Case Report, Clin Oncol Case Rep Vol: 6 Issue: 2
Severe photosensitivity resulting from an interaction between encorafenib and nirmatrelvir/ritonavir in a patient with advanced melanoma: A Case Report
Emilia Janiczek, Kevin B. Kim*
Department of Medical Oncology, California Pacific Medical Center Research Institute, San Francisco, CA, United States
*Corresponding Author: Kevin B. Kim
Department of Medical Oncology
California Pacific Medical Center Research Institute, 2333 Buchanan St. San Francisco, CA, United States
E-mail: kimkb@sutterhealth.org
Received: February 09, 2023; Manuscript No: COCR-23-89168;
Editor Assigned: February 11, 2023; PreQC Id: COCR-23-89168 (PQ);
Reviewed: February 20, 2023; QC No: COCR-23-89168 (Q);
Revised: February 22, 2023; Manuscript No: COCR-23-89168 (R);
Published: February 25, 2023; DOI: 10.4172/cocr.6(2).277
Citation: Janiczek E, Kim KB (2023) Severe Photosensitivity Resulting from an Interaction between Encorafenib and Nirmatrelvir/Ritonavir in a Patient with Advanced Melanoma: A Case Report. Clin Oncol Case Rep 6:2
Abstract
Nirmatrelvir/ritonavir (PAXLOVIDTM, Pfizer) is considered the first line treatment for mild to moderate COVID-19 infection. Ritonavir, a potent inhibitor of the cytochrome P450 3A4 (CYP3A4) isoenzyme, is susceptible to considerable drug-drug interactions with drugs that are metabolized by the CYP3A4 enzyme. Here, we report a case of severe photosensitivity in a patient taking encorafenib for metastatic melanoma after initiating nirmatrelvir/ritonavir treatment for COVID-19 infection. This case serves as a reminder that caution is required by physicians when prescribing nirmatrelvir/ritonavir to patients taking encorafenib or other molecular-targeted BRAF inhibitors for the treatment of V600 BRAF-mutant cancer.
Keywords: Nirmatrelvir/ritonavir (Paxlovid); COVID-19 infection; Encorafenib; Melanoma
Introduction
The recent COVID-19 pandemic had a major impact on the health care system throughout the world. In addition to the mortality and morbidity associated with COVID-19 infection, treatments for COVID-19 can complicate the management of pre-existing medical conditions in many patients. In December 2021, the Food and Drug Administration issued an Emergency Use Authorization for nirmatrelvir/ritonavir (PAXLOVIDTM, Pfizer), now considered the first line treatment for mild to moderate COVID-19 infection [1-3]. Ritonavir is a potent inhibitor of the cytochrome P450 3A4 (CYP3A4) isoenzyme and can have considerable drug-drug interactions with many drugs that are metabolized by the CYP3A4 enzyme. Here, we report a severe adverse event in a patient taking encorafenib for metastatic melanoma after initiating nirmatrelvir/ritonavir treatment for COVID-19 infection.
Case Presentation
The patient was a 62-year-old female diagnosed in 2016 with a V600E BRAF-mutant melanoma, metastatic to the right chest wall, liver, ribs, lungs, spleen, and brain with an extensive history of multiple primary melanomas. After her disease progressed following three cycles of a combination of nivolumab and ipilimumab, she started a combination of dabrafenib and trametinib in December 2016. Due to the development of intolerable fever, chills, and fatigue, the treatment regimen was changed to a combination of vemurafenib and cobimetinib in January 2017. Her disease responded greatly to the treatment. However, she suffered severe fatigue, dermatologic reaction (panniculitis), and arthralgia from the treatment. In August 2018, she started a combination of encorafenib and binimetinib. Her disease had been well controlled except for an isolated brain metastasis, for which she underwent a stereotactic radiosurgery in February 2019, and a chest wall metastasis, for which she received palliative radiation therapy in December 2021. She continued the combination of encorafenib and binimetinib, noting only mild fatigue and occasional headaches.
In May 2022, she contracted COVID-19 with symptoms of sore throat, runny nose, and worsening fatigue, overall mild in severity. She began a course of nirmatrelvir/ritonavir for treatment of COVID-19. On the second day of treatment, she became extremely fatigued, lethargic, and incoherent while tending her garden. She recalled leaning against a fence but could not remember anything else that had occurred. Her husband found her about one hour later with severe sun burn on the posterior neck and anterior aspects of the bilateral thighs. The next day, she developed blisters and later open wounds (Figure 1, Figure 2a and b). She was taken to the emergency room for stroke evaluation. The brain MRI showed no significant findings to explain the mental status change. She discontinued the nirmatrelvir/ritonavir after three days of treatment, after which the symptoms resolved. She continued the encorafenib and binimetinib without a dose change and has tolerated well since then.
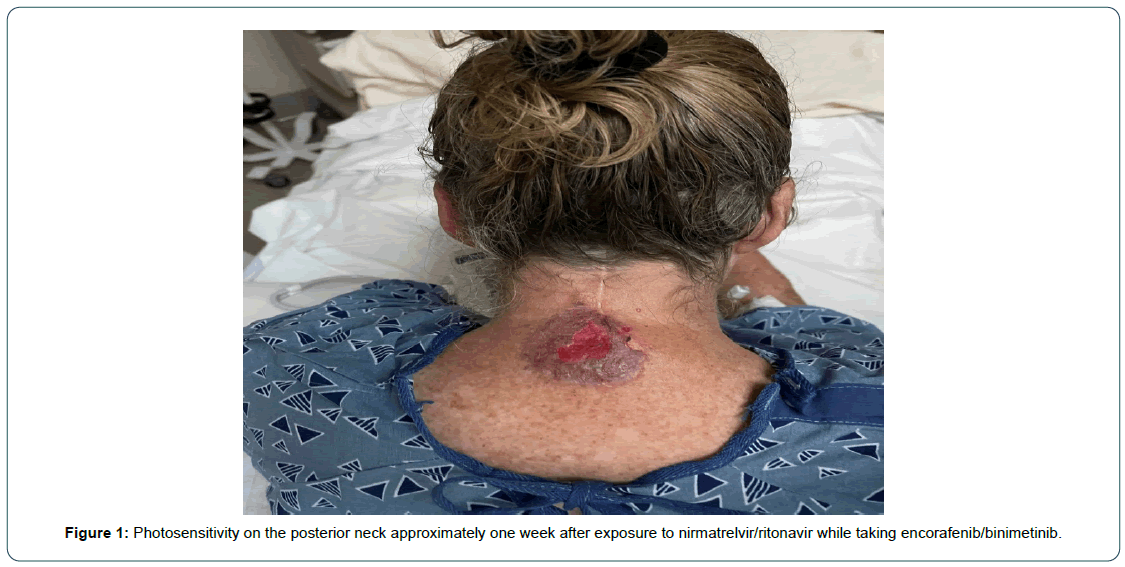
Figure 1: Photosensitivity on the posterior neck approximately one week after exposure to nirmatrelvir/ritonavir while taking encorafenib/binimetinib.
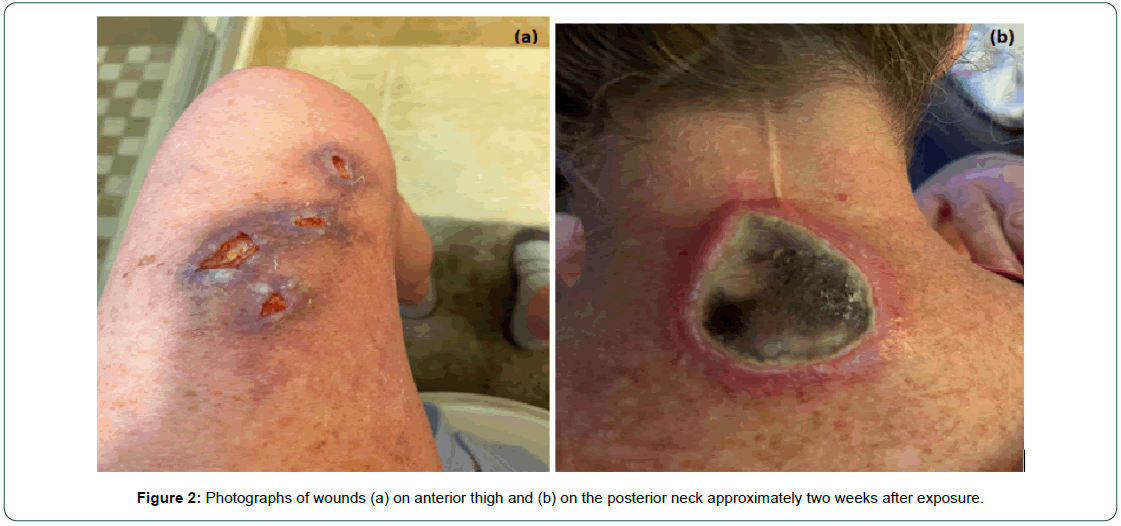
Figure 2: Photographs of wounds (a) on anterior thigh and (b) on the posterior neck approximately two weeks after exposure.
Discussion
An increase in treatment options for COVID-19 brings novel challenges in managing drug-drug interactions for those most at risk of severe disease. Currently, nirmatrelvir/ritonavir is the recommended treatment for mild to moderate COVID-19 infection [1-3]. In a randomized, controlled trial, nirmatrelvir/ritonavir reduced the risk of hospitalization in symptomatic, unvaccinated COVID-19 cases when treatment was given within 5 days of symptom onset (relative risk reduction 89.1%) [4]. The treatment is therefore initiated during the early phase of infection to prevent progression to the severe, inflammatory phase.
Nirmatrelvir is an antiviral agent which inhibits 3-chymotrypsinlike cysteine protease enzyme (Mpro), preventing polyprotein cleavage of proteins required for genome replication of the virus [5]. As a single agent, nirmatrelvir is limited by its short half-life due to extensive metabolism through the cytochrome P450 3A4 isoenzyme (CYP3A4) pathway [6]. Therefore, it is combined with ritonavir, a potent inhibitor of CYP3A4 which pharmacokinetically enhances nirmatrelvir by extending the half-life of nirmatrelvir to achieve and maintain target therapeutic concentrations [4].
CYP3A4 is a key enzyme in the metabolism of many drugs, making drug-drug interactions with ritonavir a major source of concern [7-9]. Maximum inhibition occurs within 48 hours of treatment onset [10], with 70-90% of inhibition resolving within 2-3 days of discontinuation [11]. Ritonavir has long been utilized in the treatment of HIV infection and its contribution to drug-drug interactions in that setting is well documented. Numerous studies of HIV protease inhibitors provide evidence for adverse drug reactions associated with ritonavir. For example, when rosuvastatin, a medication used to treat high cholesterol, was taken in combination with the HIV treatment lopinavir/ritonavir, there was a 2.1- fold increase in rosuvastatin AUC and 4.7- fold increase of Cmax [12]. Thus, treatment with ritonavir may increase exposure to other substances which rely heavily on CYP3A4 for metabolism and elimination.
There is emerging data which suggests nirmatrelvir/ritonavir has a similar effect in populations at higher risk of drug-drug interactions due to polypharmacy. For example, kidney transplant patients are commonly prescribed tacrolimus, an immunosuppressant medication which is metabolized by the CYP3A4 enzyme system [13]. Several case reports detail the development of supratherapeutic tacrolimus levels in kidney transplant patients prescribed nirmatrelvir/ritonavir [14-16].
Cancer patients are another population at risk of drug-drug interactions. A majority of kinase inhibitors, a widely used class of anticancer agents, are metabolized by the CYP3A4 system [17- 19]. Given their narrow therapeutic index, significant toxicities are anticipated with concurrent administration of nirmatrelvir/ritonavir [20,21].
In our case, the patient had tolerated the combination of encorafenib and binimetinib without significant toxicity for nearly four years, despite her history of significant skin toxicity (panniculitis) with another BRAF inhibitor, vemurafenib. Within two days of starting nirmatrelvir/ritonavir treatment, she experienced severe sunburn. Photosensitivity is known to be associated with encorafenib treatment. In a Phase 1 expansion trial of encorafenib alone, photosensitivity was reported in 2.9% of patients in the melanoma cohort [22], and in the pooled analysis of the combination of encorafenib and binimetinib, photosensitivity was observed in 4.0% of 274 patients [23]. The time course of the adverse event strongly suggests toxicity of encorafenib from the drug-drug interaction between the BRAF inhibitor and nirmatrelvir/ritonavir, as encorafenib is primarily metabolized by the cytochrome P450 3A4 enzyme system [23, 24]. Though coadministration of nirmatrelvir/ritonavir with encorafenib has not been studied, strong or moderate CYP3A4 inhibitors increase encorafenib plasma concentrations [23]. It is therefore highly plausible that nirmatrelvir/ritonavir induces severe adverse effects in patients taking encorafenib. Unfortunately, blood samples were not obtained from our patient to measure the serum concentration of encorafenib at the time of the adverse events; therefore, our conjecture remains speculative.
Several countries proposed general guidelines on drugdrug interactions with nirmatrelvir/ritonavir based on the drug information and expert opinions of clinical pharmacologists [18, 20, 25-27]. In the case of encorafenib, modification or discontinuation is recommended for patients treated with nirmatrelvir/ritonavir. Our case is the first to document the exacerbation of adverse events due to drug-drug interaction between nirmatrelvir/ritonavir and encorafenib and serves as a reminder that caution is required when nirmatrelvir/ritonavir is used as a COVID-19 treatment in patients who are treated with encorafenib.
Conclusion
As observed in our patient case, the drug-drug interaction between encorafenib and nirmatrelvir/ritonavir can be significant, resulting in severe adverse events such as photosensitivity in patients who are treated with molecular-targeted BRAF inhibitors for V600 BRAF-mutant cancer.
Consent for Publication
The patient’s informed consent was obtained for the publication of the case details and images.
Acknowledgments
No funding support was provided for this manuscript. Editorial assistance to the authors was provided by Ms. Lesley C. Scott-Skye at California Pacific Medical Center Research Institute.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted.
References
- PAXLOVIDTM (nirmatrelvir tablets; ritonavir tablets) For Patients
- Saravolatz LD, Depcinski S, Sharma M (2023) Molnupiravir and nirmatrelvir-ritonavir: Oral coronavirus disease 2019 antiviral drugs. Clin Infect Dis : An Off Pub Infect Dis Soc Am 76: 165-171. [Google Scholar] [Cross Ref]
- Molnupiravir and nirmatrelvir-ritonavir: Oral coronavirus disease 2019 antiviral drugs
- Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, et al. (2022) Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. Eng J Med 386: 1397-1408. [Google Scholar] [Cross Ref]
- Hilgenfeld R (2014) From SARS to MERS: Crystallographic studies on coronaviral proteases enable antiviral drug design. FEBS J 281: 4085-4096. [Google Scholar] [Cross Ref]
- Owen DR, Allerton CMN, Anderson AS, Aschenbrenner L, Avery M, et al. (2021) An oral SARS-CoV-2 mpro inhibitor clinical candidate for the treatment of COVID-19. Scien 374: 1586-1593. [Google Scholar] [Cross Ref]
- Watkins PB, Wrighton SA, Schuetz EG, Molowa DA, Guzelian PS (1987) Identification of glucocorticoid-inducible cytochromes P-450 in the intestinal mucosa of rats and man. J Clin Invest 80: 1029-1036. [Google Scholar] [Cross Ref]
- Kolars JC, Schmiedlin-Ren P, Schuetz JD, Fang C, Watkins PB (1992) Identification of rifampin-inducible P450IIIA4 (CYP3A4) in human small bowel enterocytes. J Clin Invest 90: 1871-1878. [Google Scholar] [Cross Ref]
- Sevrioukova IF, Poulos TL (2010) Structure and mechanism of the complex between cytochrome P4503A4 and ritonavir. Proceed Nat Acad Scien United States Am 107: 18422-18427. [Google Scholar] [Cross Ref]
- Katzenmaier S, Markert C, Riedel KD, Burhenne J, Haefeli WE, et al. (2011) Determining the time course of CYP3A inhibition by potent reversible and irreversible CYP3A inhibitors using a limited sampling strategy. Clin Pharmacol Therap 90: 666-673. [Google Scholar] [Cross Ref]
- Stader F, Khoo S, Stoeckle M, Back D, Hirsch HH, et al. (2020) Stopping lopinavir/ritonavir in COVID-19 patients: Duration of the drug interacting effect. J Antimicrob Chemoth 75: 3084-3086. [Google Scholar] [Cross Ref]
- Kiser JJ, Gerber JG, Predhomme JA, Wolfe P, Flynn DM, et al. (2008) Drug/drug interaction between lopinavir/ritonavir and rosuvastatin in healthy volunteers. J Acquired Immune Def Synd 47: 570-578. [Google Scholar] [Cross Ref]
- Dai Y, Hebert MF, Isoherranen N, Davis CL, Marsh C, et al. (2006) Effect of CYP3A5 polymorphism on tacrolimus metabolic clearance in vitro. Drug Metab Disposition 34: 836-847. [Google Scholar] [Cross Ref]
- Prikis M, Cameron A (2022) Paxlovid (nirmatelvir/ritonavir) and tacrolimus drug-drug interaction in a kidney transplant patient with SARS-2-CoV infection: A case report. Transplant Proceed 54: 1557-1560. [Google Scholar] [Cross Ref]
- Berar Yanay N, Bogner I, Saker K, Tannous E (2022) Paxlovid-tacrolimus drug-drug interaction in a 23-year-old female kidney transplant patient with COVID-19. Clin Drug Investig 42: 693-695. [Google Scholar] [Cross Ref]
- Young C, Papiro T, Greenberg JH (2022) Elevated tacrolimus levels after treatment with nirmatrelvir/ritonavir (Paxlovid) for COVID-19 infection in a child with a kidney transplant. Pediat Nephrol 1-2. [Google Scholar] [Cross Ref]
- Roskoski RJ (2019) Cyclin-dependent protein serine/threonine kinase inhibitors as anticancer drugs. Pharmacol Res 139: 471-488. [Google Scholar] [Cross Ref]
- Hakkola J, Hukkanen J, Turpeinen M, Pelkonen O (2020) Inhibition and induction of CYP enzymes in humans: An update. Arch Toxicol 94: 3671-3722. [Google Scholar] [Cross Ref]
- Arora A. Scholar EM (2005) Role of tyrosine kinase inhibitors in cancer therapy. Perspectives Pharmacol 315: 971-979. [Google Scholar] [Cross Ref]
- Mikus G, Foerster KI, Terstegen T, Vogt C, Said A, et al. (2022) Oral drugs against COVID-19: management of drug interactions with the use of nirmatrelvir/ritonavir. Deutsches Arzteblatt Internat 119: 263-269. [Google Scholar] [Cross Ref]
- [Google Scholar]
- Delord JP, Robert C, Nyakas M, McArthur GA, Kudchakar R, et al. (2017) Phase I dose-escalation and-expansion study of the BRAF inhibitor encorafenib (LGX818) in metastatic BRAF-mutant melanoma phase I study: Encorafenib in BRAF-mutant melanoma. Clin Can Res 23: 5339-5348. [Google Scholar] [Cross Ref]
- Braftovi (2022) Product information. European Medicines Agency.
- Shirley M (2018) Encorafenib and binimetinib: First global approvals. Drugs 78: 1277-1284. [Google Scholar] [Cross Ref]
- Azanza JR, Mensa J, Del-Castillo JG, Rufo ML, Molero JM, et al. (2022) Interactions listed in the Paxlovid fact sheet, classified according to risks, pharmacological groups, and consequences. Revista Espanola de Quimioterapia 35: 357-361. [Google Scholar] [Cross Ref]
- Lemaitre F, Gregoire M, Monchaud C, Bouchet S, Saint-Salvi B, et al. (2022) Management of drug-drug interactions with nirmatrelvir/ritonavir in patients treated for Covid-19: Guidelines from the French Society of Pharmacology and Therapeutics (SFPT). Therap 77: 509-521. [Google Scholar] [Cross Ref]
- Marzolini C, Kuritzkes DR, Marra F, Boyle A, Gibbons S, et al. (2022) Recommendations for the management of drug–drug interactions between the COVID‐19 antiviral nirmatrelvir/ritonavir (Paxlovid) and comedications. Clin Pharmacol Therap 112: 1191-1200. [Google Scholar] [Cross Ref]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi