Case Report, Clin Oncol Case Rep Vol: 8 Issue: 7
Solitary Fibrous Tumor of the Pelvis: A Case Report and Literature Review
Pelegrina Manzano Amalia*1,2, Monllau Font Vanesa1, Puértolas Pérez Alejandro2, Domingo Amela Anna3, Merlo Mas Josep1, Rodríguez Blanco Manuel1
1Department of Surgery, ServiDigest Clinic, Hospital del Pilar, 08006 Barcelona, Spain
2Department of Medicine and Life Sciences, Universitat Pompeu Fabra, 08003 Barcelona, Spain
3Pathological Anatomy Laboratory, Atrys Health, 08025 Barcelona, Spain
- *Corresponding Author:
- Pelegrina Manzano Amalia
Department of Surgery, ServiDigest Clinic, Hospital del Pilar, 08006 Barcelona, Spain
E-mail: apelegrina@hmar.cat
Received: April 18, 2025; Manuscript No: COCR-25-164632; Editor Assigned: May 8, 2025; PreQC: COCR-25-164632(PQ); Reviewed: May 28, 2025; QC No: COCR-25-164632(Q); Revised: June 10, 2025; Manuscript No: COCR-25- 164632(R); Published: July 30, 2025, DOI: 10.4172/cocr.8(7).423
Citation: Amalia PM, Vanesa MF, Alejandro PP, Anna DA, Josep MM, et al. (2025) Solitary Fibrous Tumor of the Pelvis a Case Report and Literature Review. Clin Oncol Case Rep 8(7):423
Abstract
Solitary fibrous tumor (SFT) is a rare mesenchymal neoplasm, occasionally arising in the retroperitoneal or pelvic cavities. Periprostatic SFTs are especially uncommon and can mimic primary prostatic neoplasms. While often benign, they may behave unpredictably. We report a case of a 76-year-old male with a large periprostatic SFT managed surgically, and we review the literature. Histopathological and immunohistochemical features confirmed the diagnosis, and risk stratification classified the tumor as intermediate risk. This case underscores the importance of accurate diagnosis, complete surgical resection, and long-term follow-up due to potential recurrence.
Keywords
Solitary Fibrous Tumor; Periprostatic; Pelvic Tumor; Mesenchymal Neoplasm; Surgical Resection; Immunohistochemistry; Risk Stratification
Introduction
Solitary fibrous tumors (SFTs) are rare spindle-cell neoplasms of mesenchymal origin, originally described by Klemperer and Rabin in 1931 [1]. Initially associated with the pleura, they are now recognized in extrapleural sites including the prostate [2]. Their incidence is <1/million [3], and origin from submesothelial mesenchymal cells may explain their diverse localization [4]. Retroperitoneal and pelvic SFTs are extremely rare, with fewer than 40 prostatic cases described [5,6]. SFTs exhibit spindle cells within a collagenous stroma and a staghorn vascular pattern. Immunohistochemistry (IHC) typically shows CD34, STAT6, Bcl-2, and CD99 positivity [7-9]. Recent genetic studies have identified a chromosomal rearrangement involving NAB2-STAT6 fusion on chromosome 12q13 [7-9], which appears to be a defining molecular feature of SFTs.
Prostatic SFTs can be misdiagnosed due to their similarity to other spindle-cell tumors [7-10]. Most follow a benign course, but 20% show malignant behavior [11]. Katsuno et al. estimated that ~6% of SFTs are pelvic in origin [5,12,13]. No standardized management exists; surgical resection is the mainstay [14-21].
Case Presentation
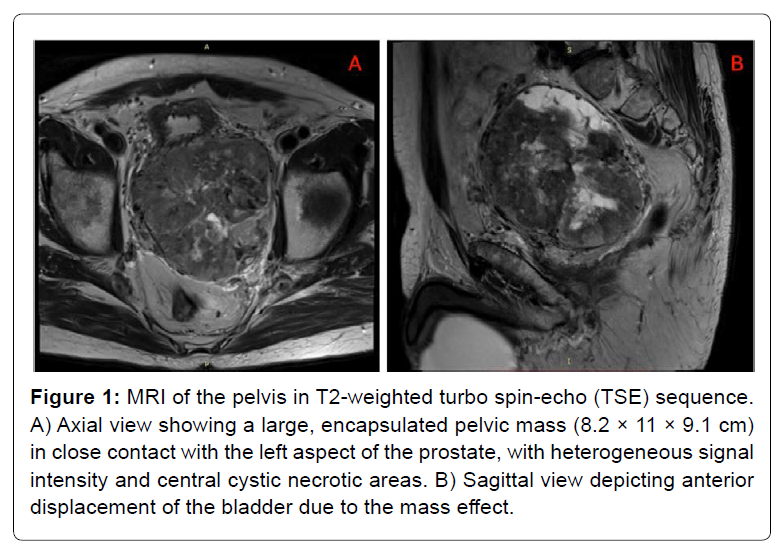
A 76-year-old male with no relevant history presented with an incidental pelvic mass on ultrasound. PSA was normal. CT and MRI revealed a large encapsulated pelvic tumor (100 × 90× 83 mm) with heterogeneous features and close contact with the prostate and bladder. The lesion caused displacement of both structures, raising suspicion of sarcoma. No lymphadenopathy was noted [Figure 1].
Figure 1: MRI of the pelvis in T2-weighted turbo spin-echo (TSE) sequence. A) Axial view showing a large, encapsulated pelvic mass (8.2 × 11 × 9.1 cm) in close contact with the left aspect of the prostate, with heterogeneous signal intensity and central cystic necrotic areas. B) Sagittal view depicting anterior displacement of the bladder due to the mass effect.
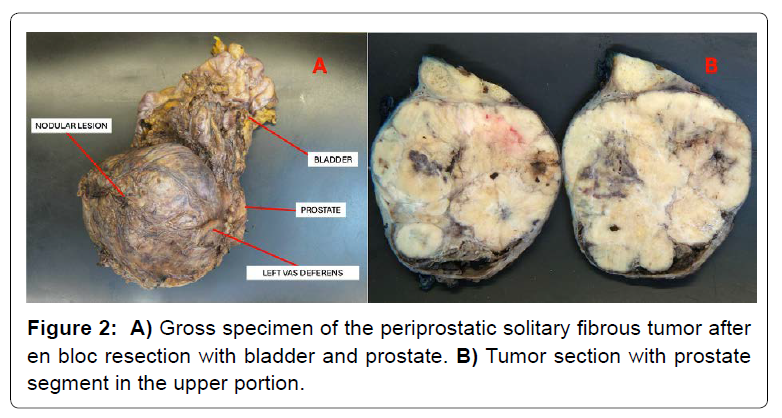
Surgical exploration revealed extensive tumor adherence to pelvic structures. En bloc resection of prostate, seminal vesicles and bladder was required [Figure 2], followed by urinary diversion (Bricker ileal conduit) and protective ileostomy. Intraoperative rectal perforation was repaired. The postoperative course was stable.
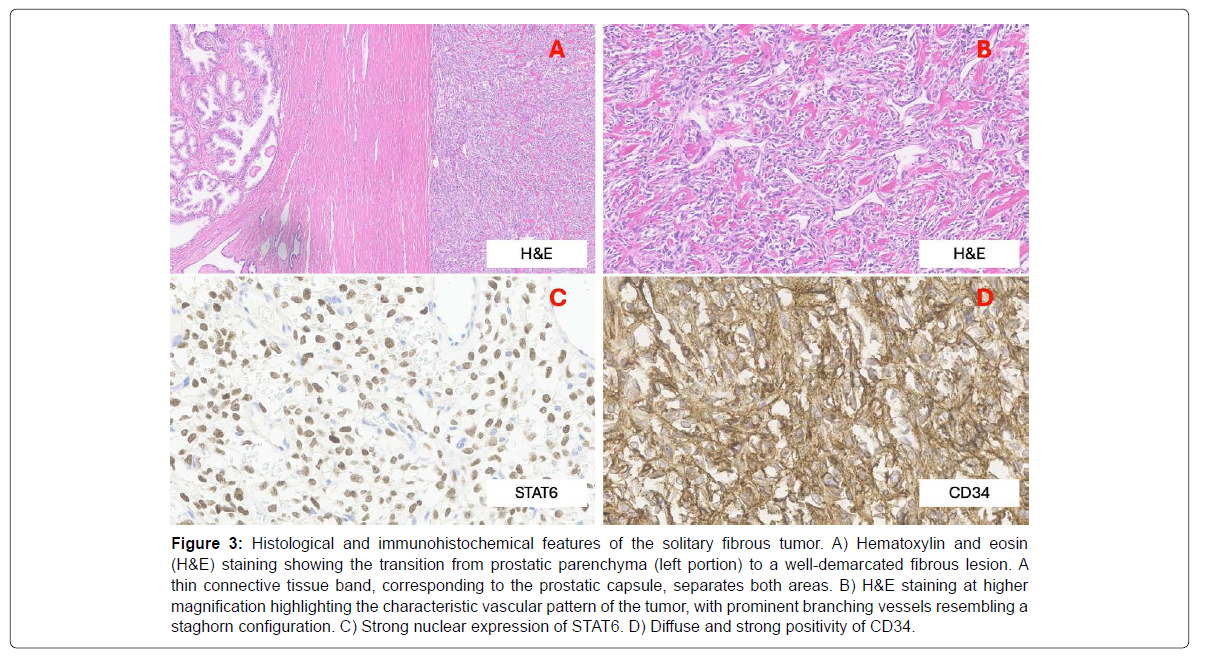
Histopathology showed a well-circumscribed, spindle-cell tumor with low mitotic index, no necrosis or atypia. IHC was positive for CD34 and STAT6 [Figure 3]. Demicco et al.'s model classified it as intermediate-risk [22]. No evidence of disease recurrence was observed at six months; follow-up is ongoing.
Figure 3: Histological and immunohistochemical features of the solitary fibrous tumor. A) Hematoxylin and eosin (H&E) staining showing the transition from prostatic parenchyma (left portion) to a well-demarcated fibrous lesion. A thin connective tissue band, corresponding to the prostatic capsule, separates both areas. B) H&E staining at higher magnification highlighting the characteristic vascular pattern of the tumor, with prominent branching vessels resembling a staghorn configuration. C) Strong nuclear expression of STAT6. D) Diffuse and strong positivity of CD34.
Discussion
SFTs are often indolent but can exhibit aggressive features. Pelvic SFTs represent ~16% of extrathoracic cases [23,24]. Preoperative imaging helps define anatomy, but diagnosis relies on pathology and IHC [8,9,25]. Molecular confirmation via NAB2-STAT6 fusion enhances diagnostic accuracy [9].
Our patient’s tumor lacked malignant features but met criteria for intermediate-risk due to size and age [22]. Similar cases report variability in presentation and outcome [26]. Complete surgical resection (R0/R1) is key, and organ sacrifice should be reserved for invasion [14-27]. In our case, resection of prostate, seminal vesicles and bladder was necessary. The role of adjuvant therapy remains unclear but may be considered in aggressive forms.
For unresectable/metastatic SFTs, systemic therapy includes tyrosine kinase inhibitors like pazopanib, as supported by the GEIS-32 trial [28-32]. ISG15 may serve as a prognostic biomarker [25]. Dedifferentiated SFTs require anthracycline-based chemotherapy or trabectedin with radiotherapy [8,33].
Conclusion
This case adds to the limited literature on per prostatic SFTs. Accurate histological and molecular diagnosis, individualized surgical planning, and long-term follow-up are essential. Future research should refine prognostic tools and explore new therapeutic strategies, particularly for aggressive SFT subtypes [Table 1].
| Author (year) | Tumor Location | Age (years) | Presentation | PSA (ng/mL) | Tumor size (cm) | Biopsy | Treatment | Margins | Necrosis | Mitosis/10 HPF | Cellularity | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Current report -2025 | Periprostatic | 76 (male) | Incidental | Normal | 10 × 9 × 8.3 | No | CP | R0 | No | 3 | Intermediate | NR 6 months |
| Kc et al. (2024)34 | Pelvic | 46 (female) | Persistent lower abdominal pain | - | 5.8 × 6.3 × 5.7 | No | Tumor resection + bilateral salpingectomy | NA | No | 1 | Low | NR 24 months |
| Pelvis | 68 (male) | Acute urinary retention | Normal | NA | Yes | Tumor resection | NA | Yes | 1 | Intermediate | Dead due to cardiac arrest under the surgery | |
| Heger et al. (2024)10 | Prostatic | 76 (male) | Obstructive urinary symptoms | Normal | NA | Yes | Radical prostatectomy + lymphadenectomy | R0 | NA | > 4 | Intermediate | NA |
| Zhou and Xu (2023)26 | Pelvic | 15 (male) | Numbness in buttock and radiating pain in LL | NA | 11.5 × 8.8× 7.7 | Yes | Complete surgical excision | R0 | NA | 7 | Intermediate | 9 months after surgery tumor recurrence: Imatinib (1 year) |
| Yilmaz et al. (2023)35 | Prostatic | 44 (male) | Lower abdominal pressure | Normal | 4.8 × 6.6 | Yes | Surveillance | - | No | 01-Feb | Intermediate | No signs of malignancy at 36 months |
| Peng et al. (2022)5 | Prostatic | 50 (male) | LUTS | Normal | 4.6 x 3.5 x 2.8 | Yes | Radical prostatectomy | R0 | NA | NA | NA | NR 3 months |
| Gordillo et al. (2022)36 | Pelvic | 47 (male) | Difficulty urinating | NA | 10.3 x 14 x 16 | Yes | Tumor resection + APR | NA | Yes | NA | NA | NA |
| Ahnou et al. (2021)37 | Prostatic | 77 (male) | LUTS | Normal | 10.8 × 8.2 × 5.9 | Yes | Radical prostatectomy + lymphadenectomy | R0 | Yes | < 1 | Low | NR 6 months |
| Takeuchi et al (2021)38 | Prostatic | 43 (male) | Incidental | Normal | 3 x 3.4 | Yes | Radical prostatectomy | R0 | NA | NA | Intermediate | NR 24 months |
| Gilbert et al. (2020)39 | Periprostatic | 78 (male) | Constipation and lower abdominal pain | Normal | 6.32 x 4.6 | NA | Tumor resection (prostate sparing technique) | R0 | NA | < 1 | Low | NR 12 months |
| Mishra et al. (2020)40 | Prostatic | 28 (male) | Obstructive urinary symptoms | NA | 5.8 x 6.4 x 6.5 | Yes | Simple suprapubic prostatectomy + suprapubic cystostomy | R0 | No | < 1 | Intermediate | NR 168 months |
| Okubo et al. (2020)41 | Prostatic | 40 (male) | Lower abdominal pain | NA | 6 x 5 x 4 | Yes | Tumor resection | R0 | No | NA | Low | NR 6 months |
| Bakhshwin et al. (2020)42 | Prostatic | Median: 62 (4 cases) | LUTS (3) and hematuria (1) | NA | Median: 8.5 (5, 8, 9 and 13) | Yes | Prostatectomy (1), CP (2), TURP (1) | NA (2), R0 (1), R1 (1) | Yes (2), no (2) | Median: 11.5 (20, 13, 7 and 6) | High (2), intermediate (2) | NA (1), metastasize at 14 months (1), relapse at 30 months (1), NR 12 months (1) |
| Matos et al. (2020)12 | Prostatic | 66 (male) | Urinary frequency, urgency and nocturia | Normal | NA | NA | Prostatectomy | NA | NA | NA | Intermediate | NR 60 months |
| Wada et al. (2019)24 | Pelvic | 72 (male) | Hypoglycemic attack | NA | 15 × 8 × 8 | NA | Tumor resection | NA | Yes | 7 | High | NR 12 months |
| Mora-Guzmán et al. (2019)43 | Pelvic | 83 (female) | Abdominal discomfort | - | 7.5 x 5.4 x 4.2 | Yes | Tumor resection | NA | Yes | 4 | NA | NR 36 months |
| Cheng et al. (2019)44 | Prostatic | 43 (male) | Gross hematuria | Normal | 8 x 4.5 x 3.5 | Yes | Resection of pelvic tumor through bladder | R0 | Yes | 4 | Intermediate | NR 24 months |
| Tanaka et al. (2018)45 | Prostatic urethra | 68 (male) | Urinary frequency and gross hematuria | Normal | 6 x 5 | Yes | Tumor resection | NA | Yes | >10 | High | Local recurrence and lung metastases at 54 months. Palliative care |
| Ronchi et al. (2017)46 | Prostatic | 62 (male) | Urinary retention and constipation | High (5.8) | 20 x 10 | Yes | Prostatectomy | R0 | Yes | 8 | Intermediate | NR 96 months |
| Osamu et al. (2017)47 | Prostatic | 65 (male) | Nocturia | Normal | 10 | Yes | Prostatectomy | R0 | Yes | <2 | Intermediate | NR 18 months |
| Gao et al (2016)48 | Pelvic | 58 (male) | Incidental | NA | 5.5 x 2.5 | Yes | Tumor resection | NA | NA | NA | NA | Relapse at 24 months: tumor resection + radiotherapy |
| Yang et al. (2015)49 | Prostatic | 46 (male) | Dysuria, irritative LUTS | Normal | 6.4 x 5.6 x 5.7 | Yes | Prostatectomy | R0 | NA | NA | Intermediate | NR 18 months |
| Dhameja et al. (2015)50 | Prostatic | 62 (male) | Dysuria, urinary urgency and increased frequency | Normal | 18 x 12 x 6 | Yes | CP | NA | Yes | 7 | Intermediate | NA |
| Moureau-Zabotto et al. (2012)6 | Prostatic | 60 (male) | Urinary tract obstruction | Normal | 15 | Yes | CP | R0 | Yes | 4 | High | NR 28 months |
| Katsuno et al. (2011)13 | Pelvic | 56 (female) | Painless | - | 9 × 7.5 × 5 | Yes | Trans-sacral approach | NA | NA | NA | NA | NR 24 months |
| Galosi et al. (2009)51 | Prostatic | 60 (male) | Urinary tract obstruction | Normal | 8 | Yes | Prostatectomy | R0 | No | 1 | Low | NR 6 months |
| Nair et al. (2007)52 | Prostatic | 37 (male) | Urinary tract obstruction | NA | 10 | Yes | Enucleation | R1 | NA | 1 | High | NR 24 months |
| Herawi et al. (2007)53 | Prostatic | Median: 65* (range 46-75) | Urinary tract obstruction | NA | Median: 10.5 (range 8.5-15) | NA | TUR (1); Prostatectomy (4); CP (2); pelvic exenteration (2); enucleation (1) | NA | Yes | 0 (5); 3-5 (5) | NA | NA (4); death at 7 months from unrelated cause (1) NR 1 to 10 years (5) |
| Oguro et al. (2006)54 | Prostatic | 35 (male) | Urinary tract obstruction | Normal | 5.2 | Yes | Enucleation | R1 | No | NA | Low | Local relapse at 12 months |
| Vossough et al. (2005)11 | Pelvic peritoneum | 61 (male) | Increased urinary frequency, left flank pain radiating to the scrotum, and deep pelvic pain | NA | 9 x 6 x 11 | NA | Tumor resection | NA | Yes | NA | High | NA |
| Vodovnik et al. (2005)55 | Prostatic | 87 (male) | Urinary tract obstruction and hematuria | Normal | > 9 | No | Emergency hemostatic prostatectomy | R1 | Yes | 15 | High | Death (first postoperative day) |
| Grasso et al. (2002)56 | Prostatic | 21 (male) | Urinary tract obstruction | NA | 2.4 | Yes | Enucleation | NA | NA | 0 | NA | NA |
| Sekine et al. (2001)57 | Prostatic | 42 (male) | Urinary tract obstruction | Normal | NA | Yes | Prostatectomy | R0 | NA | 2 | High | NR 18 months |
| Pins et al. (2001)58 | Prostatic | 73 (male) | Urinary tract obstruction | Normal | 6 | No | Prostatectomy | NA | No | 4 | High | NR 21 months |
| Prostatic | 57 (male) | None (rectal examination) | Normal | 10 | Yes | Prostatectomy | R0 | No | <1 | Low | NR 15 months | |
| Westra et al. (2000)59 | Prostatic | 65** (male) | Urinary retention | NA | 11 | No | CP + RT | R2 | Yes | NA | High | NR 2 months |
| RSV | 47 (male) | Left groin discomfort | NA | NA | NA | Excision of RSV | R0 | No | NA | NA | NR 15 months | |
| RSV | 46 (male) | Hematospermia | NA | 5 | NA | Excision of RSV | R0 | No | NA | NA | NR 8 months | |
| Bladder | 67 (male) | Incidental | NA | 4 | NA | CP | R0 | No | >10 | High | NR 9 months | |
| Bladder | 67 (male) | Incidental | NA | NA | NA | TUR | R0 | NA | NA | NA | NR 1 month | |
| Kelly & Baxter (1998)60 | Prostatic | 59 (male) | Urinary tract obstruction | Normal | NA | Yes | Observation | - | NA | NA | NA | NA |
| Takeshima et al. (1997)61 | Prostatic | 42 (male) | Difficult voiding and constipation | NA | 14×13×11 | No | CP | NA | Yes | NA | NA | NR 10 months |
| Mentzel et al. (1997)62 | Prostatic | 72 (male) | Urinary tract obstruction and pelvic pressure | NA | No | No | TUR | NA | No | <2 | Low | NA |
* 10 cases, including the case reported by Westra et al. (2000); ** This case is also included in the report of Herawi et al. (2007); APR: Abdominal-perineal resection of the rectum; MRI: Magnetic resonance imaging; CP: Cystoprostatectomy; HPF: High power field; LL: Lower limbs; LUTS: Lower urinary tract symptoms; NA: Not available; NR: No relapse; PSA: Prostate-Specific Antigen; RSV: Right seminal vesicle; TUR: Transurethral resection; TURP: Transurethral resection of the prostate; RT: Radiotherapy.
Table 1: SFT of the pelvic cavity cases.
References
- Klemperer P, Coleman BR (2025) Primary neoplasms of the pleura. A report of five cases. Am J Ind Med 22: 4-31.
- Park MS, Ravi V, Conley A, Patel SR, Trent JC, et al (2013) The role of chemotherapy in advanced solitary fibrous tumors: a retrospective analysis. Clin Sarcoma Res 3: 7.
- Dagrada GP, Spagnuolo RD, Mauro V, Tamborini E, Cesana L, et al (2015) Solitary fibrous tumors: loss of chimeric protein expression and genomic instability mark dedifferentiation. Modern Pathology 28: 1074-1080.
- Hu Y, Mahar TJ, Hicks DG, Raymond D, Jones C, et al (2009) Malignant solitary fibrous tumor: Report of 3 cases with unusual features. Applied Immunohistochemistry and Molecular Morphology 17(5):451–457.
- Peng Y, Jiang Y, Ding S, Zheng Y, Tang W, et al (2022) Solitary fibrous tumors in prostate: a case report with review of the literature. Aging Male 25: 219-222.
- Moureau-Zabotto L, Chetaille B, Bladou F, Dauvergne PY, Marcy M, et al (2012) Solitary fibrous tumor of the prostate: Case report and review of the literature. Case Rep Oncol 5: 22-29.
- Doyle LA, Vivero M, Fletcher CDM, Mertens F, Hornick JL (2014) Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod Pathol 27: 390-395.
- Martin-Broto J, Mondaza-Hernandez JL, Moura DS, Hindi N (2021) A Comprehensive Review on Solitary Fibrous Tumor: New Insights for New Horizons. Cancers (Basel) 13: 2913.
- Salguero-Aranda C, Martínez-Reguera P, Marcilla D, de Álava E, Díaz-Martín J (2021) Evaluation of nab2-stat6 fusion variants and other molecular alterations as prognostic biomarkers in a case series of 83 solitary fibrous tumors. Cancers (Basel) 13(20): 5237.
- Heger P, Hill A, Charchenko C (2024) CD-34 negative solitary fibrous tumor of the prostate: A case report. Urol Case Rep 57: 102855.
- Vossough A, Torigian DA, Zhang PJ, Siegelman ES, Banner MP (2005) Extrathoracic solitary fibrous tumor of the pelvic peritoneum with central malignant degeneration on CT and MRI. Journal of Magnetic Resonance Imaging 22: 684-686.
- Matos J, Paparo F, Calcagno T, Marinaro E, Introini C, et al (2020) Solitary Fibrous Tumor of the Prostate. Urology 141: e43-44.
- Katsuno H, Maeda K, Hanai T, Sato H, Masumori K, et al (2011) Trans-sacral resection of a solitary fibrous tumor in the pelvis: Report of a case. Surg Today 41: 1548-1551.
- Swallow CJ, Strauss DC, Bonvalot S, Rutkowski P, Desai A, et al (2021) Management of Primary Retroperitoneal Sarcoma (RPS) in the Adult: An Updated Consensus Approach from the Transatlantic Australasian RPS Working Group. Ann Surg Oncol 28: 7873-7888.
- Dingley B, Fiore M, Gronchi A (2019) Personalizing surgical margins in retroperitoneal sarcomas: an update. Expert Rev Anticancer Ther 19: 613-631.
- Gronchi A, Strauss DC, Miceli R, Bonvalot S, Swallow CJ, et al (2016) Variability in patterns of recurrence after resection of primary retroperitoneal sarcoma (RPS). A report on 1007 patients from the multi-institutional collaborative RPS working group. Ann Surg 263: 1002-1009.
- Gronchi A, Lo Vullo S, Fiore M, Mussi C, Stacchiotti S, et al (2009) Aggressive Surgical Policies in a Retrospectively Reviewed Single-Institution Case Series of Retroperitoneal Soft Tissue Sarcoma Patients. Journal of Clinical Oncology 27: 24-30.
- Bonvalot S, Rivoire M, Castaing M, Stoeckle E, Le Cesne A, et al (2009) Primary Retroperitoneal Sarcomas: A Multivariate Analysis of Surgical Factors Associated With Local Control. Journal of Clinical Oncology 27: 31-77.
- Bonvalot S, Raut CP, Pollock RE, Rutkowski P, Strauss DC, Hayes AJ, et al (2012) Technical considerations in surgery for retroperitoneal sarcomas: Position paper from E-Surge, a master class in sarcoma surgery, and EORTC-STBSG. Ann Surg Oncol 19: 2981-2991.
- Gilbert NF, Cannon CP, Lin PP, Lewis VO (2009) Soft-tissue sarcoma. J Am Acad Orthop Surg 17: 40-47.
- Chao AH, Mayerson JL, Chandawarkar R, Scharschmidt TJ (2015) Surgical management of soft tissue sarcomas: Extremity sarcomas. J Surg Oncol 111: 540-545.
- Demicco EG, Wagner MJ, Maki RG, Gupta V, Iofin I, et al (2017) Risk assessment in solitary fibrous tumors: validation and refinement of a risk stratification model. Modern Pathology 30: 1433-1442.
- Gold JS, Antonescu CR, Hajdu C, Ferrone CR, Hussain M, et al (2002) Clinicopathologic correlates of solitary fibrous tumors. Cancer 94: 1057-1068.
- Wada Y, Okano K, Ando Y, Uemura J, Suto H, et al (2019) A solitary fibrous tumor in the pelvic cavity of a patient with Doege-Potter syndrome: a case report. Surgical Case Reports 5(1): 1-5.
- Mondaza-Hernandez JL, Moura DS, Lopez-Alvarez M, Sanchez-Bustos P, Blanco-Alcaina E, et al (2022) ISG15 as a prognostic biomarker in solitary fibrous tumour. Cell Mol Life Sci 79: 434.
- Zhou P, Xu X (2023) Recurrent malignant solitary fibrous tumor of pelvis: A case report and treatment approach. Medicine (United States) 102: E34520.
- O’Donnell PW, Griffin AM, Eward WC, Sternheim A, Catton CN, et al (2014) The effect of the setting of a positive surgical margin in soft tissue sarcoma. Cancer 120: 2866-2875.
- Martin-Broto J, Cruz J, Penel N, Le Cesne A, Hindi N, et al (2020) Pazopanib for treatment of typical solitary fibrous tumours: a multicentre, single-arm, phase 2 trial. Lancet Oncol 21: 456-466.
- Stacchiotti S, Ferrari S, Redondo A, Hindi-Muniz N, Palmerini E, et al (2019) Pazopanib for treatment of advanced extraskeletal myxoid chondrosarcoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol 20: 1252-1262.
- Martin-Broto J, Stacchiotti S, Lopez-Pousa A, Redondo A, Bernabeu D, et al (2019) Pazopanib for treatment of advanced malignant and dedifferentiated solitary fibrous tumour: a multicentre, single-arm, phase 2 trial. Lancet Oncol 20: 134-144.
- Stacchiotti S, Tortoreto M, Baldi GG, Grignani G, Toss A, et al (2014) Preclinical and clinical evidence of activity of pazopanib in solitary fibrous tumour. Eur J Cancer 50: 3021-3028.
- Van Der Graaf WTA, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, et al (2012) Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. The Lancet 379: 1879-1886.
- Martin-Broto J, Hindi N, Lopez-Pousa A, Peinado-Serrano J, Alvarez R, et al (2020) Assessment of Safety and Efficacy of Combined Trabectedin and Low-Dose Radiotherapy for Patients With Metastatic Soft- Tissue Sarcomas: A Nonrandomized Phase 1/2 Clinical Trial. JAMA Oncol 6: 535-541.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi