Case Report, Clin Oncol Case Rep Vol: 6 Issue: 4
Utilization of Dual Immunotherapy for Metastatic Pulmonary Pleomorphic Giant Cell Carcinoma: A Case Report
Breanna Hinman*, Godsfavour Umoru, Ethan Burns, Cynthia El Rahi, Jun Zhang
Department of Medical Oncology, Houston Methodist Hospital, 6565 Fannin St. DB 1-09, Houston, TX 77030, United States
*Corresponding Author: Breanna Hinman
Department of Medical Oncology,, Houston Methodist Hospital, 6565 Fannin St. DB 1-09, Houston, TX 77030, United States.
E-mail: bshinman@houstonmethodist.org
Received: March 28, 2023; Manuscript No: COCR-23-93329;
EditorAssigned: March 30, 2023; PreQC Id: COCR-23-93329 (PQ);
Reviewed: April 07, 2023; QC No: COCR-23-93329 (Q);
Revised:April 14, 2023; Manuscript No: COCR-23-93329 (R);
Published: April 20, 2023; DOI: 10.4172/cocr.6(4).284
Citation: Hinman B, Umoru G, Burns E, Rahi CE, Zhang J (2023) Utilization of Dual Immunotherapy for Metastatic Pulmonary Pleomorphic Giant Cell Carcinoma: A Case Report. Clin Oncol Case Rep 6:4
Abstract
Introduction: Pulmonary Pleomorphic Carcinomas (PPC) are a rare histologic subgroup of sarcomatoid Non-Small Cell Lung Carcinomas (NSCLC). They are characterized by more aggressive growth and elicit a poorer response to antitumor chemotherapy. Furthermore, PPC is associated with an increased likelihood of early metastasis as well as a high risk of relapse. Here, we report the clinical course of a patient with PPC treated with dual immune checkpoint inhibitor therapy and highlight the partial response observed with this approach.
Case Presentation: A 48-year-old male presented with metastatic high-grade pleomorphic giant cell carcinoma.
The liquid biopsy from the original brain lesion revealed no actionable mutations. Next generation sequencing results showed PD-L1 50% and Tumor Mutational Burden (TMB) of 13 mutations per megabase. He was initiated on nivolumab 3 milligrams (mg)/kilogram (kg) every 2 weeks and ipilimumab 1 mg/kg every 6 weeks in a 42-day cycle. He also underwent Stereotactic Radiosurgery (SRS) to the brain lesions. After completing two cycles of treatment, his repeat PET scan showed an overall interval partial response.
Conclusion: Dual immune-checkpoint inhibitor therapy is effective for the treatment of PPC and prospective clinical trials are warranted.
Keywords: Pulmonary pleomorphic carcinoma; PD-L1 expression; PD-1 inhibitors; PD-L1 inhibitors; Tumor mutational burden
Introduction
PPC is an uncommon subgroup of Non-Small Cell Lung Cancer (NSCLC), comprising less than one percent of cases [1]. Tobacco smoking and advanced age are risk factors for the development of PPC, and compared to other histologic classifications of NSCLC, pleomorphic carcinomas are characterized by more highly aggressive growth patterns, poorer response to systemic chemotherapy regimens, increased likelihood of early metastasis, and ultimately dismal outcomes [1,2]. At the time of this report, there are no known randomized clinical trials that have evaluated specific antineoplastic regimens for PPC, and treatment options are limited to retrospective studies and case reports. Given the paucity of specific chemotherapy options for PPC in available guidelines, there is a strong need for the exploration of efficacious treatment strategies in this patient population. Herein, we report the clinical course of a patient with PPC treated with dual immune checkpoint inhibitor therapy and highlight the partial response observed with this approach.
Case Presentation
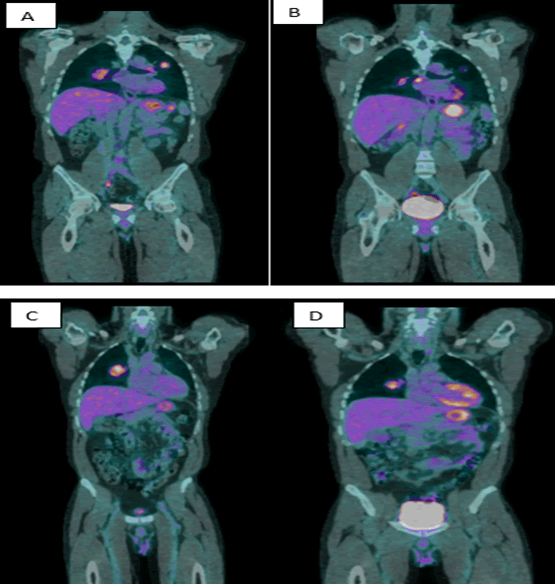
A 48-year-old male with a 40 pack-year smoking history and family history of hepatocellular carcinoma presented with seizure activity. Computed Tomography (CT) of the head and neck revealed a left temporo-occipital mass with perilesional edema. A full body Positron Emission Tomography (PET)/CT showed a dominate right upper lung mass measuring 6.4 x 4.3 cm and demonstrated a Standardized Uptake Value (SUV) of 6.9. Moreover, there were other bilateral pulmonary nodules and suspicious subcarinal adenopathy concerning for metastatic disease (Figure 1 A to C). The patient underwent a left temporal parietal craniotomy and resection of the intracranial mass. Initial suspicions were for a primary lung malignancy given the dominate lesion. However, immunohistochemical screening, positive staining for CK7, S100P, CK19, CK8/18, GATA3, and Inhibin, also suggested possible urothelial carcinoma, adrenal cortical carcinoma, and/or upper gastrointestinal/pancreatobiliary tract carcinoma in the differential diagnosis. He later received gamma knife Stereotactic Radiosurgery (SRS) to the right insula and to the postoperative resection cavity. He underwent endobronchial ultrasound with biopsy of a station 11R lymph node. Histopathologic examination revealed a high-grade pleomorphic giant cell carcinoma. Immunohistochemistry screening demonstrated 2% PD-L1 expression on tumor cells and a negative HER-2 overexpression score of 0. The tumor cells were positive for CK AE1/AE3, CK7, CK19, CK8/18, S100, GATA3, and Inhibin. Notably, tumor cells did not stain for CK20, PAX8, p63, p40, TTF-1, Napsin, ALK, PD-L1, androgene, calretinin, WT1, CEA, CDX2, OCT3/4, Arginase, HepPar, BHCG, SAL4, or CD117. Moreover, the brain lesion was absent for PD-L1 expression, and the liquid biopsy revealed no actionable mutations. He was ultimately started on nivolumab 3 milligram(mg)/kilogram(kg) every 2 weeks and ipilimumab 1 mg/kg every 6 weeks in a 42-day cycle. With regard to the duration of therapy, a total of 4 cycles of dual immunotherapy was initially planned. During the work-up for intermittent left amaurosis fugax, a transthoracic echocardiogram showed a large complex mitral mass with 2 separate components measuring up to 4cm in length. The mass was not biopsied, and the patient wasinitiated on therapeutic enoxaparin for two months. The cardiac mass finding did not result in any delay to ICI treatment. A repeat transthoracic echocardiogram 2 months later revealed a significant decrease in size (from 4 cm to 1 cm). After completion of cycle 2, next generation sequencing results showed PD-L1 50% and Tumor Mutational Burden (TMB) of 13 mutations per megabase. However, a repeat brain MRI showed a new metastatic lesion at the posterior right parietal lobe for which the patient received additional SRS. After completing two cycles of treatment, his repeat PET scan showed an overall interval partial response with a stable right suprahilar mass while the other pulmonary lesions showed an interval decrease in size (Figure 1 B, D).
Figure 1: PET scan at diagnosis and after two cycles of dual ICI, coronal views. (A). 2.3 cm left upper lobe pulmonary nodule with SUV of 5.0. A 9 mm short axis right sided subcarinal lymph node demonstrates SUV of 2.7. (B). 1.4 cm left upper lobe nodule demonstrates an SUV of 3, decreased in size and avidity. The right subcarinal lymph node demonstrates an SUV of 6.6 and is now 1.3 cm x 0.7 cm in size. (C). Dominate right medial upper lobe nodule, 6.4 cm, with SUV of 6.9. (D). Necrotic right suprahilar measures 4.3 cm, with an SUV of 6.3.
The patient returned to his home country after receiving Cycle three, Day fifteen of nivolumab and was hospitalized for a traumatic head injury which resulted in a two-month delay of ICI treatment. A PET scan completed two weeks post resumption of nivolumab showed progressive disease. He was then switched to therapy with MAID (doxorubicin 15 mg/m2 days 1-4, dacarbazine 250 mg/m2 days 1-4, ifosfamide 2000 mg/m2 days 1-3, and mesna). The patient transferred care back to our institution after receiving 3 cycles of MAID. His therapy was subsequently modified to omit dacarbazine and the doses of ifosfamide and doxorubicin were changed to 2000 mg/m2 on days 1-4 and 25 mg/m2 on days 1-3 respectively. PET imaging after cycle 4 showed continued improvement in the right suprahilar mass, mediastinal metastases, and bilateral pulmonary nodules with no evidence of new visceral metastases. Although there was no adequate detection of FDG within the CNS, brain MRI showed a progressive enhancing lesion at the left parietal lobe. At this time, the plan is for the patient to complete two more cycles of MAI and receive SRS for the progressive parietal lesion.
Discussion
This is a unique case of a young patient presenting with metastatic PPC that had a partial response to dual therapy with the Immune Checkpoint Inhibitor (ICI) agents nivolumab and ipilimumab. PPC is a rare histology that is often resistant to conventional chemotherapy with an Objective Response Rate (ORR) of 17% and Progression- Free Survival (PFS) of 2 months with first-line chemotherapy [3]. Patients with this histology often have a poor prognosis with a median overall survival between 5-10 months [3-7]. Moreover, guidelines do not provide any specific guidance for PPC, and these carcinomas are typically treated like other histologic subtypes of NSCLC. A retrospective study by Karim and colleagues showed no significant difference in survival of patients treated with systemic chemotherapy alone compared to patients that did not receive any treatment [8]. Thus, novel treatment strategies are needed to address this unmet need.
ICIs have demonstrated significant efficacy and tolerable safety profiles across the spectrum of NSCLC histologic subtypes. A recognized predictor of ICI response is the degree of programmed death-ligand 1 (PD-L1) expression, although this response varies across tumor types. PPC histology has been shown to display PDL1 expression at a higher rate than other NSCLC histologies. Kim et al. reported that 90.2% of patients with pulmonary pleomorphic carcinomas had positive PD-L1 expression [9, 10]. Chang et al. reported that 70.5% of PPC patients had high PD-L1 expression [11]. Although uncommon NSCLC histologies have been included in large clinical trials, specific characterization is infrequently reported [12-13]. Retrospective studies, case reports, and case series (Table 1) have shown that ICI therapy utilized as either monotherapy or in combination with chemotherapy offers promising clinical efficacy for patients who highly express PD-L1 (PD-L1 ≥50%) [14-30]. Miyashita et al. performed a retrospective analysis that included propensity score-matched patients with common NSCLC histology and uncommon histology, including 10 patients with PPC treated with ICI monotherapy [28]. Results from this study found that median ORR, PFS, and OS were not significantly different based on the dichotomization of common versus uncommon histology groups. Subgroup analysis of pleomorphic carcinoma showed a median PFS and OS were 7.7 months and 9.5 months, respectively, and was similar to the primary group [28]. Domblides et al. performed a retrospective study of 37 patients with pulmonary sarcomatoid carcinoma with a median PD-L1 expression of 70% that received ICI as second-line therapy or beyond [29]. The ORR and median OS were 40.5% and 12.7 months, respectively. The ORR was higher in patients who were PD-L1 positive [29]. Lee et al. performed a retrospective analysis of 49 pulmonary pleomorphic carcinoma that were treated with PD-1/ PD-L1 inhibitor therapy [30]. The ORR, median PFS, and OS were 49.0%, 7.2 months, and 22.2 months, respectively [30]. The outcome differences between the results by Domblides and Lee et al. may be due to differences in the number of patients included with high PDL1 expression.
Table 1: ICI therapy utilized as either monotherapy or in combination with chemotherapy offers promising clinical efficacy for patients who highly express PD-L1.
| Case Reports and Case Series | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author/Year | Age/sex | Ever Smoker | Histologic Subtype | Stage | PD-L1 TPS (%) | Surgery | Radiation | Prior Lines of Treatment | Treatment | Response | Duration of ICI treatment | irAE | Ref | ||||||||||||
| Kanazu et al., 2017 | 66/M | NR | Pleomorphic | IV | ≥95 | NR | Y | 1 | Nivolumab | PD | 6 cycles/ | NR | [13] | ||||||||||||
| discontinued | |||||||||||||||||||||||||
| Kanazu et al., 2017 | 59/F | NR | Pleomorphic | IIIA | 80-90 | N | NR | 2 | Nivolumab | PR | 19 cycles/ | NR | [13] | ||||||||||||
| ongoing | |||||||||||||||||||||||||
| Kanazu et al., 2017 | 83/M | NR | Pleomorphic | IIIA | 60-70 | N | N | 3 | Nivolumab | PR | 10 cycles/ | NR | [13] | ||||||||||||
| ongoing | |||||||||||||||||||||||||
| Marchand et al., 2017 | 55/M | Y | Pleomorphic | IV | 90 | N | N | 1 | Nivolumab | PR | 9 cycles/ | Diabetes mellitus, | [14] | ||||||||||||
| discontinued | Hypophysitis | ||||||||||||||||||||||||
| Senoo et al., 2019 | 62/M | Y | Pleomorphic | IV | 70 | N | N | 1 | Nivolumab | PR | 12 cycles/ | Hypophysitis | [15] | ||||||||||||
| ongoing | |||||||||||||||||||||||||
| Ito et al., 2016 | 67/M | Y | Pleomorphic | IV | ≥50 | N | N | 6 | Nivolumab | PR | 8 cycles/ | N | [16] | ||||||||||||
| ongoing | |||||||||||||||||||||||||
| Salati et al., 2018 | 74/M | N | Pleomorphic | IV | ≥50 | Prior to ICI | N | 2 | Nivolumab | PR | 28 months/ | Grade 1 Rash | [17] | ||||||||||||
| ongoing | |||||||||||||||||||||||||
| Okamura et al., 2018 | 75/M | Y | Pleomorphic | IV | 90 | N | N | 2 | Nivolumab | CR | 3 cycles/ | NR | [18] | ||||||||||||
| ongoing | |||||||||||||||||||||||||
| Tozuka et al., 2018 | 82/M | Y | Pleomorphic | IIB | 75 | Prior to ICI | N | 0 | Pembrolizumab | PR | 3 cycles/ | Agranulocytosis, | [19] | ||||||||||||
| discontinued | Grade 1 pneumonitis, | ||||||||||||||||||||||||
| Ocular myasthenia gravis | |||||||||||||||||||||||||
| Matsumoto et al., 2017 | 51/M | Y | Pleomorphic | IVB | ≥50 | Prior to ICI | N | 0 | Pembrolizumab | PR | 3 cycles/ | N | [20] | ||||||||||||
| ongoing | |||||||||||||||||||||||||
| Roesel et al., 2019 | 57/M | NR | Sarcomatoid | IV | 80-90 | Prior to ICI | N | 1 | Nivolumab | CR | 3 months/ | NR | [21] | ||||||||||||
| ongoing | |||||||||||||||||||||||||
| Roesel et al., 2019 | 60/M | NR | Sarcomatoid | IV | 100 | Prior to ICI | N | 1 | Nivolumab | PR | 8 months/ | NR | [21] | ||||||||||||
| ongoing | |||||||||||||||||||||||||
| Kakimoto et al., 2019 | 69/F | Y | Giant cell | IV | 75 | Post ICI | Y | 0 | Pembrolizumab | CR | 4 cycles/ | Renal dysfunction, | [22] | ||||||||||||
| discontinued | Adrenal insufficiency | ||||||||||||||||||||||||
| Ikematsu et al., 2017 | 71/M | Y | Sarcomatoid | IV | 80 | N | N | 0 | Pembrolizumab | PR | 5 cycles/ | N | [23] | ||||||||||||
| ongoing | |||||||||||||||||||||||||
| Hayashi et al., 2021 | 73/M | NR | Pleomorphic | IV | 80-90 | Prior to ICI | Y | 0 | Pembrolizumab | NR | 2 cycles | NR | [24] | ||||||||||||
| Hayashi et al., 2021 | 66/M | NR | Pleomorphic | IIIA | 90-100 | N | N | 0 | Pembrolizumab/Carboplatin/ nab-paclitaxel àpembrolizumab maintenance | PR | 4 cycles/ | NR | [24] | ||||||||||||
| ongoing | |||||||||||||||||||||||||
| Hayashi et al., 2021 | 49/M | NR | Pleomorphic | IV | 60-70 | N | Y | 1 | Pembrolizumab | PR | 11 months/ | NR | [24] | ||||||||||||
| ongoing | |||||||||||||||||||||||||
| Rajdev et al., 2018 | 56/F | Y | Sarcomatoid | IIB | Unknown | N | Y | 1 | Nivolumab | PR | Ongoing | NR | [25] | ||||||||||||
| Sukrithan et al., 2019 | 46/M | Y | Pleomorphic | IV | 90 | N | Y | 2 | Pembrolizumab | PR | 22 months/ | NR | [26] | ||||||||||||
| discontinued (death) | |||||||||||||||||||||||||
| Sukrithan et al., 2019 | 64/F | Y | Pleomorphic | IV | 80 | N | Y | 0 | Pembrolizumab | PR | 11 months/ | NR | [26] | ||||||||||||
| ongoing | |||||||||||||||||||||||||
| Sukrithan et al., 2019 | 57/M | Y | Sarcomatoid | IV | 100 | N | N | 0 | Pembrolizumab | SD | 8 months/ | NR | [26] | ||||||||||||
| ongoing | |||||||||||||||||||||||||
| Sukrithan et al., 2019 | 67/F | Y | Sarcomatoid | IV | >75 | N | N | 0 | Pembrolizumab | PR | 7 months/ | NR | [26] | ||||||||||||
| ongoing | |||||||||||||||||||||||||
| F: Female; M: Male; Y: Yes; N: No; NR: Not reported; ICI: Immune Checkpoint Inhibitor; PD-L1: Programmed Death Ligand 1; TPS: Tumor Proportion Score (TPS); CR: Complete Response; PR: Partial Response; SD: Stable Disease; PD: Progressive Disease; irAE: Immune-related Adverse Events; Ref: Reference | |||||||||||||||||||||||||
| Retrospective Cohort and Observational Studies | |||||||||||||||||||||||||
| Author/Year | Population Studied | n | Men | Ever Smoker | Stage | Prior Lines of Treatment | PD-L1 TPS | Treatment | ORR | Median PFS in PPC Patients | Median OS in PPC Patients | irAE | Ref | ||||||||||||
| Miyashita et al., 2021 | Common and uncommon NSCLC histologies | 44 | 95% | 95% | III: 32% | 2: 50% | ≥50%: 39% | Nivolumab: 50% | NR | 7.7 months | 9.5 months | 8 patients experienced an irAE | [27] | ||||||||||||
| (10 PPC patients) | IV: 48% | ≥3: 34% | 1–49%: 23% | Pembrolizumab: 36% | |||||||||||||||||||||
| Not available: 25% | |||||||||||||||||||||||||
| Domblides et al., 2020 | Pulmonary sarcomatoid carcinoma | 37 | 73% | 95% | III: 32% | 2: 54% | Median PD-L1: 70% | Nivolumab: 87% | 40.50% | NR | 12.7 months | 6 patients experienced an irAE | [28] | ||||||||||||
| IV: 46% | ≥3: 46% | Pembrolizumab: 8% | |||||||||||||||||||||||
| Lee et al., 2020 | Pulmonary pleomorphic carcinoma | 49 | 73.50% | 73.50% | III: 27% | 2: 77% | ≥50%: 83.7% | Nivolumab: 14.3% | 49% | 7.2 months | 22.2 months | 13 irAE reported | [29] | ||||||||||||
| IV: 60% | ≥3: 18% | Pembrolizumab: 81.6% | |||||||||||||||||||||||
| NR: Not reported; PD-L1: Programmed Death Ligand 1; TPS: Tumor Proportion Score (TPS); ORR: Objective Response Rate; PRF: Progression-Free Survival; OS: Overall Survival; irAE: Immune-related Adverse Events; Ref: Reference | |||||||||||||||||||||||||
TMB is another important cancer biomarker that has been utilized to predict responsiveness to ICI. Like PD-L1 expression, TMB can vary between patients and cancers. High TMB (TMB-H) is associated with a higher proportion of patients with better response to ICI monotherapy compared to patients with non-TMB-H carcinomas [31]. Dual ICI therapy has also been associated with improved clinical outcomes in patients with NSCLC and TMB-H [32]. A genomic profiling study of 125 PPCs by Schrock et al demonstrated intermediate to high TMB status in 43% of patients, showing this is an important biomarker of interest in this patient population [33]. There are fewer data available evaluating TMB status and ICI responsiveness in PPC patients compared with PD-L1 expression. A case report and a retrospective cohort study have demonstrated that patients with TMB-H status may have higher response rates to ICI therapy [29]. Domblides et al. included 8 patients who had TMB status assessed [29]. The median TMB was 18 mutations per megabase, with 87.5% of the cases displaying a high TMB. This study found a higher TMB was associated with a trend toward higher survival, with a median 18-month OS in TMB-H populations versus a 1.8-month OS in low TMB populations.
This is the first known report describing the utilization of dual ICI therapy in a patient with metastatic pulmonary pleomorphic giant cell carcinoma. Partial response was the best response achieved. Akin to the previously described literature, our patient had PD-L1 expression of 50% and a Tumor Mutational Burden (TMB) of 13 mutations per megabase, which may have culminated in the partial response observed. It is important to highlight the differences in PD-L1 expression between the CNS lesion, lymph node, and NGS results for this patient. These differences are reflective of the known heterogeneity of a tumor specimen. Although we were unable to determine if the eventual progression on immunotherapy was due to the 2-month delay in therapy while the patient was hospitalized for a traumatic head injury, we found the partial response to be encouraging given the poor response of this disease state to traditional chemotherapy options.
Conclusion
There is an unmet need to fi nd no vel tr eatment st rategies fo r PPC, a subtype of NSCLC that is notoriously resistant to conventional chemotherapy, has a paucity of standardized recommendations for clinical management, and a strong under representation in clinical trials. This study adds to the limited literature regarding the potential benefit of adding ICI therapy in PD-L1 high expressing PPCs and is to our knowledge, the first employing the utilization of dual ICI therapy. Prospective studies are needed to corroborate findings limited by case reports and case series, and there is also a continued need to identify potentially targetable biomarkers to guide treatment or predict therapeutic response.
Availability of Data and Materials
Data sharing not applicable to this case report as no datasets were generated or analyzed for this study.
Conflicts of Interests
The authors declare that there is no conflict of interest regarding the publication of this article.
Funding
No funding.x
References
- Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JH, et al. (2015) The 2015 World Health Organization classification of lung tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J Thor Oncol 10: 1243-1260. [Google Scholar] [Cross Ref]
- Yendamuri S, Caty L, Pine M, Adem S, Bogner P, et al. (2012) Outcomes of sarcomatoid carcinoma of the lung: A surveillance, epidemiology, and end results database analysis. Surg 152: 397-402. [Google Scholar] [Cross Ref]
- Vieira T, Girard N, Ung M, Monnet I, Cazes A, et al. (2013) Efficacy of first-line chemotherapy in patients with advanced lung sarcomatoid carcinoma. J Thor Oncol 8:1574-1577. [Google Scholar] [Cross Ref]
- Fishback NF, Travis WD, Moran CA, Guinee Jr DG, McCarthy WF, et al. (1994) Pleomorphic (spindle/giant cell) carcinoma of the lung. A clinicopathologic correlation of 78 cases. Cancer 73: 2936-2945. [Google Scholar] [Cross Ref]
- Bae HM, Min HS, Lee SH, Kim DW, Chung DH, et al. (2007) Palliative chemotherapy for pulmonary pleomorphic carcinoma. Lung Can 58: 112-115. [Google Scholar] [Cross Ref]
- Hong JY, Choi MK, Uhm JE, Park MJ, Lee J, et al. (2009) The role of palliative chemotherapy for advanced pulmonary pleomorphic carcinoma. Med Oncol 26: 287-291. [Google Scholar] [Cross Ref]
- Tamura Y, Fujiwara Y, Yamamoto N, Nokihara H, Horinouchi H, et al. (2015) Retrospective analysis of the efficacy of chemotherapy and molecular targeted therapy for advanced pulmonary pleomorphic carcinoma. BMC Res Notes 8: 1-7. [Google Scholar] [Cross Ref]
- Karim NA, Schuster J, Eldessouki I, Gaber O, Namad T, et al. (2018) Pulmonary sarcomatoid carcinoma: University of Cincinnati experience. Oncotarget 9: 4102. [Google Scholar] [Cross Ref]
- Kim S, Kim MY, Koh J, Go H, Lee DS, et al. (2015) Programmed death-1 ligand 1 and 2 are highly expressed in pleomorphic carcinomas of the lung: Comparison of sarcomatous and carcinomatous areas. Europ J Can 51: 2698-2707. [Google Scholar] [Cross Ref]
- Chang YL, Yang CY, Lin MW, Wu CT, Yang PC (2016) High co-expression of PD-L1 and HIF-1α correlates with tumour necrosis in pulmonary pleomorphic carcinoma. Eur J Cancer 60: 125-135. [Google Scholar] [Cross Ref]
- Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, et al. (2018) Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. New Eng J Med 378: 2288-2301. [Google Scholar] [Cross Ref]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, et al. (2018) Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. New Eng J Med 378: 2078-2092. [Google Scholar] [Cross Ref]
- Kanazu M, Uenami T, Yano Y, Nakatsubo S, Hosono Y, et al. (2017) Case series of pleomorphic carcinomas of the lung treated with nivolumab. Thor Can 8: 724-728. [Google Scholar] [Cross Ref]
- Marchand L, Paulus V, Fabien N, Perol M, Thivolet C, et al. (2017) Nivolumab-induced acute diabetes mellitus and hypophysitis in a patient with advanced pulmonary pleomorphic carcinoma with a prolonged tumor response. J Thor Oncol 12: e182-e184. [Google Scholar] [Cross Ref]
- Senoo S, Ninomiya T, Makimoto G, Nishii K, Kano H, et al. (2019) Rapid and long-term response of pulmonary pleomorphic carcinoma to nivolumab. Inter Med 58: 985-989. [Google Scholar] [Cross Ref]
- Ito K, Hataji O, Katsuta K, Kobayashi T, Gabazza EC, et al. (2016) “Pseudoprogression” of pulmonary pleomorphic carcinoma during nivolumab therapy. J Thor Oncol 11: e117-e119. [Google Scholar] [Cross Ref]
- Salati M, Baldessari C, Calabrese F, Rossi G, Pettorelli E, et al. (2018) Nivolumab-induced impressive response of refractory pulmonary sarcomatoid carcinoma with brain metastasis. Case Rep Oncol 11: 615-621. [Google Scholar] [Cross Ref]
- Okamura K, Fukuda Y, Soda H, Ogawara D, Iwasaki K, et al. (2018) Pulmonary pleomorphic carcinoma with few PD‐1‐positive immune cells and regulatory T cells that showed a complete response to nivolumab. Thora Can 9: 193-196. [Google Scholar] [Cross Ref]
- Tozuka T, Sugano T, Noro R, Takano N, Hisakane K, et al. (2018) Pembrolizumab-induced agranulocytosis in a pulmonary pleomorphic carcinoma patient who developed interstitial lung disease and ocular myasthenia gravis. Oxford Med Case Rep 2023. [Google Scholar] [Cross Ref]
- Matsumoto Y, Miura T, Horiuchi H, Usui K (2017) The successful treatment of pulmonary pleomorphic carcinoma with pembrolizumab: A case report. Case Rep Oncol 10: 752-757. [Google Scholar] [Cross Ref]
- Roesel C, Kambartel K, Kopeika U, Berzins A, Voshaar T, et al. (2019) Lazarus-type tumour response to therapy with nivolumab for sarcomatoid carcinomas of the lung. Current Oncol 26: 270-273. [Google Scholar] [Cross Ref]
- Kakimoto T, Sasaki M, Yamamoto T, Iwamaru A, Ogata K, et al. (2019) A histologically complete response to immunotherapy using pembrolizumab in a patient with giant cell carcinoma of the lung: an additional report and literature review. Case Rep Oncol Med 2019. [Google Scholar] [Cross Ref]
- Ikematsu Y, Yoneshima Y, Ijichi K, Tanaka K, Harada T, et al. (2017) Marked response to pembrolizumab in a patient with pulmonary pleomorphic carcinoma highly positive for PD-L1. Lung Cancer 112:230-231. [Google Scholar] [Cross Ref]
- Hayashi K, Tokui K, Inomata M, Azechi K, Mizushima I, et al. (2021) Case series of pleomorphic carcinoma of the lung treated with immune checkpoint inhibitors. In Vivo 35: 1687-1692. [Google Scholar] [Cross Ref]
- Rajdev K, Siddiqui AH, Ibrahim U, Agarwal S, Ding J, et al. (2018) Sarcomatoid carcinoma of the lung presenting as localized bronchiectasis: A case report and review of literature. Respirat Med Case Rep 24:143-146. [Google Scholar] [Cross Ref]
- Sukrithan V, Sandler J, Gucalp R, Gralla R, Halmos B (2019) Immune checkpoint blockade is associated with durable responses in pulmonary sarcomatoid carcinoma. Clin Lung Can 20: e242-e246. [Google Scholar] [Cross Ref]
- Miyashita K, Karayama M, Inoue Y, Hozumi H, Suzuki Y, et al. (2021) Efficacy of immune checkpoint inhibitors in non-small cell lung cancer with uncommon histology: A propensity-score-matched analysis. BMC Pulmon Med 21: 1-9. [Google Scholar] [Cross Ref]
- Domblides C, Leroy K, Monnet I, Mazières J, Barlesi F, et al. (2020) Efficacy of immune checkpoint inhibitors in lung sarcomatoid carcinoma. J Thor Oncol 15: 860-866. [Google Scholar] [Cross Ref]
- Lee J, La Choi Y, Jung HA, Lee SH, Ahn JS, et al. (2020) Outstanding clinical efficacy of PD-1/PD-L1 inhibitors for pulmonary pleomorphic carcinoma. Europ J Can 132:150-158. [Google Scholar] [Cross Ref]
- Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, et al. (2020) Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 21: 1353-1365. [Google Scholar] [Cross Ref]
- Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, et al. (2018) Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. New Eng J Med 378: 2093-2104. [Google Scholar] [Cross Ref]
- Schrock AB, Li SD, Frampton GM, Suh J, Braun E, et al. (2017) Pulmonary sarcomatoid carcinomas commonly harbor either potentially targetable genomic alterations or high tumor mutational burden as observed by comprehensive genomic profiling. J Thor Oncol 12: 932-942. [Google Scholar] [Cross Ref]
- Tokuyasu H, Ishikawa S, Sakai H, Ikeuchi T, Miura H (2019) Single pembrolizumab treatment causing profound durable response in a patient with pulmonary pleomorphic carcinoma. Respir Med Case Rep 28: 100879. [Google Scholar] [Cross Ref]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi