8) and Week 3 and Week 4 no treatment. This was followed by staging investigations at Week 9 demonstrating stable disease (RECIST 1.1) (Table 1). Due to stabilisation of disease at the completion of the PRIME001 treatment protocol, the patient recommenced treatment with pembrolizumab. LDH was normal at the start of treatment and was elevated to 357 at completion of the 2 cycles of epigenetic and platinum induction therapy. LDH levels started to decrease with every cycle of Pembrolizumab with levels recorded every 3 weeks of 290, 260, 254 and eventually 250 over a 12 week period. PET scan demonstrated central necrosis in the single remaining large right external iliac nodal area with stable measurements on CT scan (RECIST 1.1) and stable overall FDG activity on PET scan (Figure 2). The disease in the left external iliac nodal station remained stable in size and there was a slight decrease in the FDG activity. Brain metastasis were not detected at any stage of disease on serial imaging throughout the course of treatment. In summary, the left and right iliac nodal areas that had previous disease progression on Pembrolizumab, stabilized after the PRIME001 treatment protocol of 2 × 4 week cycles of decitabine followed by carboplatin.
Table 1: RECIST, LDH and PET avidity in response to decitabine, carboplatin and maintenance pembrolizumab.
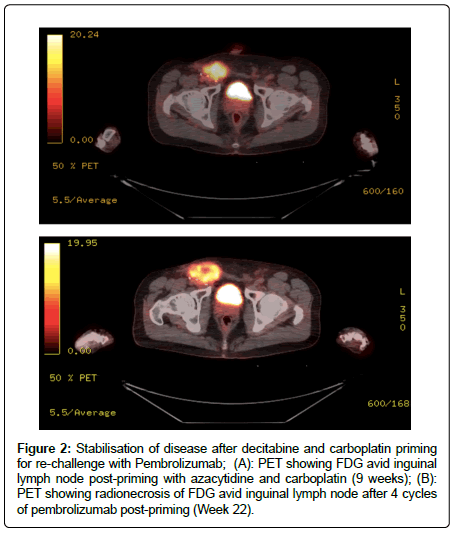
Figure 2: Stabilisation of disease after decitabine and carboplatin priming for re-challenge with Pembrolizumab; (A): PET showing FDG avid inguinal lymph node post-priming with azacytidine and carboplatin (9 weeks); (B): PET showing radionecrosis of FDG avid inguinal lymph node after 4 cycles of pembrolizumab post-priming (Week 22).
The patient remained well during participation on the PRIME001 clinical trial as well as on subsequent Pembrolizumab. He had minimal grade 1 intermittent diarrhoea after starting decitabine treatment. He developed neutropenia grade 2 (ANC 0.9) after the first cycle of decitabine and carboplatin which recovered to ANC 1.2. His ANC was 1.9 when he completed his final cycle of chemotherapy. Normal renal and liver function was confirmed during the 2 months of epigenetic and platinum therapy. He was asymptomatic during the course of treatment and had no fevers or any symptoms of infection during the short period of neutropenia. The patient declined a repeat surgical biopsy after the 2 months of epigenetic and platinum therapy.
PET scans 6 months after re-commencing Pembrolizumab demonstrated stable disease with mild decrease in the SUV values and no evidence of new metastatic disease. Stable disease continued from the first cycle of treatment on trial for 10 months.
10 months after commencing the PRIME001 trial, the patient presented with symptomatic progression with increasing discomfort and increase in size of the right inguinal lymphadenopathy. LDH increased to 274 and slowly increased over the next 3 months to 405. CT and PET scans demonstrated disease progression, confirmed by RECIST 1.1 (>20%) in the right external iliac nodal chain, with increase in FDG activity. Debulking surgery occurred to improve local symptoms and LDH subsequently returned to normal. Similar to the mesenteric lymph node collected after first progression on Pembrolizumab, there was a modest tumour lymphocyte infiltrate restricted to the periphery of the lymph node and increased expression of PD-L1 on the border of the tumour tissue (Figure 1C and 1D).
To assess clonal heterogeneity and tumour mutation load tissue collected from the mesenteric lymph node at baseline and the right inguinal lymph node collected during debulking surgery 12 months after commencing the PRIME001 trial were assessed by 2 independent pathologists, DNA extracted and sequenced using the Oncomine Comprehensive v3 and oncomine tumour mutation load assays on an ion torrent S5 sequencer, mutations were identified using Ion Reporter Software. Genomic analysis was performed at the Kinghorn Centre for Clinical Genomics Core Facility, Garvan Institute of Medical Research, Sydney, Australia. Four genetic alterations were identified in common to both lymph nodes and the tumour mutation load was high at both timepoints (Table 2). The mesenteric lymph node contained an additional NF1 mutation and amplification of IGF1R which was indicative of this area of disease occurring during the first disease progression on Pembrolizumab. Conversely, the inguinal nodal disease was present earlier in disease, confirming the NF1 mutation and IGF1R amplification were acquired during the course of disease progression. The role of the mutations identified in anti-PD1 immunotherapy resistance is largely unknown and requires further investigations.
1799 T>A (Val600Glu)PresentPresentHRAS
37G>C
(Gly13Arg)PresentPresentCDKN2A
39_40insT (Asp14Ter)PresentPresentPTCH1
1777C>T (Pro593Ser)PresentPresentTERT
1-46C>TPresentPresentNF1
2087G>A
(Trp696Ter)PresentAbsentIGF1R
Amplification (10.45 copies)PresentAbsentTumour Mutation Load (mutations/Mb)27.94 (high)25.12 (high)
Table 2: Genomic analysis of pembrolizumab-resistant biopsies before and after decitabine and carboplatin.
To assess peripheral immune response to Pembrolizumab before and after priming with decitabine and carboplatin, serial trough blood collections occurred for cycles 26 to 30 during the initial treatment with Pembrolizumab, and again for cycles 1 to 6 and cycle 10 of Pembrolizumab re-challenge. Analysis of changes in peripheral T-cell subsets during initial Pembrolizumab treatment leading up to confirmed progressive disease at cycle 30 showed wide variation and a sharp decrease in both CD4+ and CD8+ T-cells and CXCR6+ subsets at progression (Figure 3). Conversely, for the first 10 cycles of Pembrolizumab re-challenge CD4+, CD8+ and both CD4+CXCR6+ and CD8+CXCR6+ subsets increased and were stably maintained (Figure 3).
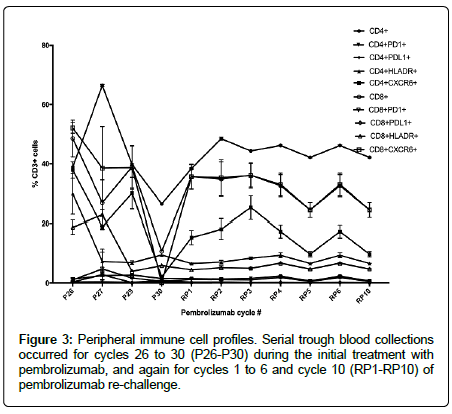
Figure 3: Peripheral immune cell profiles. Serial trough blood collections occurred for cycles 26 to 30 (P26-P30) during the initial treatment with pembrolizumab, and again for cycles 1 to 6 and cycle 10 (RP1-RP10) of pembrolizumab re-challenge.
After de-bulking surgery of the right inguinal node area, the patient continued on Pembrolizumab and had stable disease on repeat PET scans with no significant size increase in lymph nodes and no evidence of disease progression in other nodal areas or visceral sites for a further 12 months. At 12 months, PET scans showed increase in FDG activity in a peripheral rim around the site where the debulking surgery was performed. PD was also confirmed at this time by RECIST 1.1. No areas of new disease were detected by CT or PET, the recurrent disease remained contained to the inguinal node area.
Discussion and Conclusion
To our knowledge this is the first reported case of clinical benefit from sequential decitabine and carboplatin for metastatic melanoma. A phase Ib-IIa study in 30 platinum-resistant ovarian cancer patients used 75 mg/m2 azacitidine for 5 days followed by AUC4 or AUC5 carboplatin on Day 2 of a 28 day cycle to achieve an Overall Response Rate (ORR) of 13.7% and Clinical Benefit Rate (CBR) of 48.3% [14]. Similarly, Fang and colleagues reported a ORR of 35% to sequential decitabine and carboplatin in a Phase 1 dose escalation study of high-grade serous ovarian cancer [15]. The use of decitabine and carboplatin in combination to prime for increased response to immunotherapy has not been tested before this case report.
Sequential decitabine and carboplatin is a feasible treatment regime, with a tolerable safety profile for patients with advanced melanoma who are resistant to prior immunotherapy. The sequential combination treatment limits the burden of disease and possibly increases tumour immunogenicity to allow effective ongoing treatment with immune checkpoint inhibitors. The role for priming with demethylating and platinum chemotherapy is being further investigated in a larger follow-up early phase 2 investigator-initiated clinical trial (ACTRN12618000053224).
