Research Article, J Clin Exp Radiol Vol: 1 Issue: 1
The Optimal Efficacy of a Single Therapeutic Dose of Sm- 153 EDTMP in the Treatment of Painless Skeletal Metastases
Mai Elzahry1*, Waleed Diab2 and Helmut Sinzinger3
1Department of Clinical Oncology and Nuclear Medicine, Faculty of Medicine, South Valley University, Qena 83523, Egypt
2Departments of Clinical Oncology and Nuclear Medicine, Faculty of Medicine, Assiut University, Egypt
3Department of Nuclear Medicine, Medical University of Vienna, Austria
*Corresponding Author : Mai Elzahry, Ph.D.
Department of Mechanical Engineering, Faculty of Engineering, Assiut University, Egypt
Tel: +2 01010720929
E-mail: mai.rifat@hotmail.com
Received: November 09, 2017 Accepted: February 22, 2018 Published: February 28, 2018
Citation: Elzahry M, Diab W, Sinzinger H (2018) The Optimal Efficacy of a Single Therapeutic Dose of Sm-153 EDTMP in the Treatment of Painless Skeletal Metastases. J Clin Exp Radiol 1:1.
Abstract
Background: Sm-153 EDTMP is a widely accessible radiopharmaceutical for systemic radionuclide therapy in cancer patients with painful skeletal disseminations; its use is expressed to any metastatic bone lesions which has been avid to Tc-99m MDP. In this study we obtained a clinical experience with a single therapeutic dose of Sm-153 EDTMP on cancer patients showed painless bone metastases on conventional bone scintigraphy, its bone response so far has not been studied in a large number of patients. Objective: evaluation of the overall therapeutic bone response in 103 cancer patients showed a painless skeletal metastases whom underwent a single therapeutic dose of Sm-153 EDTMP and elaborate if there is a significant difference in bone response depending on gender, patients age, pathology of primary cancer, number of the metastatic bone lesions. Methods: 103 patients were included in this retrospective analysis, 78(75.7%) males and 25(24.3%) females, their age range (19-92y, mea= ± SD=64.3 ± 13.7), their diagnosis were prostate (60.2%), breast(19.4%), lung(7.8%), other primaries(12.6%), 64.1% of cancer patients showed less than 10 bone lesions, 35.9% showed more than 10 bone lesions on conventional bone scintigraphy performed pretherapy. Results: Out of 103 cancer patients received a single dose of Sm- 153 EDTMP therapy, 67% (69/103) showed overall therapeutic bone stabilization/regression and 33% (34/103) showed bone progression, the rate of bone progression/regression showed a statistical significant difference among cancer patients dependent on their gender, age, type of cancer (P-values<0.005), while the rate of bone progression/stabilization differed significantly among those with more than 10 bone lesions as compared with patients showed less than 10 bone lesions on conventional bone scintigraphy. Conclusion: This study showed that as Sm-153 EDTMP offers a riskless and effective therapy options in patients with painful bone disseminations, it also provides encouraging results among those with painless skeletal metastases.
Keywords: Optimal efficacy; Sm-153 EDTMP; Painless skeletal metastases
Introduction
Bone is the most common site for metastasis in cancer particularly in breast and prostate cancers because of the predominance of these diseases [1]. ~70% of patients dying of these cancers have evidence of metastatic bone disease. However, bone metastases may result in morbidity. Carcinomas of the thyroid, kidney, and bronchus also commonly give rise to bone metastases, with an incidence of 30% to 40% [2].
Although some malignancies give rise principally to plastic or lytic lesions (prostate and multiple myeloma, respectively), most malignancies exhibit a mixed plastic-lytic phenotype [3].
The therapeutic options are rarely (if ever) curative and at some point of time the vast majority of patients suffering from osseous metastasis will develop progressive disease, leading to a series of disease related events that have the most significant impact on the quality of life in these patients. Chemotherapy or hormonal therapy may be used for both soft tissue and bone metastases and can be effective until the disease becomes refractory to these agents [4]. Systemic therapy with radionuclides avid to bone seeking agents, is a therapeutic option for patients with painful skeletal metastases, owing to its efficacy, economic and safety issues [5]. Bone seeking radiopharmaceuticals like Samarium‑153 EDTMP, Sr‑89, etc., have affinity for skeletal tissue and when administered intravenously they concentrate in areas of increased bone turnover [6]. Localizing in active bone and mainly at metastatic lesions, allowing site‑directed radiotherapy [7]. Samarium-153 is widely used in the field of metastatic bone dissemination has been obtained in this study, it localizes in the skeleton by chemo-absorption of the tetraphosphonate by hydroxyapatite and by the formation of Samarium oxide involving an oxygen on the hydroxyapatite molecule [8]. There is a few number of systemic studies deal with the efficacy of significant bone response induced by therapeutic doses of Sm-153 EDTMP in cancer patients. Therefore, it was the aim of our study to evaluate the rate of bone regression/stabilization after a single therapeutic dose of Sm-153 EDTMP in 103 cancer patients with pain - free referred to the Nuclear Medicine Unit at The Medical University of Vienna.
Design and Methodology
Nature of the study
• This is a retrospective clinical study on 103 cancer patients with pain free who underwent single dose of Sm-153 EDTMP for therapy of metastatic bone dissemination.
• Concomitant chemo/radiotherapy or even repeated application of bisphosphonates are possible.
• Aim of the study was to evaluate the overall therapeutic bone response and elaborate if there is a significant difference in bone response depending on gender, patients age, pathology of primary cancer, number of the metastatic bone lesions among those patients.
Treatment design
Sm-153 EDTMP administration was performed according to the Vienna protocol [9]. The protocol is defined as follows: 30 mCi (1.1 GBq) of Sm-153 EDTMP was administered by slow intravenous injection, Red and white blood cell as well as platelet count was determined (3 and 6 weeks). Scintigraphy was performed usually on the next day anyway, more than 6 hours after radionuclide application to achieve complete blood clearance.
A recent conventional whole body Tc-99 m (MDP) bone scan (less than 8 wk.) proving osteoplastic activity in the metastatic sites. Total body scans were obtained after the intravenous administration of 20 mCi of technetium-99 m methylene bisphosphonate. The scan was performed 2 or more hours after the injection of the radionuclide.
Whole body bone Scintigraphy after radionuclide application was performed usually on the next day (to achieve complete blood clearance) showed enhanced tracer uptake in the known metastatic lesions using large field of view double headed γ-camera, LEHRcollimation, energy window 20%,103 Kev, acquisition mode contionusely 15 cm/min, early images(<4 hours) showed significantly lower quality.
Lesion response assessment
For assessment of the lesion response to 153Sm-EDTMP therapy, we reviewed the bone centigram (Tc-99 m MDP) of each patient at the time of first detection of bone metastases and compared it with the post-153Sm-EDTMP bone centigram, the number of lesions on the Tc-99 m MDP bone centigram was counted into 2 groups; “group 1” had 1-10 bone lesions, “group 2” had >10 bone lesions.
Data reported in this study were from patients receiving only a single dose of 153Sm-EDTMP. The study included patients had been treated at Department of Nuclear Medicine, Medical University of Vienna, Austria.
Ethical approval
This study was approved by the Ethics Commission at the Medical University of Vienna and the Vienna General Hospital (AKH), each patient was illustrated the details of the procedure, benefits and side effects of therapy and the follow-up protocol and all patients provided written informed consents.
Statistical analysis
Date entry and data analysis were done using SPSS version 16. The data of the patients were retrospectively collected. Continuous variables were reviewed as mean ± standard deviations, while categorical variables were summarized as numbers and percentage. Chi square test was used to test for significance. For all P-values <0.05 were selected as significant.
Patients and Methods
103 patients were included in this retrospective analysis, 78(75.7%) males and 25(24.3%) females, their age range (19-92y, mean ± SD=64.3 ± 13.7), their diagnosis were prostate, breast, lung, other primaries (rectum, pancreases, renal cell carcinoma, osteosarcoma, bladder, oesophagus, nasopharynx, multiple myeloma, uterus, adenocarcinoma of the jaw, respectively, with the majority of the patients have prostate and breast cancer representing (79.6%). 64.1% of the patients showed less than 10 bone lesions on conventional bone scintigraphy performed before initiating the treatment with 153Sm- EDTMP while 35.9% showed more than 10 bone lesions (Table 1).
Before initiating treatment, all patients underwent whole body bone scintigraphy, which showed evidence of significant metastatic bone dissemination obtained after the intravenous administration of 20 mCi of Tc-99 m methylene bisphosphonate (MDP). The scan was performed two or more hours after the injection of the radionuclide. Patients were scanned by large field of view double headed camera LEHR-collimation, energy window 20%, 140 Kiev acquisition modes continuously 15 cm/min. Another whole Tc-99 m MDP bone scintigraphy was performed 7 months later to evaluate the rate of bone response among those patients.
Results
A total of 103 cancer patients received a single dose of Sm- 153 EDTMP therapy referred to the Nuclear Medicine Unit with painless disseminated bone metastases, 67% (69/103) showed overall therapeutic bone stabilization/regression and 33% (34/103) showed bone progression (Table 1).
| Personal characteristics | ||
|---|---|---|
| age: (years) | ||
| • mean ± SD | 64.3 ± 13.7 | |
| • range | 19-92 y | |
| Sex: | n. | % |
| male female | 78/103 25/103 | 75.7 24.3 |
| Pathology of primary cancer | n. | % |
| prostate breast lung others | 62/103 60.2 20/103 19.4 8/103 7.8 13/103 12.6 | |
| n. of bone lesions = 10 lesions >10 lesions |
n. 66/103 37/103 | % 64.1 35.9 |
| bone response progression(n/total) stabilization(n/total) regression(n/total) | n. 34/103 37/103 32/103 | % 33 35.9 31.1 |
Table 1: Demographic characteristics.
Bone response is assessed in all 103 cancer patients after administration of the single dose of 153Sm-EDTMP depending on their gender age, type of cancer and the number of metastatic bone lesions.
Gender
There is a significant difference between the number of male patients in this study as compared to the number of female patients (P-value=0.000), male patients who showed bone progression/bone regression on scintigraphy differ significantly than female patients showed the same bone response (P-value=0.017,0.001, respectively) (Table 2).
| gender male female p-value |
|---|
| n/% 78/75.7 25/24.3 *0.000 |
| bone progression 33/42.3 4/16 *0.017 |
| bone stabilization 25/32.1 5/20 0.522 |
| bone regression 20/25.6 16/64 *0.001 |
| *Statistical significant difference (P<0.05) |
Table 2: Sm153-EDTMP therapy induced bone response vs. patient’s gender
Age
All patients are divided into two groups according to their ages with considering of 64 years old is the cut-off point between both groups. The number of the young patients is nearly similar to the oldest one with no statistical difference between them, there is a significant difference in the rate of bone progression/regression between both groups of the patients (P-value=0.000) with no significant difference in the rate of bone stabilization among the both groups (Table 3).
| Age = 64 is>64ys p-value |
| n/% 46/44.6 57/55.4 0.278 |
| bone progression 23/50 32 *0.000 |
| bone stabilization 17/36.9 15 0.261 |
| bone regression 6/13.1 10 *0.0003-Pathology of primary cancer |
Table 3: Sm153-EDTMP therapy induced bone response vs. patient’s age
The rate of bone progression and bone regression in prostate and breast cancer patients showed a significant difference among them (P-value=0.005, 0.000, respectively) as compared to patients with lung cancer and other primaries who showed no significant difference in bone response (Table 4).
| Type of cancer CA prostate CA breast p-value CA lung others p-value |
| n/% 62/60.3 20/19.4 *0.000 8/7.7 13/12.6 *0.000 |
| bone progression 29/46.7 3/15 *0.005 2/25 1/7.6 0.294 |
| bone stabilization 20/32.3 5/25 0.731 1/12.5 6/46.2 0.124 |
| bone regression 13/21 12/60 *0.000 5/62.5 6/ 46.2 0.491 |
Table 4: Sm153-EDTMP therapy induced bone response vs. type of cancer.
Number of metastatic bone lesions
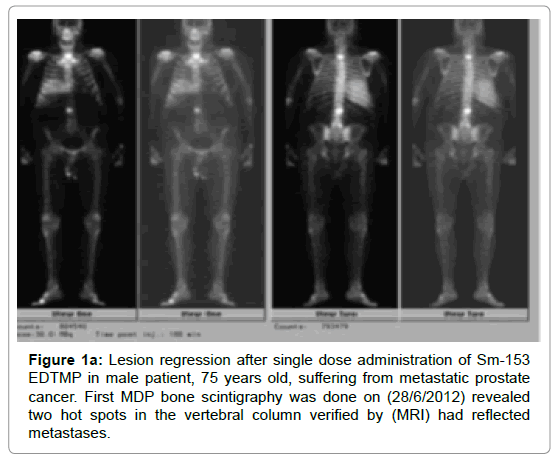
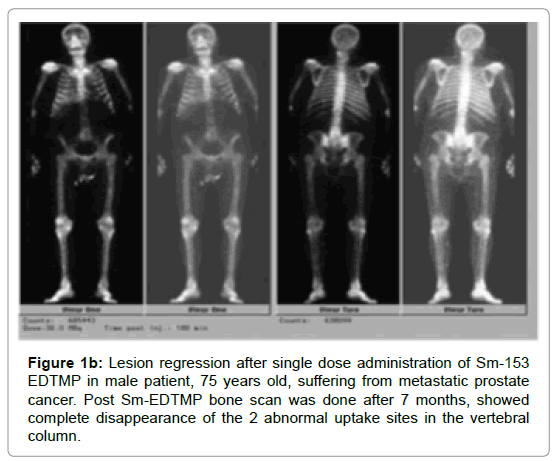
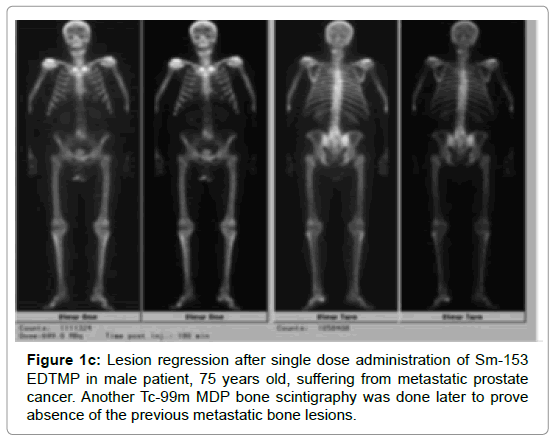
Before initiating treatment, all patients were submitted to whole body 99m Tc-MDP bone scintigraphy, which showed evidence of significant metastatic bone dissemination. After reviewing of their bone scintigraphies, The cancer patients are divided according to the number of metastatic bone lesions to two groups, with the majority of cancer patients having more than 10 bone lesions showed a significant difference (P-value=0.004) as compared with the other group. Another whole bone scintigraphy (Tc-99 m MDP) was performed later after 7 months to assess the rate of bone response. The rate of bone progression/stabilization showed a significant difference among both groups of cancer patients (P-value=0.023, 0.016) while, the rate of bone regression showed no statistical difference (Table 5, Figures 1a, 1b, 1c and 2).
| bone lesions = 10 lesions>10 lesions p-value |
| (n/total) 66/103 37/103 *0.004 |
| bone progression 16 17 *0.023 |
| bone stabilization 32 9 *0.016 |
| bone regression 18 11 0.793 |
Table 5: Sm153-EDTMP therapy induced bone response vs. patient’s age.
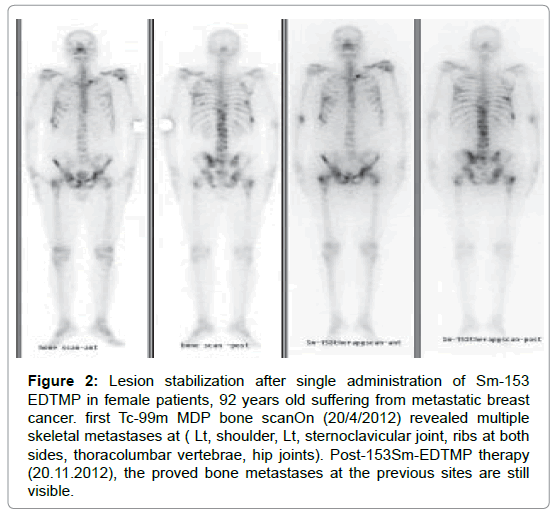
Figure 2: Lesion stabilization after single administration of Sm-153 EDTMP in female patients, 92 years old suffering from metastatic breast cancer. first Tc-99m MDP bone scanOn (20/4/2012) revealed multiple skeletal metastases at (Lt, shoulder, Lt, sternoclavicular joint, ribs at both sides, thoracolumbar vertebrae, hip joints). Post-153Sm-EDTMP therapy (20.11.2012), the proved bone metastases at the previous sites are still visible.
Discussion
Several studies (including multicentre trials) support the efficacy of Sm-153 Lexidronam treatment for palliative metastatic bone pain, including its ability to improve patients’ life quality with a very limited number of studies reported the optimal efficacy of Sm-153 EDTMP therapy in lesion regression/stabilization among cancer patients suffered from painless skeletal metastases. This study is part of a wide study included 213 cancer patients received a single dose of Sm-153 EDTMP for palliation of pain pain, in 110/213 cancer patients, the overall therapeutic response was evaluated and elaborated if there is a significant difference in bone pain response depending on gender, type of cancer, patients age, the number of the metastatic bone lesions [10]. Seraing AN, was the first author reported the first prospective, multicentre, double-blind, randomized, and placebo-controlled study to evaluate the efficacy of Sm-153 EDTMP for the palliation of painful bone metastases [11]. Many authors later assessed the therapeutic efficacy and the haematological toxicity of Sm-153 EDTMP for bone pain palliation in cancer patients suffered from multiple skeletal metastases, especially Angelo G [12-21]. Who reported that this therapy may have a therapeutic potential beyond simple palliation of bone pain [22].
In March 1998, intravenous therapy with 153Sm-EDTMP was performed at Department of Nuclear Medicine, University of Vienna, Austria on male patients with metastatic prostate cancer in the spine and the pelvis. The post single therapeutic scintigraphy showed increased tracer uptake in the known metastatic lesions. Tc-99 m DPD scintigraphy performed 7 months later (October1998) revealed complete regression and the PSA level was and still in the normal range(<0.5 mg/ml) with the patients still free of pain, concluded that Sm-153 EDTMP is not only effective in pain palliation, but might also have significant curative action in selected patients with metastatic bone lesions [23]. To complete the previous study but on a wide sample size, we obtained a clinical experience with a single therapeutic dose of Sm-153 EDTMP on 103 cancer patients showed a painless skeletal metastases on conventional bone scintigraphy. This study included patients having been treated at Department of Nuclear Medicine, Medical University of Vienna, Austria, between March 2002 and May 2013.
Out of 103 cancer patients, 33% showed bone progression, 35.9% showed bone stabilization and 32.1% showed bone regression. Breelong A confirmed also that Sm-153 EDTMP may be useful as anti-neoplastic therapy apart from pain palliation in a variety of malignancies. For prostate cancer patients, several phase I and II clinical trials have shown that combined Sm-153 EDTMP and docetaxel-based chemotherapy can result in>50% decrease in prostate-specific antigen with manageable myeloid suppression. Since many patients with metastatic solid tumours’ are end-stage, or go on to receive other systemic therapy after Sm153 EDTMP, few studies evaluate overall or even progression-free survival [24]. In Turner’s initial study of 35 patients (15 prostate, 10 breasts, 10 other) who received a single dose of Sm-153 EDTMP, 15 of 34 patients showed regression or stabilization in bone scans three months after therapy [25]. Two other studies reported similar findings but did not include dosimeter or survival data [26,27]. In our study of 103 patients (62 prostate, 20 breast, 21 others), 50 patients showed bone stabilization/ regression in bone scans 7 months after therapy. In prostate cancerspecific studies, results may be a bit more encouraging. In a study of 32 men with CRPC who received 1.1 mCi / kg 153Sm-EDTMP, 88% of patients had an improved or stable bone scan one month post therapy and 78% of patients had improved or stable scans at 3 months [28]. Another study of 52 patients with CRPC received Sm-153 EDTMP in doses from 0.5-3 mCi, one month following therapy, 36 of 50 men had stable or improved in bone scans, while in our study performed on 62 men with prostate cancer, 20 men showed bone stabilization and 13 men showed improvement in bone response in bone scans after 7 months [29].
Similar strategies in other metastatic solid tumors have been investigated in very few studies. In a recent study of 43 breast cancer patients with osteoplastic or mixed osteoplastic/osteocytes bone lesions who received 1-1.5 mCi/kg Sm-153 EDTMP, where bone scans, three months later, showed improvement in 12% and stable disease in 70% of patients, as compared to our study which was performed on 20 females with breast cancer, 25% showed stabilization and 60% showed improvement in bone scan after 7 months [30].
Reported use of Sm-153 EDTMP in hematologic malignancies falls into two paradigms - low-dose as a single agent for pain control or in combination with radio sensitizing drugs, or high-dose with a myeloablative agent for pre-transplant conditioning [31]. Despite the classical teaching that myeloma bone lesions are primarily osteocytes. Some patients have enhancing lesions on bone scan that possess osteoplastic components, enabling Sm-153 EDTMP uptake, in this study we have only one patient with multiple myeloma showed bone progression on bone scan after receiving the single therapeutic dose of 153Sm-EDTMP 30mci (1.1 Gabs) [32]. Further studies in multiple myeloma with a large sample size may be needed to perform a significant statistical analysis.
In osteosarcoma, Sm-153 EDTMP infusion delivers radiation to multiple unrespectable lesions simultaneously and provides local cytotoxicity without soft tissue damage that can be combined with chemotherapy or radiation [33]. Several studies reported significant improvement in neurologic dysfunction related to spinal cord compression and resolution of patient’s pain that lasted for six months [34-36]. In this study we have one patient aged 19 years old with osteosarcoma showed less than 10 bone lesions in bone scan, after receiving a single therapeutic dose of Sm-153 EDTMP(30 mci), his bone scintigraphy after 7 months showed improvement.
Based on what Vegan et al, have reported, the cumulated activity of administered Sm-153 EDTMP in bone and red marrow are significantly higher in patients of prostate cancer (in which bone metastases are osteoplastic in nature), than in patients with breast cancer (where bone metastases are more of an osteocytes or mixed lytic/plastic component) [37]. As per this finding, the rate of bone regression among male prostate patients showed a statistical significant difference as compared to female breast cancer (P-values<0.05). However, several studies examined the response rate to Sm-153 EDTMP depending on the type of radiopharmaceutical administered, underlying cancer, age, number of metastases, and some other determinants as well as Beaky et al who also confirmed that the type of the underlying malignancy, gender, age and the number of metastases are not predictors of response to therapy [38-44]. Our study revealed a significant improved/stabilized bone scan among younger patients (≤ 64 years old) as compared to the oldest patients as well as in patients showed less than 10 bone lesion in bone scintigraphy (P-values<0.05).
Another recent study confirmed the efficacy of Sm-153 EDTMP as antineoplastic therapy as part of pain palliation in different malignancies, on a 73-year-old woman with a permanent postoperative hyperparathyroidism. They concluded that in case of persistent hypocalcaemia caused by the combination of osteoplastic metastases and a permanent postoperative hyperparathyroidism after failure of the conventional replacement therapy, the administration of radionuclide therapy with Sm-153 EDTMP could be an effective option [45].
Conclusion
The results of this study showed that, as 153Sm-EDTMP offers a riskless and effective treatment option in patients with painful bone metastases, it also provides encouraging results among those with painless skeletal metastases.
References
- Robert EC (2006) Clinical Features of Metastatic Bone Disease and Risk of Skeletal Morbidity. Clin Cancer Res 12: 6243-6249.
- Galasko C, Weiss L, (1981) the anatomy and pathways of skeletal metastases. In Gilbert A (editors) Bone metastases, GKHall, Boston.
- Stoll BA (1983) Natural History. Prognosis and staging of bone metastases. In Szoll BA. Parboo S. (eds), Bone metastases: Monitoring and treatment, Ravein, New York.
- Hoskin PJ (1995) Radiotherapy for bone pain. Pain 63: 137-139.
- Dearnaley DP, Bayly RJ, A’Hern RP, Gadd J, Zivanovic MM, et al. (1992) Palliation of bone metastases in prostate cancer. Hemi body irradiation or strontium-89? Clin Oncol 4: 101-107.
- Goeckeler WF, Edwards B, Volkert WA, Holmes RA, Simon J, et al. (1987) Skeletal localization of samarium‑153 chelates: Potential therapeutic bone agents. J Nucl Med 28: 495‑504.
- Bayouth JE, Macey DJ, Kasi LP, Fossella FV (1994) Dosimetry and toxicity of Sm‑153‑EDTMP administered for bone pain due to skeletal metastases. J Nucl Med 35: 63‑69.
- Tripathi M, Singhal T, Chandrasekhar N, Kumar P, Bal C, et al. (2006) Samarium-153 ethylenediamine tetra methylene phosphate therapy for bone pain palliation in skeletal metastases. Indian J Cancer 43: 86-92.
- Sin zinger H, Weiss K, Hiltune J (2009) Background, reasons and benefits using the Vienna Protocol for the treatment of painful bone recurrences with 153Sm-EDTMP. Anticancer Res 29: 3393-3396.
- Elzahry M, Diab W, Sin zinger H (2017) Assessment of Bone Pain Response in Cancer Patients Receiving Single Dose of Sm-153 EDTMP Therapy. J Nucl Med Radiate Ther 8: 349.
- Serafini AN, Houston SJ, Resche I, Quick DP, Grund FM, et al. (1998) Palliation of Pain Associated With Metastatic Bone Cancer Using Samarium-153 Lexidronam: A Double-Blind Placebo-Controlled Clinical Trial. J Clin Oncol 16: 1574-1581.
- Krishnamurthy G, Krishnamurthy S (2000) Radionuclides for metastatic bone pain palliation: A need for rational re-evaluation in the new millennium. J Nucl Med 41: 688-691.
- Tian J , Cao L, Zhang J, Ouyang Q, Hou Q, et al. (2002) Comparative analysis of patients not responding to a single dose of 153Sm-EDTMP palliative treatment for painful skeletal metastases. Chin Med J 115: 824-828.
- Wang RF, Zhang CL, Zhu SL, Zhu M (2003) A comparative study of 153Samarium-ethylenediaminetetramethylene phosphoric acid with pamidronate disodium in the treatment of patients with painful metastatic bone cancer. Med Princ Pract 12: 97-101.
- Szefer J, Zuchora Z (2004) Radionuclide therapy for painful bone metastases. Wiad Lek 57: 280-283.
- Coronado M, Redondo A, Coya J, Espinosa E, Couto RM, et al. (2006) Clinical role of Sm-153 EDTMP in the treatment of painful bone metastatic disease.Clin Nucl Med 31: 605-610.
- Baczyk M, Czepczyński R, Milecki P, Pisarek M, Oleksa R, et al. (2007) 89Sr versus 153Sm-EDTMP: comparison of treatment efficacy of painful bone metastases in prostate and breast carcinoma. Nucl Med Commun 28: 245-250.
- Dolezal J (2009) Efficacy and toxicity of 153 Samarium-EDTMP in painful breast cancer bone metastases. Onkologie 32: 35-39.
- Iakovou I, Doumas A, Badiavas K, Mpalaris V, Frangos S, et al. (2014) Pain palliative therapy in women with breast cancer osseous metastatic disease and the role of specific serum cytokines as prognostic factors. Cancer Biother Radio pharm 29: 116-123.
- Guerra Liberal FD, Tavares AA, Tavares JM (2016) Palliative treatment of metastatic bone pain with radiopharmaceuticals: A perspective beyond Strontium-89 and Samarium-153. Appl Radiate I sot 110: 87-99.
- Roqué I, Figuls M, Martinez-Zapata MJ, Scott-Brown M, Alonso-Coello P (2017) Radioisotopes for metastatic bone pain. Cochrane Database Syst Rev 23: 3.
- D'angelo G, Sciuto R, Salvatori M, Sperduti I, Mantini G, et al. (2012) Targeted "bone-seeking" radiopharmaceuticals for palliative treatment of bone metastases: a systematic review and meta-analysis. Q J Nucl Med Mol Imaging 56: 538-543.
- Weiss K, Köck HH, Atefie K, Sin zinger H (2001) Complete scintigraphic lesion regression after single 153Sm-EDTMP therapy in prostate cancer. Rev Esp Med Nucl 20: 311-312.
- Breelyn A, Wilky BA, Loeb DM (2013) Beyond Palliation: Therapeutic Applications of 153Samarium-EDTMP. Clin Exp Pharmacol 3:3.
- Turner JH, Claringbold PG, Hetherington EL, Sorby P, Martindale AA (1989) A phase I study of samarium-153 ethylenediaminetetramethylene phosphonate therapy for disseminated skeletal metastases. J Clin Oncol 7: 1926-1931.
- Tripathi M, Singhal T, Chandrasekhar N, Kumar P, Bal C, et al. (2006) Samarium-153 ethylenediamine tetramethylene phosphate therapy for bone pain palliation in skeletal metastases. Indian J Cancer 43: 86-92.
- Liepe K, Runge R, Kotzerke J (2005) The benefit of bone-seeking radiopharmaceuticals in the treatment of metastatic bone pain. J Cancer Res Clin Oncol 131: 60-66.
- Loeb DM, Hobbs RF, Okoli A, Chen AR, Cho S, et al. (2010) Tandem dosing of samarium-153 ethylenediamine tetra methylene phosphoric acid with stem cell support for patients with high-risk osteosarcoma. Cancer 116: 5470-5478.
- Collins C, Eary JF, Donaldson G, Vernon C, Bush NE, et al. (1993) Samarium- 153-EDTMP in bone metastases of hormone refractory prostate carcinoma: a phase I/II trial. J Nucl Med 34: 1839-1844.
- Dolezal J (2009) Efficacy and toxicity of 153samarium-EDTMP in painful breast cancer bone metastases. Onkologie 32: 35-39.
- Goel A, Dispenzieri A, Geyer SM, Greiner S, Peng KW, et al. (2006) Synergistic activity of the proteasome inhibitor PS-341 with non-myeloablative 153-Sm-EDTMP skeletally targeted radiotherapy in an orthotropic model of multiple myeloma. Blood 107: 4063-4070.
- Abruzzese E, Iuliano F, Trawinska MM, Di Maio M (2008) 153Sm: its use in multiple myeloma and report of a clinical experience. Expert Opin Investig Drugs 17: 1379-1387.
- Lattimer JC, Corwin LA Jr, Stapleton J, Volkert WA, Ehrhardt GJ, et al. (1990) Clinical and clinicopathologic response of canine bone tumor patients to treatment with samarium-153-EDTMP. J Nucl Med 31: 1316-1325.
- Bruland OS, Skretting A, Solheim OP, Aas M (1996) Targeted radiotherapy of osteosarcoma using 153 Sm-EDTMP. A new promising approach. Acta Oncol 35: 381-384.
- Franzius C, Bielack S, Flege S, Eckardt J, Sciuk J, et al. (2001) High-activity samarium-153-EDTMP therapy followed by autologous peripheral blood stem cell support in unrespectable osteosarcoma. Nuklearmedizin 40: 215-220.
- Anderson P, Wiseman G, Dispenzieri A, Arndt C, Hartmann L, et al. (2002) High-dose samarium-153 ethylene diamine tetramethylene phosphonate: low toxicity of skeletal irradiation in patients with osteosarcoma and bone metastases. J Clin Oncol 20: 189-196.
- Vigna L, Matheoud R, Ridone S, Arginelli D, Della Monica P, et al. (2011) Characterization of the [(153)Sm]Sm-EDTMP pharmacokinetics and estimation of radiation absorbed dose on an individual basis. Phys Med 27: 144-152.
- Finlay IG, Mason MD, Shelley M (2005) Radioisotopes for the palliation of metastatic bone cancer: a systematic review. Lancet Oncol 6: 392- 400.
- Maxon HR, Schroder LE, Thomas SR, Hertzberg VS, Deutsch EA, et al (1990) Re-186(Sn) HEDP for treatment of painful osseous metastases: initial clinical experience in 20 patients with hormone-resistant prostate cancer. Radiology 176: 155-159.
- Liepe K, Kotzerke J (2007) A comparative study of 188Re-HEDP, 186Re-HEDP, 153Sm-EDTMP and 89Sr in the treatment of painful skeletal metastases. Nucl Med Commun 28: 623-630.
- Baczyk M, Czepczynski R, Milecki P, Pisarek M, Oleksa R, et al (2007) 89Sr versus 153Sm-EDTMP: comparison of treatment efficacy of painful bone metastases in prostate and breast carcinoma. Nucl Med Commun 4:245-250.
- Lewington VJ (1993) Targeted radionuclide therapy for bone metastases. Eur J Nucl Med. 20: 66-74.
- Dickie GJ, Macfarlane D (1999) Strontium and samarium therapy for bone metastases from prostate carcinoma. Australas Radiol 43: 476-479.
- Beiki D, Haddad P, Fallahi B, Keyvan A, Gholamrezanezhad A, et al. (2013) Effectiveness and complications of 153Sm-EDTMP in palliative treatment of diffuse skeletal metastases. Iran J Nucl Med 21:26-32.
- Kassi E, Kapsali I, Kokkinos M, Gogas H (2017) Treatment of severe hypocalcaemia due to osteoblastic metastases in a patient with post thyroidectomy hypoparathyrodism with 153 Sm-EDTMP. BMJ Case Rep.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi