Case Report, Clin Oncol Case Rep Vol: 5 Issue: 2
Utilizing Liquid Biopsy for Treatment Management in Bone Dominant Metastatic Breast Cancer
Shirley Cheng1, Edward Nguyen1, and Jami Fukui2*
1University of Hawai’i John A. Burns School of Medicine, Honolulu, Hawai
2University of Hawai’i Cancer Center, Honolulu, Hawai
*Corresponding Author:
Jami Fukui
University of Hawaii Cancer Center,
701 Ilalo Street, Rm 319, Honolulu, HI 96813
E-mail: jfukui@cc.hawaii.edu
Received: January 17, 2022, Manuscript No: COCR-22-51800;
Editor Assigned: January 22, 2022, PreQC No: P-51800;
Reviewed: February 21, 2022, QC No: Q-51800;
Revised: February 27, 2022, Manuscript No: R-51800;
Published: February 28, 2022, DOI: 10.4172/cocr.5(2).220
Citation: Cheng S, Nguyen E, Fukui J (2022) Utilizing Liquid Biopsy for Treatment Management in Bone Dominant Metastatic Breast Cancer. Clin Oncol Case Rep 5:2.
Abstract
Applications of liquid biopsy are primarily to monitor treatment response and to detect secondary treatment resistant mutations. In patients with bone-dominant metastatic breast cancer it can be challenging to evaluate for new mutations. Here, we present two patient cases with bone-dominant hormone positive metastatic breast cancer in whom we utilized liquid biopsy testing to guide therapy upon disease progression. Liquid biopsies were used to evaluate circulating-tumor DNA and analyzed for mutations using next generation sequencing. PIK3CA mutations were identified in both cases allowing the use of FDA approved PIK3CA-directed treatment with alpelisib. These cases demonstrate the clinical use of liquid biopsy in identifying actionable mutations, offering patients targeted treatment options without undergoing a metastatic biopsy. More real world reports are needed to further understand current liquid biopsy utilization in oncology patient care.
Keywords: Biopsy; Mutations; Metastatic breast cancer; Bone dominant hormone; Next generation sequencing
Introduction
Bone metastasis occurs in up to 70% of patients with advanced breast cancer [1]. In clinical practice, the standard way to confirm metastatic disease is to biopsy a metastatic site. Obtaining a biopsy from a metastatic site can have limitations depending on the location, these can be technically challenging and may not yield enough specimen to be analyzed [2]. Furthermore, biopsy procedures are painful and undesirable for patients [3]. When evaluating metastatic disease in the bone, the decalcification step can lead to decreased DNA/RNA purity, poor DNA/RNA integrity, and loss of mutations [4]. Given the challenges in acquisition and analysis of bone samples in Metastatic Breast Cancer (mBC), a liquid biopsy is a less invasive alternative that can reveal clinically relevant alterations.
Many studies have demonstrated that plasma derived circulating tumor DNA (ctDNA) can be utilized as an effective surrogate marker in mBC providing spatial and longitudinal information regarding tumor status and treatment response [5,6]. In July 2020, the U.S. Food and Drug Administration (FDA) approved two comprehensive liquid biopsy tests for solid tumors, Guardant360 CDx and FoundationOne Liquid CDx [7]. The utilization of next-generation sequencing (NGS) technology can help identify tumor mutations and guide clinicians in determining clinical benefit from targeted therapy.
PIK3CA is the most commonly mutated gene in hormone positive (HR+) mBC (approx 40%) and has been associated with resistance to endocrine therapy [8]. According to the National Comprehensive Cancer Network® (NCCN) guidelines, a tumor or liquid biopsy is indicated to identify PIK3CA mutations in patients with HR+/HER2- BC and recurrent unresectable (local or regional) or stage IV (M1) disease for treatment with alpelisib [9]. Despite progress in blood-based technologies, there are still no standard guidelines for its use in breast cancer and is it left for the clinician to determine when to best utilize liquid biopsy [10]. Although tissue biopsies are still the conventional method for testing tumor mutational status, clinicians should first consider liquid biopsies in bone-dominant mBC as it is more convenient and less invasive for patients, and then reflex to standard tissue biopsy if no actionable mutation is found.
Case Reports
Here we report two cases of bone-dominant HR+mBC, in which liquid biopsies were obtained and identified molecular targets for FDA approved treatment. Patient 1 (MA) is a 42 year-old premenopausal woman with a history of high risk HR+BC (pT3N3-stage 3C, initial diagnosis at age 38, no genetic mutations) who developed lower back pain while on adjuvant endocrine therapy. Staging scans showed multiple bone lesions and biopsy confirmed HR+mBC, with no other sites of disease. During her metastatic work up, we obtained a liquid biopsy for NGS, the Guardant360® CDx liquid tumor biopsy testing (Guardant Health, Redwood City, CA, http://www.guardanthealth.com/) and did not detect any ctDNA (Figure 1). She was started on standard treatment for mBC with alternate endocrine therapy, CDK4/6 inhibitor and bisphosphonate therapy. During interval scans she was noted to have additional bone lesions along with increasing tumor markers, indicating progression of disease. Another liquid biopsy was performed at that time with the same NGS platform to evaluate potential actionable molecular abnormalities and a PIK3CA E542K mutation was found (Figure 1). She subsequently was started on alpelisib along with alternate endocrine therapy and bisphosphonate treatment.
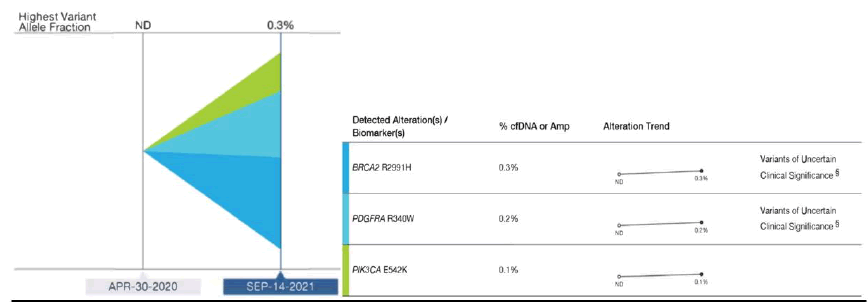
Figure 1: Guardant360® CDx liquid biopsy testing performed. Each color represents a different genetic alteration (BRCA2 R2991H, PDGFRA R340W and PIK3CA E542K). At disease progression, a repeat liquid biopsy was performed. PIK3CA mutations were not identified in the initial testing (April 30, 2020) but identified in the repeat testing (September 14, 2021).
Patient 2 (PR) is a 77-year old post-menopausal woman with a history of early stage HR+BC (pT1cN0-stage IA, initial diagnosis at age 65) who did not tolerate adjuvant endocrine therapy. After 5 years, she had a new abnormality on breast imaging and staging scans showed multiple bone lesions. She then underwent palliative radiation and started on endocrine treatment and bisphosphonate therapy. After another 4 years and multiple therapies she underwent FoundationOne® Liquid CDx (Foundation Medicine, Cambridge, MA, https://www.foundationmedicine.com/) NGS testing which identified PTEN I101N, ESR1 Y537N and D538G, CDH1 F810fs, PIK3CA E545K mutations (Figure 2). She evaluated by a major academic institution and underwent a T10 biopsy that confirmed HR+ mBC. The bone tissue sample was also sent for NGS with FoundationOne® (Foundation Medicine, Cambridge, MA, https://www.foundationmedicine.com/) which again identified a PTEN I101N, ESR1 Y537N, CDH1 F810fs, PIK3CA E545K mutations, TMB-4Mut/Mb and Microsatellite-stable (Figure 2). At that time she was started on oral chemotherapy with capecitabine. After progression of disease and FDA approval in May 2019, she started alpelisib.
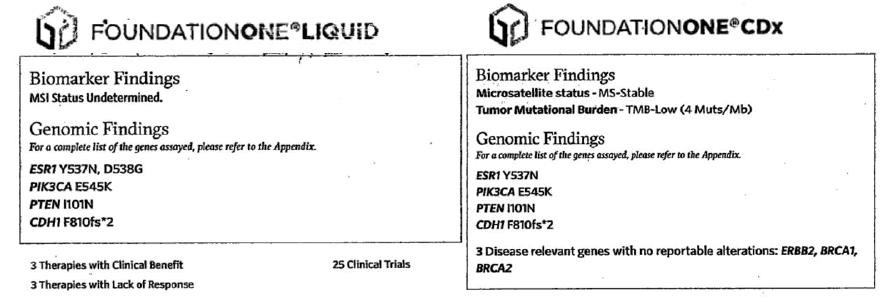
Figure 2: FoundationOne® Liquid testing performed by local oncologist. PTEN I101N, ESR1 Y537N and D538G, CDH1 F810fs, PIK3CA E545K mutations were found (December 19, 2018). A FoundationOne® CDx testing on tissue (bone) biopsy was performed after academic center consultation, PTEN I101N, ESR1 Y537N, CDH1 F810fs, PIK3CA E545K mutations, TMB-4Mut/Mb and Microsatellite-stable were reported (January 21, 2019).
Discussion
In recent years, the availability of NGS through liquid biopsies have revolutionized the clinical management of patients with metastatic cancer. Blood-based sampling is non-invasive and accessible making it more favorable to tissue biopsies. We have presented two cases of bone-dominant mBC highlighting the use of liquid biopsy using two separate NGS platforms that were able to detect actionable PIK3CA mutations. This demonstrates a feasible real-world clinical application to liquid biopsies. Serial sampling in our first patient case showed the dynamic change found in tumor mutational status upon disease progression. In the second patient case, both tissue (bone) and liquid biopsy provided similar results. Although tissue biopsy is recommended when there are no actionable mutations detected in the blood, liquid biopsy has utility in the community practice and should be considered especially at disease progression and/or when tissue biopsies are difficult to obtain. This report provides an example of precision medicine in breast cancer oncology and how genomic characterization using liquid biopsies can help clinicians tailor treatment.
Funding
This work was completed as part of the funding from the University of Hawaii Cancer Center P30 CA071789 Grant from the National Institutes of Health. The funding body had no role in the design; in the collection, analysis, or interpretation of data; in writing of the manuscript; or in the decision to submit the manuscript for publication.
Disclosure
The authors declare no conflicts of interest.
References
- Fang J, Xu Q (2015) Differences of osteoblastic bone metastases and osteolytic bone metastases in clinical features and molecular characteristics. Clin Transl Oncol 17: 173-179.
Google Scholar Cross Ref - Hilton JF, Amir E, Hopkins S, Nabavi M, DiPrimio G, et al. (2011) Acquisition of metastatic tissue from patients with bone metastases from breast cancer. Breast Cancer Res Treat 129: 761-765.
Google Scholar Cross Ref - Hjortholm N, Jaddini E, HaÅ?aburda K, Snarski E (2013) Strategies of pain reduction during the bone marrow biopsy. Ann Hematol 92: 145-149.
Google Scholar Cross Ref - Miquelestorena-Standley E, Jourdan ML, Collin C, et al. (2020) Effect of decalcification protocols on immunohistochemistry and molecular analyses of bone samples. Mod Pathol 33: 1505-1517.
Google Scholar Cross Ref - Alimirzaie S, Bagherzadeh M, Akbari MR (2019) Liquid biopsy in breast cancer: A comprehensive review. Clin Genet 95: 643-660.
Google Scholar Cross Ref - Tay TKY, Tan PH (2021) Liquid biopsy in breast cancer: A focused review. Arch Pathol Lab Med 145: 678-686.
Google Scholar Cross Ref - Cavallo J (2021) The evolution of liquid biopsy in cancer care. The ASCO Post.
- Fusco N, Malapelle U, Fassan M, Marchiò C, Buglioni S, et al. (2021) PIK3CA mutations as a molecular target for hormone receptor-positive, HER2-negative metastatic breast cancer. Front Oncol 11: 644737.
Google Scholar Cross Ref - NCCN (2021) NCCN clinical practice guidelines in oncology: Breast cancer. National Comprehensive Cancer Network. Version 8.2021.
- Van Poznak C, Somerfield MR, Bast RC, Cristofanilli M, Goetz MP, et al. (2015) Use of biomarkers to guide decisions on systemic therapy for women with metastatic breast cancer: american society of clinical oncology clinical practice guideline. J Clin Oncol 33: 2695-2704.
Google Scholar Cross Ref
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 