In summary, this is the first report that sequential decitabine and carboplatin has potential as an effective, readily available and costeffective combination treatment for melanoma and can be used to stabilise disease for re-challenge with immunotherapy.
Declarations
Ethics approval and consent to participate
Ethics approval for Early Phase II study of Decitabine and Carboplatin in patients with advanced melanoma: A new combination treatment protocol for metastatic melanoma was obtained from Hunter New England Health Human Ethics Committee reference number 15/12/16/3.08. Patients in this study provided fully informed signed consent.
Consent for publication
The patient and study members have provided consent for publication.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Competing interests
The authors declare no competing interests.
Funding
MG and NAB were supported by a Cancer Institute NSW Career Development Fellowship (14CDF117); NAB and the study supported by Vanessa McGuigan HMRI Fellowship and HMRI project grant (supported by Mrs Valerie Ryan). MG and the study were supported by a Ramaciotti Foundation Health Investment Grant and the Maitland Cancer Appeal, NSW Australia. Patient blood samples were collected as part of the Hunter Cancer Biobank Sequential Blood Collection Project funded by the Cancer Institute NSW Hunter Cancer Research Alliance and NSW Pathology.
Authors' contributions
AvdW designed the study, identified, consented and treated the patient as principal investigator and wrote the manuscript. MG collected and analysed the peripheral immune cell data, RL collected tissue samples, LH performed and analysed DNA sequencing, REV performed IHC and reported PDL-1 levels and wrote the manuscript and NAB designed the study, analysed data and wrote the manuscript.
References
- Perera D, Poulos RC, Shah A, Beck D, Pimanda JE, et al. (2016) Differential DNA repair underlies mutation hotspots at active promoters in cancer genomes. Nature 532: 259-263.
- Poulos RC, Olivier J, Wong JWH (2017) CpG methylation accounts for genome-wide C>T mutation variation and cancer drive formation across cancer types. BioRxiv 106872.
- Matei D, Fang F, Shen C, Schilder J, Arnold A, et al. (2012) Epigenetic resensitization to platinum in ovarian cancer. Cancer Res 72: 2197-2205.
- Budden T, van der Westhuizen A, Bowden NA (2018) Sequential decitabine and carboplatin treatment increases the DNA repair protein XPC, increases apoptosis and decreases proliferation in melanoma. BMC Cancer 18: 100.
- Benson EA, Skaar TC, Liu Y, Nephew KP, Matei D (2015) Carboplatin with decitabine therapy, in recurrent platinum resistant ovarian cancer, alters circulating mirnas concentrations: A pilot study. PLoS One 10: e0141279.
- Fang F, Zuo Q, Pilrose J, Wang Y, Shen C, et al. (2014) Decitabine reactivated pathways in platinum resistant ovarian cancer. Oncotarget 5: 3579-3589.
- Li X, Mei Q, Nie J, Fu X, Han W (2015) Decitabine: A promising epi-immunotherapeutic agent in solid tumors. Expert Rev Clin Immunol 11: 363-375.
- Keung E, Burton EA, Amaria RN, Glitza IS, Patel SP, et al. (2017) A phase II study of oral azacitidine (CC-486) in combination with pembrolizumab (PEMBRO) in patients with metastatic melanoma (MM). J Clin Oncol 37: s15.
- Williams SD, Birch R, Einhorn LH, Irwin L, Greco FA, et al. (1987) Treatment of disseminated germ-cell tumors with cisplatin, bleomycin, and either vinblastine or etoposide. N Engl J Med 316: 1435-1440.
- Glover D, Glick JH, Weiler C, Fox K, Guerry D (1987) WR-2721 and high-dose cisplatin: An active combination in the treatment of metastatic melanoma. J Clin Oncol 5: 574-578.
- Siddik ZH (2003) Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene 22: 7265-7279.
- Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, et al. (2007) Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 13: 1050-1059.
- Bracci L, Schiavoni G, Sistigu A, Belardelli F (2014) Immune-based mechanisms of cytotoxic chemotherapy: Implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ 21: 15-25.
- Fu S, Hu W, Iyer R, Kavanagh JJ, Coleman RL, et al. (2011) Phase 1b-2a study to reverse platinum resistance through use of a hypomethylating agent, azacitidine, in patients with platinum-resistant or platinum-refractory epithelial ovarian cancer. Cancer 117: 1661-1669.
- Fang F, Balch C, Schilder J, Breen T, Zhang S, et al. (2010) A phase 1 and pharmacodynamic study of decitabine in combination with carboplatin in patients with recurrent, platinum-resistant, epithelial ovarian cancer. Cancer 116: 4043-4053.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 