Case Report, Clin Oncol Case Rep Vol: 3 Issue: 4
The Combination of Ponatinib and Alpha-Interferon can Improve Outcome of Resistant Chronic Myeloid Leukemia Patients
Claudia Barate1*, Simona Soverini2, Elisabetta Abruzzese3, Francesca Guerrini4, Susanna Grassi4, Maria Immacolata Ferreri5, Federica Ricci1, Serena Balducci1, Mario Petrini4 and Sara Galimberti4
1Section of Hematology, AOUP, Pisa, Italy
2Department of Experimental, Diagnostic and Specialty Medicine, Hematology/Oncology 'L. e A. Seragnoli', University of Bologna
3Department of Hematology, S Eugenio Hospital, Tor Vergata University, Rome, Italy
4Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Italy
5Laboratory of Medical Genetics, AOUP, Pisa, Italy
*Corresponding Author : Claudia Barate
Section of Hematology, AOUP, Pisa,
Italy
E-mail: claudia.barate@gmail.com
Received: May 15, 2020 Accepted: June 21, 2020 Published: August 15, 2020
Citation: Barate C, Soverini S, Abruzzese E, Guerrini F, Grassi S, et al. (2020) The Combination of Ponatinib and Alpha-Interferon can Improve Outcome of Resistant Chronic Myeloid Leukemia Patients. Clin Oncol Case Rep 3:4.
Abstract
Abstract
The most of patients affected by chronic myeloid leukemia is cured with Tyrosine Kinase Inhibitors (TKI). However, about 30% must change therapies for intolerance or poor efficacy. The failure of therapy is associated in the majority of cases to the appearance of ABL1 mutations. Ponatinib (ICLUSIG, INCYTE®) is able to overcome most of the known mutations, including the T315I. But when compound mutations appear, the TKIs alone are not enough and a combination therapy is required to overcome this condition.
We describe here the evolution of the mutational status of 3 patients with compound mutations, treated with the association of ponatinib plus alfa interferon. This combination resulted safe and effective for all.
In the first patient, the T315I plus the V289A and the M244V mutations were discovered; the second patient showed the T315I plus the A288T and the G390R and the third one, without T315I, the K419E plus the G254R were found. After one year of the combination therapy, all cases showed the disappearanced of the compound mutations and the T315I. In addition, the use of next generation sequencing resulted fundamental for detecting and monitoring resistant patients.
Keywords: Ponatinib; Alpha-interferon; Chronic myeloid leukemia
Introduction
Chronic Myeloid Leukemia (CML), defined by the Philadelphia chromosome (Ph’) and BCR-ABL1 fusion gene, is a myeloid disorder characterized by release of immature myeloid cells in the bloodstream. In the past, Alpha Interferon (IFNa) offered not rare cytogenetic remissions with a median survival of 5 years [1]. Tyrosine Kinase Inhibitors (TKIs) significantly improved CML prognosis [2], but during treatment 30% of patients develop intolerance or resistance [3], often due to ABL1 mutations [4,5]. Despite its sensitivity to Ponatinib, the T315I remains the mutation associated to the worst prognosis [6]. Indeed, when different mutations co-exist (compound mutations), Ponatinib is not able to overcoming resistance [7]. Furthermore, in many resistant patients, the strong multi tyrosin-kinase inhibition of Ponatinib, added to the immunomodulatory effect of IFNa, can help improving the response, also when mutations are not found. We report here our experience of Ponatinib and IFNa combination in 3 ABL1-mutated patients.
Case Presentation
Case 1
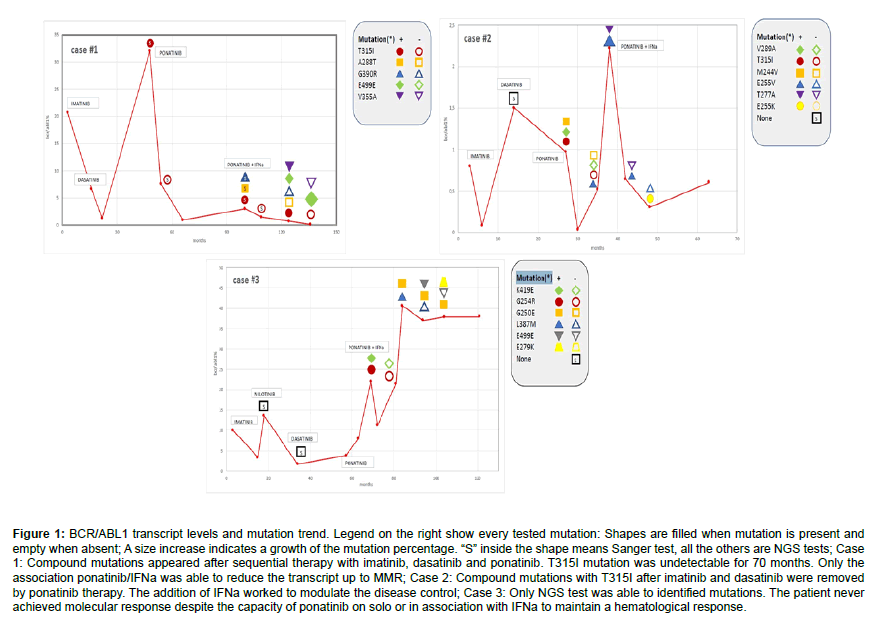
A 54-year old male was diagnosed with Sokal low-risk Chronic Phase (CP) CML in July 2007. He carried an e1a2 BCR-ABL1 transcript and t (8;11). Previously, he underwent a kidney transplantation and a coronary bypass. Imatinib 400 mg daily was started; after 3 months, the BCR-ABL1/ABL1 ratio was 20,66%. During the first 16 months of treatment, patient never achieved the Major Molecular Response (MMR); cytogenetics documented a partial response (PCyR) and the disappearance of the t (8;11). Because of failure, in December 2008 Dasatinib 50 mg daily was started. After 6 months, Complete Cytogenetic Response (CCyR) and a substantial reduction of the BCR-ABL1 transcript (1.2%) were achieved. In July 2010, a cytogenetic relapse occurred and BCR-ABL1/ABL1 ratio increased up to 32%. Mutational analysis by Sanger revealed the presence of the T315I mutation. Transplantation option was discarded due to the high transplant-related mortality risk, but in April 2012 Ponatinib became available in the context of the PACE trial [8]. After one month, Ponatinib was reduced from 45 mg to 30 mg daily due to WHO grade-4 pancreatic toxicity; twelve months later, BCR-ABL1/ABL1 ratio was 0.86% with CCyR. The T315I mutation became undetectable for two years. In November 2015, Sanger sequencing identified 3 co-existing mutation: T315I plus A288T and G390R. Patient was still in CP, the BCR-ABL1/ABL1 ratio was 2.93% and no cytogenetic abnormalities were detected. We added IFNa to Ponatinib; to avoid further cardiovascular toxicity, so from December 2015 patient received Ponatinib 15 mg alternate days plus IFNa 1.5 MU, three times a week. After 9 months, the T315I disappeared (by Sanger). With the introduction of Next Generation Sequencing (NGS), we tested our patient after two years of the combined treatment. Three mutations were identified: T315I (61,47%), E499E (29,7%) and V355A (20,71%). Combination therapy was continued and after 3 years BCR-ABL1/ABL1 ratio was 0,087%; NGS showed the E499E mutation only (44,7%) (Figure 1A-1C). Patient died for heart failure 55 months after CML diagnosis and 38 months from starting combination therapy.
Case 2
In September 2013, a 61 year old male with diabetes, hypertension, dyslipidemia, hyperuricemia and recent coronary artery disease was diagnosed with high Sokal risk CML in Accelerated Phase (AP). Imatinib 400 mg daily was started because of the patient’s high comorbidities. After three months, PCyR but early molecular response (e13a2 BCR-ABL1/ABL1 ratio 0.8% IS) were obtained. WHO grade 1-2 toxicities (anemia, fluid retention, cramps, edema and renal dysfunction) were reported. In December 2014 MMR and CCyR were suddenly lost. ABL1 Sanger sequencing resulted negative and Dasatinib 80 mg daily was started, without efficacy. In January 2016, a progressive increase of BCR-ABL1/ABL1 ratio (0.97% IS) and Ph’ metaphases (30%) were observed and NGS discovered three mutations in the new e1a2 clone: T315I (99.69%), V289A (1.20%) and M244V (1.19%). In February 2016, 45 mg daily Ponatinib was started. After 3 months, e13a2 and e1a2 BCR-ABL1/ABL1 ratio were 0.03% IS and 0% respectively; cytogenetics showed the absence of Ph’ and the appearance of+8. Because of WHO grade-2 abdominal pain and grade-4 skin toxicities, Ponatinib was reduced to 30 mg daily and then stopped after 2 months because of angina and high levels of troponin. One month later, Ponatinib was reintroduced at 15 mg every other day, strictly monitoring troponin. Then, taking into account the T315I mutation and the high cardiovascular risk, IFNa 1.5 MU 3 times a week was added. After 6 months, the ABL1 mutational scenario significantly improved, with E255V (10%) being the only detected mutation. One year later, patient presented an acute myocardial infarction, and therapy was stopped. After 3 weeks, the CCyR was lost (Ph’ 35%); NGS showed increased levels of E255V (100%) and appearance of a sub-clonal further mutation, the T277A (1,68%). Ponatinib 15 mg every two days was restarted in combination with pegylated interferon 50 mcg/week plus double antiplatelet therapy. In 2017, E255V and T277A were not detectable but a new mutation, E255K, appeared (97,05%). The patient, with the persistence of +8, became transfusion dependent, with evidence of myelodysplastic features. He died for heart attack in November 2018, 62 months from CML onset and 27 after starting combination therapy. At that moment, e13a2 and e1a2 BCR-ABL1/ABL1 ratio were 0,6% and 0% respectively, and T315I was no detectable by NGS (Figure 1).
Case 3
In February 2010, a 65 year old woman was diagnosed with a Sokal high risk AP-CML. Controlled hypertension was the only comorbidity. Imatinib 600 mg daily was started, giving significant anemia, and after 6 months subcutaneous alpha-erythropoietin was added. After 15 months, e13a2 BCR-ABL1/ABL1 ratio was still 3.34% IS; cytogenetics showed the absence of Ph’ but also+8. At month 18, Ph’ reappeared while the ABL1 mutations were absent (after Sanger sequencing). Nilotinib 600 mg daily was started and rapidly CCyR was achieved; nevertheless, after 16 months, a second loss of CCyR occurred. The cytogenetics showed a-7 in the Ph’ clone; no ABL1 mutations were detected. Because of severe persistent cytopenia, Dasatinib 100 mg daily was started, without any significant molecular response. In November 2014, Ponatinib 30 mg daily was introduced but soon reduced to 15 mg daily due to grade 3 hematologic toxicity. After 6 months, patient was in CCyR but the BCR-ABL1/ABL1 ratio was still 8% IS and NGS showed the presence of 2 sub-clonal mutations, the K419E (4.12%) and the G254R (2.63%). Then, from December 2015, IFNa 1.5 MU three times a week was added to Ponatinib 15 mg daily. After one year, NGS was negative but few months later another two mutations appeared (G250E 2.89% and L387M 1.65%). Therapy was continued; after 34 months, G250E mutation increased to 25.7% and a new mutation, the E499E, was detected (10.6%); 3 months later they dropped to 18.99% and 1.03% respectively, while L387M disappeared. After 50 months of the combined therapy, BCR-ABL1/ABL1 ratio was 37,8614% IS; NGS showed an increase of the G250E mutation (67%), disappearance of E499E and appearance of E279K (7%) (Figure 1). In February 2020, after 10 years from diagnosis and 50 months from starting combination therapy, patient presented a myeloid blastic phase and is now in treatment with CPX-351.
Figure 1: BCR/ABL1 transcript levels and mutation trend. Legend on the right show every tested mutation: Shapes are filled when mutation is present and empty when absent; A size increase indicates a growth of the mutation percentage. “S” inside the shape means Sanger test, all the others are NGS tests; Case 1: Compound mutations appeared after sequential therapy with imatinib, dasatinib and ponatinib. T315I mutation was undetectable for 70 months. Only the association ponatinib/IFNa was able to reduce the transcript up to MMR; Case 2: Compound mutations with T315I after imatinib and dasatinib were removed by ponatinib therapy. The addition of IFNa worked to modulate the disease control; Case 3: Only NGS test was able to identified mutations. The patient never achieved molecular response despite the capacity of ponatinib on solo or in association with IFNa to maintain a hematological response.
Discussion and Conclusion
Our experience highlights some interesting issues in the context of CML resistance: 1) the acceptable toxicity profile of Ponatinib and IFNa combination, except for cardiovascular events. Nevertheless, both patients with heart failure had a very high CV risk already at diagnosis. In a series of 656 patients receiving second-and third-generation TKIs, age over 65 years and a previous CV disease were the two factors conditioning CV-free survival [9]. Regarding the hematological toxicity that forced us to reduce Ponatinib dose, we must consider that+8 (case #2 and #3) and-7 (case #3), were a sign of underlying myelodysplasia, that could contribute to the clinical manifestation, when Ph+clone is cleared by TKI. 2) the efficacy of combination therapy, probably sustained by the pro-immune effect of IFNa. Indeed, after combination therapy, T315I, involved in compound mutations, disappeared (#1 and #2), as E499E in case #3. In this patient, we want to underline that she maintained hematological response for long time, independently from poor cytogenetic and molecular responses. This result supports the hypothesis of an immunogenic role of IFNa, probably modulating cytokine/chemokine production and reducing myeloid- derived suppressor cells and Tregs [10]. The fundamental role of NGS in the monitoring of ABL1 mutations in CML resistant cases, as recently stated in an Italian position paper [11]. In our cases, the TKI failure was concomitant to the appearance of compound mutations, not revealed by Sanger, in line with a previous observation that 2/3 of patients failing 2 TKIs presented mutations detected by NGS [12] only the good Quality of Life (QoL) offered for a long time. Our approach has prolonged patients life without reduce QoL significantly, so that our patients did not spend their remaining years in a compromised state.
In conclusion, at our knowledge this is the first paper describing the long-term efficacy and safety of the combination of IFNa with Ponatinib, analogously to that already reported for Imatinib [13] and Dasatinib [14]. These results might be the starting point for further biological and clinical researches.
References
- Kantarjian HM, O'Brien S, Cortes JE, Shan J (2003) Complete cytogenetic and molecular responses to interferon-alpha-based therapy for chronic myelogenous leukemia are associated with excellent long-term prognosis. Cancer 97: 1033-1041.
- Hochhaus A, Larson RA, Guilhot F, Radich JP (2017) Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med 376: 917-927.
- Jabbour EJ, Cortes JE, Kantarjian HM (2013) Resistance to tyrosine kinase inhibition therapy for chronic myelogenous leukemia: A clinical perspective and emerging treatment options. Clin Lymphoma Myeloma Leuk 13: 515-529.
- Machova PK, Kulvait V, Benesova A, Linhartova J (2015) Next-generation deep sequencing improves detection of BCR-ABL1 kinase domain mutations emerging under tyrosine kinase inhibitor treatment of chronic myeloid leukemia patients in chronic phase. J Cancer Res Clin Oncol 141: 887-899.
- Soverini S, Branford S, Nicolini FE, Talpaz M (2014) Implications of BCR-ABL1 kinase domain-mediated resistance in chronic myeloid leukemia. Leuk Res 38: 10-20.
- Frankfurt O, Licht JD (2013) Ponatinib a step forward in overcoming resistance in chronic myeloid leukemia. Clin Cancer Res 19: 5828-5834.
- Zabriskie MS, Eide CA, Tantravahi SK, Vellore NA (2014) BCR-ABL1 compound mutations combining key kinase domain positions confer clinical resistance to ponatinib in Ph chromosome-positive leukemia. Cancer Cell 26: 428-442.
- Cortes JE, Kim DW, Pinilla-Ibarz J, Coutre P (2013) A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med 369: 1783-1796.
- Caocci G, Mulas O, Annunziata M, Luciano L, Abruzzese E, et al. (2020) Long-term mortality rate for cardiovascular disease in 656 chronic myeloid leukaemia patients treated with second- and third-generation tyrosine kinase inhibitors. Int J Cardiol 301: 163-166.
- Alves R, McArdle S, Vadakekolathu J, Gonçalves A (2020) Flow cytometry and targeted immune transcriptomics identify distinct pro les in patients with chronic myeloid leukemia receiving tyrosine kinase inhibitors with or without interferon-α. J Transl Med 18: 2.
- Soverini S, Abruzzese E, Bocchia M, Bonifacio M, Galimberti S, et al. (2019) Next-generation sequencing for BCR-ABL1 kinase domain mutation testing in patients with chronic myeloid leukemia: A position paper. J Hematol Oncol 12: 131.
- Soverini S, De Benedittis C, Polakova KM, Linhartova J (2016) Next-generation sequencing for sensitive detection of BCR-ABL1 mutations relevant to tyrosine kinase inhibitor choice in imatinib-resistant patients. Oncotarget 7: 21982-21990.
- Palandri F, Castagnetti F, Iacobucci I, Martinelli G (2010) The response to imatinib and interferon-alpha is more rapid than the response to imatinib alone: A retrospective analysis of 495 Philadelphia-positive chronic myeloid leukemia patients in early chronic phase. Haematologica 95: 1415-1419.
- Polivkova V, Rohon P, Klamova H, Cerna O (2016) Interferon-α revisited: Individualized treatment management eased the selective pressure of tyrosine kinase inhibitors on BCR-ABL1 mutations resulting in a molecular response in high-risk CML patients. PLoS One 11: e0155959.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi