Case Report, Clin Oncol Case Rep Vol: 6 Issue: 7
Unraveling a Rare Complication - Pembrolizumab-Induced Scleroderma: The Index Case in a Breast Cancer Patient
Shiva J Gaddam*, Yousef Hasan, Bohdan Zoshchuk, Gary V Burton
Department of Oncology, Louisiana State University of Health Sciences, Shreveport, USA.
*Corresponding Author: Shiva J Gaddam
Department of Oncology
Louisiana State University of Health Sciences, Shreveport, USA.
E-mail: gaddam@lsuhs.edu
Received: July 15, 2023; Manuscript No: COCR-23-106397;
Editor Assigned: July 17, 2023; PreQC Id: COCR-23-106397 (PQ);
Reviewed: July 22, 2023; QC No: COCR-23-106397 (Q);
Revised: July 24, 2023; Manuscript No: COCR-23-106397 (R);
Published: July 28, 2023; DOI: 10.4172/cocr.6(7).301
Citation: Gaddam SJ, Hasan Y, Zoshchuk B, Burton GV (2023) Unraveling a Rare Complication - Pembrolizumab-Induced Scleroderma: The Index Case in a Breast Cancer Patient. Clin Oncol Case Rep 6:7
Abstract
Pembrolizumab is a Programmed Cell Death protein-1 (PD-1) inhibitor which leads to increased T-cell activity which in turn increases anti-tumor immune response. Although immune-related Adverse Events (irAEs) are well-known complications to ICI therapy, limited evidence is available in the current literature regarding the incidence of scleroderma. We report a case of pembrolizumab-induced limited cutaneous scleroderma in a 37-year-old triple negative breast cancer patient.
Keywords: Pembrolizumab; Breast cancer; Tumor cells; Immune checkpoint inhibitor
Introduction
Programmed Death-Ligand (PD-L1) on tumor cells and programmed cell death protein-1 (PD-1) on T cells play a critical role in regulating immune responses. Pembrolizumab is a humanized IgG4 monoclonal antibody Immune Checkpoint Inhibitor (ICI) directed against cell surface PD-1 on lymphocytes. It blocks the interaction between PD-L1 and PD-1 leading to increased T-cell activity which in turn increases anti-tumor immune response. It has proven to be vital in modern-day cancer therapeutics. Although immunerelated adverse events (irAEs) are well-known complications to ICI therapy, new cutaneous manifestations continue to emerge, and limited evidence is available in the current literature regarding the incidence of scleroderma. We report a case of pembrolizumab-induced limited cutaneous scleroderma in a 37-year-old triple negative breast cancer patient.
Case Presentation
A previously healthy 37-year-old African American female was diagnosed with Triple Negative Breast Cancer (TNBC) stage III. As per the national standard guidelines, she was started on neoadjuvant chemotherapy along with pembrolizumab every 3 weeks.
After the 3rd dose of pembrolizumab, the patient reported swelling and tightness in her fingers, with stiffness at the interphalangeal joints bilaterally. On physical examination, she had skin thickening and tightness involving her fingers bilaterally (Figure 1). Range of motion of her interphalangeal and metacarpophalangeal joints was limited due to stiffness. She did not have similar complaints in her feet, nor did she have other systemic manifestations.
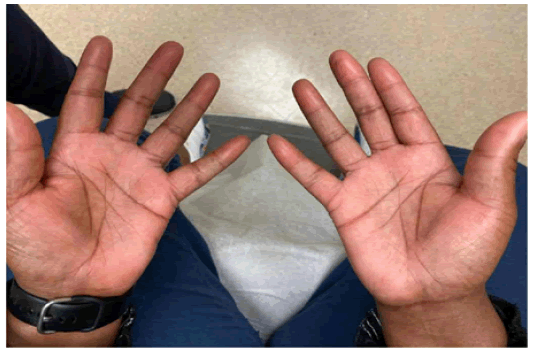
Figure 1: The bilateral pointy, thickened and swollen fingers, most notably at the fingertips.
She was referred to dermatology and a punch biopsy was obtained of her right index finger, which showed mild dermal fibrosis with thickened collagen bundles (Figure 2 A and B).
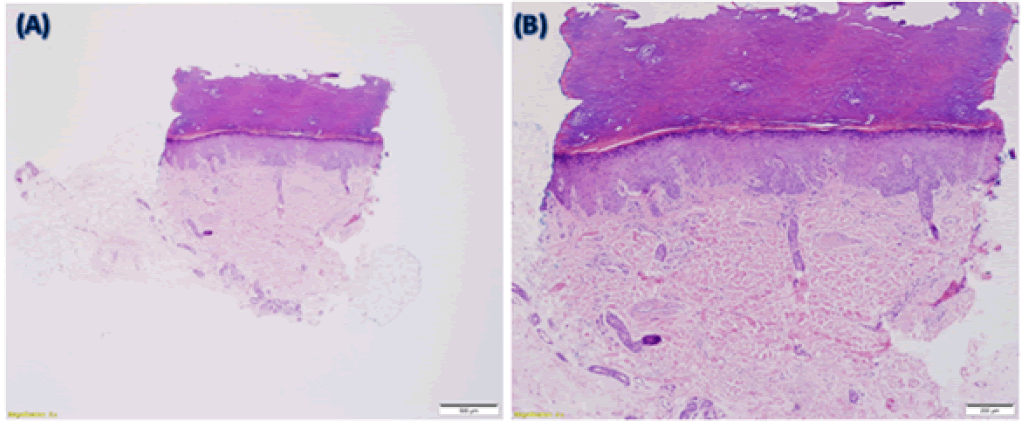
Figure 2: (A) 2x magnified image showing the overall appearance of the punch biopsy. (B) 4x magnified image demonstrating the dermis. Both are stained with Hematoxylin and Eosin (H&E). Sections show acral skin. The epidermis and the overlying stratum corneum are unremarkable. Within the mid-reticular dermis, there are thickened, hyalinized collagen bundles with associated edema. The dermis is hypocellular. Colloidal iron and elastin stain images are unavailable, but colloidal iron stain showed minimally increased dermal mucin. Elastic stain showed decreased fragmented elastic fibers within the reticular dermis.
Considering the clinical correlation with history, physical examination findings, and the dermatopathology results, a diagnosis of localized scleroderma was made. Due to the temporal relationship with the treatment, it was thought to be secondary to immunotherapy and further treatment with pembrolizumab was held. She was started on oral prednisone 40mg daily with improvement noted within 1 week. Because she developed an irAE early in the treatment course, it was felt that continuation posed an excess risk for permanent injury. Our concerns were discussed with the patient, and pembrolizumab was permanently discontinued per the patient’s wishes. She had complete resolution of her symptoms at 6 weeks after stopping the drug. She continued and completed neoadjuvant chemotherapy without any complications, strengthening our hypothesis that the side effect was secondary to ICI.
Discussion
Various cutaneous manifestations of Immune Checkpoint Inhibitors (ICIs) have been described in the literature, including lichenoid, bullous, urticarial, eczematous, macular, morbilliform, and psoriasiform lesions [1]. However, data on localized scleroderma (or morphea) is scarce.
Very few cases of ICI-induced morphea have been reported in the literature, and the majority of them were secondary to nivolumab, and mostly in melanoma and lung cancer patients [2]. The incidence of morphea in patients receiving pembrolizumab is not well established. To our knowledge, this is the fifth reported case of pembrolizumab-induced morphea, and the index case for a patient receiving it for breast cancer.
Morphea is an uncommon inflammatory condition of the skin and the subcutaneous tissue. The affected skin is typically shiny and tight, and the involvement is usually symmetric with restricted movement. The inciting pathophysiology is thought to be T-cell activation, resulting in the increased CD4+ T-cell-mediated release of cytokines (IL-2, IL-4, IL-6 and the IL2 receptor). This further causes increase in Transforming Growth Factor beta (TGF beta) and Platelet Derived Growth Factor (PDGF) which leads to progressive fibrosis [3].
The exact mechanism causing scleroderma in patients treated with pembrolizumab is not well established. PD1, and the ligands PDL1 and PDL2 have a regulatory role in the inhibition of T cell activation. It is thus hypothesized that by inhibiting PD1, Pembrolizumab leads to the loss of this PD1- mediated inhibitory regulation of T cell immune activity [4, 5]. As a result, cytokines and growth factors contributing to sclerosis are upregulated, resulting in scleroderma.
Severity of the manifestations may vary among patients, ranging from mild localized symptoms to full-blown diffuse systemic sclerosis [2]. Consensus guidelines for the management of scleroderma in patients receiving ICI are lacking, considering the rarity of the phenomenon. Some experts recommend treating it as any other cutaneous irAE. Accordingly, grade 1-2 scleroderma can be treated with topical and/or systemic steroids, and the ICI treatment can be continued with close monitoring for worsening symptoms. If the irAE is at grade 3 or higher, systemic steroids and/or other immunosuppressant modalities can be used, and ICI therapy should be permanently discontinued [6]. However, there is a lack of high-quality evidence supporting the extrapolation of this recommendation primarily made for other cutaneous irAEs [6], to scleroderma.
Conclusion
Our case emphasizes the need for clinicians to be vigilant of scleroderma as an AE while using ICIs. Although the risk is higher in patients with a known history of the disease, it can also develop in patients with no previous history (as was the case in our patient). Moreover, the clinical presentation of morphea can overlap with that of concurrent chemotherapy induced neuropathy. Hence, a detailed history and physical exam is of utmost importance. Prompt dermatology/rheumatology referrals may be warranted for an accurate diagnosis and comanagement. Further data is required to formulate optimal approaches for managing this complication.
References
- Shen J, Chang J, Mendenhall M, Cherry G, Goldman JW, et al. (2018) Diverse cutaneous adverse eruptions caused by anti-programmed cell death-1 (PD-1) and anti-programmed cell death ligand-1 (PD-L1) immunotherapies: Clinical features and management. Therap Adv Med Oncol 10: 1758834017751634. [Google Scholar] [Cross Ref]
- Terrier B, Humbert S, Preta LH, Delage L, Razanamahery J, et al. (2020) Risk of scleroderma according to the type of immune checkpoint inhibitors. Autoimmunity Rev 19: 102596. [Google Scholar] [Cross Ref]
- Rongioletti F, Ferreli C, Atzori L, Bottoni U, Soda G (2018) Scleroderma with an update about clinico-pathological correlation. Organo Ufficiale, Soc Ital Dermat Sifilog 153: 208-215. [Google Scholar] [Cross Ref]
- Panariello L, Fattore D, Annunziata MC, Piantedosi F, Gilli M, et al. (2018) Bullous pemphigoid and nivolumab: Dermatologic management to support and continue oncologic therapy. Europ J Cancer 103: 284-286. [Google Scholar] [Cross Ref]
- Dai S, Jia R, Zhang X, Fang Q, Huang L (2014) The PD-1/PD-Ls pathway and autoimmune diseases. Cellular Immunol 290: 72-79. [Google Scholar] [Cross Ref]
- Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, et al. (2018) National comprehensive cancer network management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol 36: 1714-1768. [Google Scholar] [Cross Ref]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 