Case Report, Clin Oncol Case Rep Vol: 8 Issue: 7
Vertebral Giant Cell Tumor in a Young Male with Early Aggressive Recurrence and Pulmonary Metastases: A Rare Case Report and Literature Review
Muralidhar Idamakanti1*, Rani Indrani Bijjam2, Alexei Bakhirev3, Ethan Binder4
1Attending Hospitalist, Adult Inpatient Medical Services (AIMS), Presbyterian Healthcare Services (PHS), 1100 Central Ave SE, Albuquerque, New Mexico (NM), USA, 87106
2Attending Hospitalist, Adult Internal Medicine Services (AIMS), Presbyterian Healthcare Services (PHS), 1100 Central Ave SE, Albuquerque, NM, USA, 87106
3Surgical Pathologist, President, Pathology Associates of Albuquerque (PAA), 1100 Central Ave SE, Albuquerque, New Mexico (NM), USA, 87106
4Attending Physician, Department of Hematology/ Oncology, Presbyterian Healthcare Services (PHS), 1100 Central Ave SE, Albuquerque, NM, USA, 87106
- *Corresponding Author:
- Muralidhar Idamakanti
Attending Hospitalist, Adult Inpatient Medical Services (AIMS), Presbyterian Healthcare Services (PHS), 1100 Central Ave SE, Albuquerque, New Mexico (NM), USA, 87106
E-mail: imurali44@gmail.com
Received: June 02, 2025; Manuscript No: COCR-25-166264; Editor Assigned: June 9, 2025; PreQC: COCR-25-166264(PQ); Reviewed: June 24, 2025; QC No: COCR-25-166264(Q); Revised: June 31, 2025; Manuscript No: COCR-25- 166264(R); Published: July 30, 2025, DOI: 10.4172/cocr.8(7).421
Citation: Idamakanti M, Bijjam RI, Bakhirev A, Binder E (2025) Vertebral Giant Cell Tumor in a Young Male with Early Aggressive Recurrence and Pulmonary Metastases: A Rare Case Report and Literature Review. Clin Oncol Case Rep 8(7):421
Abstract
Background: Giant cell tumor of bone (GCTB) is one of the most common benign tumors of bone, accounting for 15-20% of such tumors. The peak age of incidence is between 25 and 35 years. GCTB typically affects the epiphyseal regions of long bones, with at least 40% occurring around the knee. Vertebral involvement is rare, occurring in approximately 2-9% of GCTB cases. Local recurrence after excision is common and is generally observed around one to two years post-surgery. Pulmonary metastasis is unusual and is seldom encountered within the first year of diagnosis. Case Presentation: A 20-year-old Caucasian/African American male with no medical history presented with acute lower back pain. Imaging revealed a T12 lytic mass with a pathological compression fracture. He underwent corpectomy with tumor resection and surgical stabilization via posterior T9-L3 spinal fusion and placement of an Ulrich expandable cage graft. The biopsy was positive for osseous giant cell tumor. He was discharged in stable condition to acute rehab. The patient was lost to follow-up and returned to the hospital three months later with back pain and fatigue. He exhibited dehiscence of the surgical wound, new extensive pulmonary metastases, and recurrence of a larger mass in the post-corpectomy space. He underwent extensive neurosurgical intervention involving wound washout, re-resection of the tumor, and removal and replacement of the cage graft. He received a dose of denosumab as per the oncologist’s instructions and was discharged with close outpatient follow-ups. Conclusion: Vertebral GCTB is a rare bone tumor in a 20-yearold male. The aggressive local recurrence and extensive pulmonary metastases occurring within three months after the initial tumor debulking make this case an exceptionally rare presentation. Vertebral GCTB poses a higher risk of recurrence and pulmonary metastasis compared to other types of GCTB, necessitating a multidisciplinary approach for optimal management. Abbreviations: GCTB: Giant Cell Tumor of Bone; MRI: Magnetic Resonance Imaging; IV: Intravenous; IR: Interventional radiology; PRBC: Packed Red Blood Cells; CT: Computed Tomography; MRSA: Methicillin Resistant Staphylococcus Aureus; B-HCG: Beta-Human Chorionic Gonadotropin; RANKL: Receptor Activator of Nuclear Factor-κB Ligand; OPG: Osteoprotegerin; H3F3: Histone H3.3 protein; G34W: Glycine to Tryptophan Substitution at Codon 34; MMP: Matrix Metalloproteinases; TNF-α: Tumor Necrosis Factor-Alpha; IL-6: Interleukin-6; PET: Positron Emission Tomography; PMMA: Polymethylmethacrylate; RT: Radiotherapy.
Keywords
Giant cell tumor of bone; RANKL (Receptor Activator of Nuclear Factor-κB Ligand); Pulmonary metastasis; Expandable cage graft; Denosumab; Zoledronic acid; Local tumor recurrence.
Introduction
Giant cell tumor of bone (GCTB) is one of the most common benign tumors of the bone and accounts for approximately 15-20% of such tumors. The peak age of incidence is between 25 and 35 years, and it is rarely seen in patients younger than 20 or older than 50 years of age [1-4, 5]. GCTB typically affects the epiphyseal regions of long bones, with at least 40% occurring around the knee (femur > tibia) [1-4]. Vertebral involvement is very rare and seen in roughly 2-9% of GCTB cases [1-4]. Local recurrence after excision is common and usually occurs around one to two years after the surgery [6, 7]. Metastatic disease is rarely seen and does not directly influence the long-term prognosis. The lung is the usual organ of metastasis and is noticed 1.5 to 2 years after the initial diagnosis [8, 9]. Vertebral GCTBs have a higher risk of local recurrence and a small but significant risk of pulmonary metastasis compared to the GCTB of long bones. Prompt diagnosis and multidisciplinary management are crucial to preserving spinal stability and neurological function [1-4]. The rarity of involvement, proximity to critical structures, and risk of spinal instability or cord compression make vertebral GCTB a distinct clinical entity.
Case Presentation
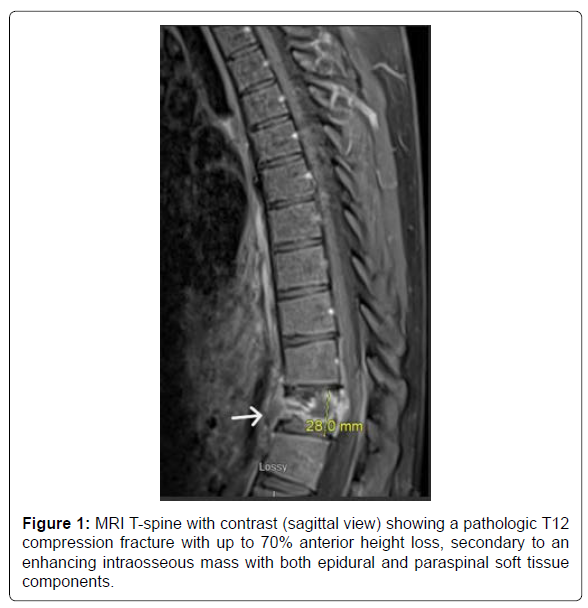
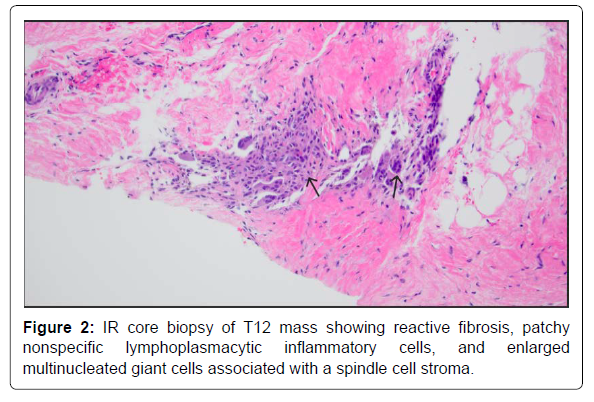
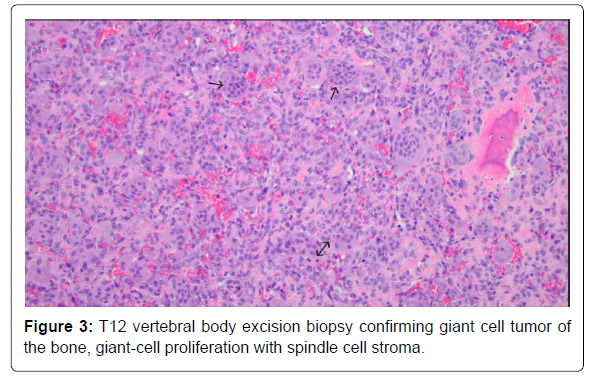
A 20-year-old male with no known significant past medical history presented with a 2-month history of lower back pain that acutely worsened on the day of presentation. Physical examination was positive for lower thoracic and upper lumbar spinal and paraspinal tenderness and negative for any focal neurological signs. Initial labs were only positive for mild leucocytosis (Table 1). MRI T-spine with contrast revealed a solitary intraosseous lytic mass at T12 with both epidural and paraspinal components and a pathological compression fracture (Figure 1). Pediatric Oncology and Neurosurgery were consulted, based on the tumor characteristics, Langerhans’ cell histiocytosis and Ewing sarcoma were sought higher on the differential. The patient was started on high-dose steroids, dexamethasone 20 mg IV every 6 hours. An IR soft tissue biopsy was performed, which showed giant cell proliferation concerning for GCTB (Figure 2). On day 12, the patient underwent corpectomy with tumor resection and surgical stabilization via posterior T9-L3 spinal fusion and placement of an Ulrich expandable cage graft. The biopsy confirmed the diagnosis of osseous giant cell tumor (Figure 3).
| Laboratory data | Day 1 | 3 months | Normal Range |
|---|---|---|---|
| White blood cells | 12.4 | 8.7 | 4.8-10.8 x 103 cells/µL |
| Hemoglobin | 15.1 | 15.1 | 13.5-17.5 g/dL |
| Platelet count | 287 | 388 | 130-400 x 103 cells/µL |
| Sodium | 138 | 142 | 136-145 mmol/L |
| Potassium | 4 | 4.7 | 3.5-5.1 mmol/L |
| Chloride | 105 | 104 | 98-107 mmol/L |
| Bicarbonate | 24 | 26 | 22-29 mEq/L |
| Blood urea nitrogen | 11 | 10 | 8-22 mg/dL |
| Creatinine | 0.85 | 0.87 | 0.7-1.2 mg/dL |
| Glucose | 102 | 105 | 70-104 mg/dL |
| Total Bilirubin | 67 | 0.3 | 0.20-1.00 mg/dL |
| AST (Aspartate transaminase)) | 24 | 19 | 10-34 U/L |
| ALT (Alanine transaminase) | 6 | 12 | 10-44 U/L |
| Calcium | 9.8 | 9.5 | 8.5-10.2 mg/dL |
| Total protein | 7.3 | 8.2 | 6.1-8.2 gm/dL |
| Albumin | 4.3 | 4.4 | 3.3-5.2 gm/dL |
| Vitamin B12 | 405 | 540 | 204-1245 pg/mL |
| B-HCG | <1 | --- | <2 mIU/ml |
Table 1: Initial laboratory data on the two admissions.
The Immediate post-operative period was complicated by postoperative ileus, urinary retention, and acute blood loss anemia from surgical blood loss and hematoma at the surgical site. The patient required two units of PRBC and a dose of IV iron (Venofer 500 mg), and hemoglobin remained stable above 9 gm/dL after that. The neurosurgery team did not recommend surgical evacuation or aspiration of the hematoma, as follow-up imaging showed stable to improved findings. Ileus and urinary retention improved with supportive care. Oncology recommended denosumab therapy and radiation therapy, but not until 6-8 weeks post-surgery to allow for adequate healing. On hospital day 20, the patient was discharged in a stable condition to an acute rehabilitation center with appropriate follow-ups scheduled for medical oncology, radiation oncology, and neurosurgery.
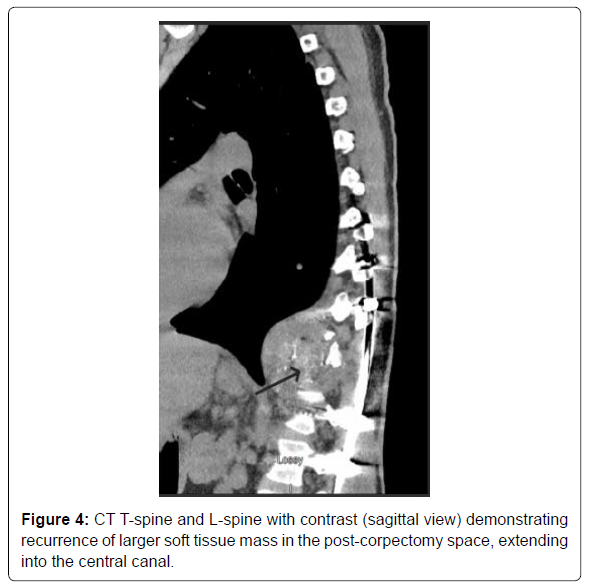
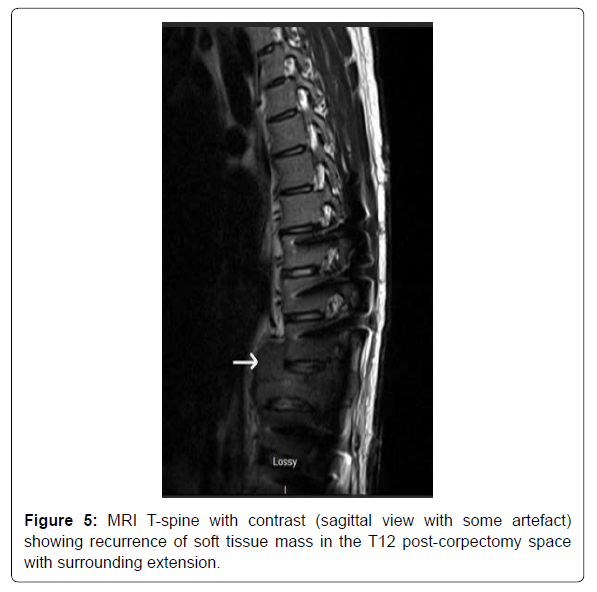
The patient did not follow up with any of the specialties after the discharge owing to poor socioeconomic support. He presented back to the hospital exactly three months after the discharge with symptoms of left and right-sided flank pain, worsening lower back pain, and fatigue. He was noted to have the dehiscence of the lower surgical incision site on examination. The patient was admitted for evaluation and management of a non-healing surgical wound. The patient was empirically started on IV ceftriaxone and Vancomycin for soft tissue infection. CT T and L-spine with contrast demonstrated recurrence of the soft tissue mass in the post-corpectomy space, extending into the central canal and measuring 8.3 x 6.5 cm, bilateral paraspinal fluid collections, and probable destructive lesions or bone erosions in the inferior endplate of T10-11 and the superior endplate of L1 (Figure 4). MRI T-spine with contrast had motion artifact related to cage graft, but within the limitations, showed similar findings of tumor recurrence (Figur 5). CT chest with contrast demonstrated extensive new soft tissue lung nodules, the largest nodule of 6mm, compared to CT from the previous admission, consistent with extensive lung metastases. The nodules were deemed too small for biopsy by the IR and the pulmonologist.
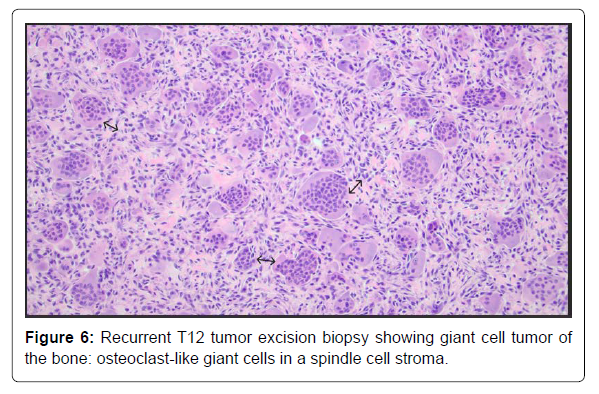
The neurosurgery team was consulted, and they took him for surgery on Day 5 of hospitalization. The operative findings were a massive recurrence of the tumor encircling the spinal cord and the expandable cage graft, and pseudoarthrosis of the graft. The patient underwent extensive surgical intervention involving washout and lavage of the infected thoracic wound, extensive re-resection of the tumor to decompress the neural elements, removal of the graft and replacement with a larger Ulrich cage graft, placement of crosslink at the laminectomy site, and reclosure of the wound. The biopsy returned positive for the giant cell tumor without evidence of malignant transformation (Figure 6). Wound cultures only grew Staphylococcus epidermidis, and antibiotics were narrowed down to ceftriaxone in the context of a negative nasal MRSA screen and eventually stopped after 8 days.
Postoperatively, the patient had acute blood loss anemia and hemorrhagic shock requiring aggressive fluid resuscitation and three units of PRBC. A follow-up X-ray of the thoracolumbar spine showed spinal instability in the thoracolumbar region. On hospital day 9, the patient was taken for surgical stabilization by the neurosurgery team, operatively noted to have pseudoarthrosis of the recently placed vertebrectomy expandable cage graft and underwent exploration and reposition of the failed expandable cage graft. The patient later received a dose of 120 mg of denosumab infusion as per the oncologist and was discharged with outpatient referrals to medical oncology, radiation oncology, pulmonology, and neurosurgery. In the immediate discharge period, the patient followed up with the pulmonologist at a higher center and underwent robotic-assisted flexible bronchoscopy and transbronchial ultrasound-guided cryo biopsy of the right lower lobe nodule. The pathology results came positive for metastatic tumor consistent with the patient’s known diagnosis of GCTB.
Discussion
Giant cell tumor of bone (GCTB) is one of the most common benign tumors of the bone and accounts for approximately 5-10% of all primary bone tumors and 15-20% of benign bone tumors [1-3]. It has a slight female predominance of 1.4:1, which is even higher (~2:5 1) in younger patients aged 20 or less, stressing the point that GCTB develops predominantly after skeletal maturity when epiphyses are closed [1, 4]. GCTB has a higher incidence in Asians compared to Westerners. GCTB is commonly seen in the 20 to 40 year age group, with a peak incidence rate between 25 and 35 years, and it is rarely seen in patients under 20 years of age or older than 50 years of age. GCTB typically affects the epiphyseal regions of long bones, with at least 40% occurring around the knee (femur > tibia). Other commonly involved bones in the order of occurrence are the distal radius, the proximal humerus, the hands (phalanges), the feet, and the pelvis. Axial skeleton is rarely affected, and when affected, the sacrum is the most common location. Spine involvement is seen in roughly 2-9% of GCTB cases, and when the spine is involved, the thoracic and lumbar spine are the most involved regions, and the vertebral body is the favorable location. Common bones involved in the skull include the mandible and maxilla [1-4].
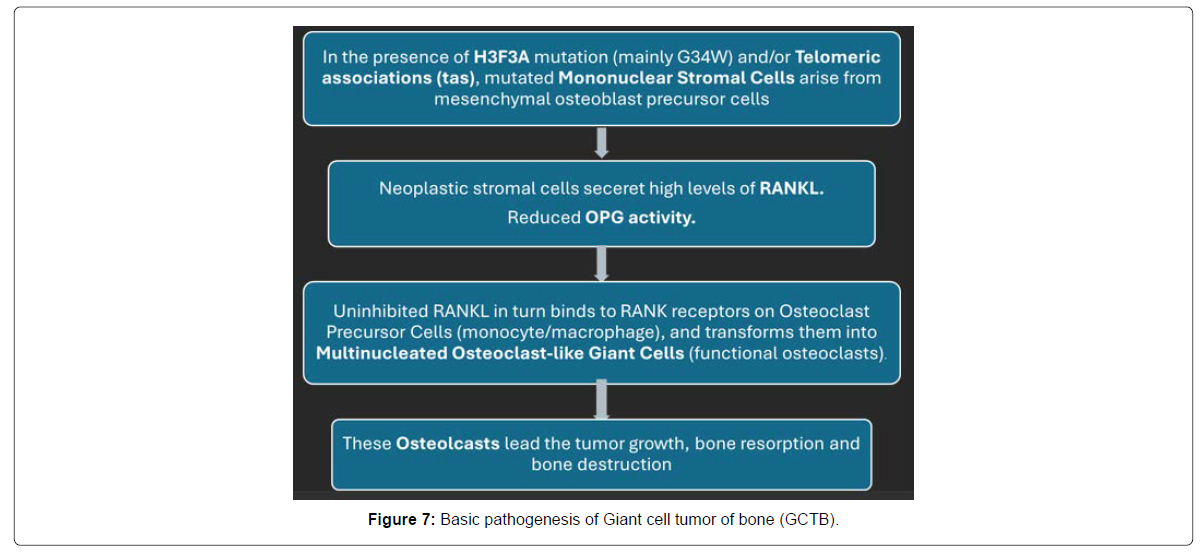
The pathogenesis of GCTB is complex and orchestrated through molecular and cellular mechanisms centered around the RANK/RANKL signaling axis (Figure 7). The gross specimen shows evidence of high vascularity and aggressive bone destruction. GCTB is a heterogeneous tumor composed of neoplastic mononuclear stromal cells (true neoplastic components), multinucleated osteoclast-like giant cells, and mononuclear histiocytic cells [1-4, 10, 11]. The interaction between these cell populations drives tumor growth and bone destruction. Neoplastic mononuclear stromal cells express high levels of RANKL (Receptor Activator of Nuclear Factor-κB Ligand), which in turn binds to RANK receptors on osteoclast precursor cells and transforms them into multinucleated osteoclast-like giant cells. These osteoclast-like giant cells are functionally active osteoclasts that are responsible for the degradation of the bone [1, 10, 11]. Denosumab targets this pathway by binding to RANKL. Reduced OPG activity (osteoprotegerin, a decoy receptor that binds RANKL and inhibits its activity) is also observed in GCTB, resulting in unchecked osteoclast activation.
Molecular and genetic analysis showed that more than 90% of GCTBs harbor a H3F3A gene mutation, specifically G34W (glycine to tryptophan substitution at codon 34), and its detection is diagnostic and can distinguish GCTB from other giant cell-rich tumors [1-4, 9-12]. Cytogenic aberrations are also frequently implicated in the pathogenesis of GCTB, and the most common aberration that is seen in approximately 70% of GCTB cases is telomeric associations (tas). Common chromosomal locations affected by tas are 1q, 11p, 12p, 13p, 14p, 15p, 19q, 21p, and 22p [9-12]. Other molecular agents involved include MMP-9 and MMP-14 (matrix metalloproteinases involved in extracellular matrix degradation), VEGF (Vascular Endothelial Growth Factor, promotes angiogenesis within the tumor) and TNF-α & IL-6 (cytokines involved in inflammatory and osteolytic processes) [1-4, 9-11]. GCTB could rarely undergo malignant transformation into giant cell-rich osteosarcoma or undifferentiated pleomorphic sarcoma. This transformation is commonly encountered in recurrent tumors, particularly in those that have received radiation therapy. The molecular genetics behind this transformation are not fully elucidated but may involve additional mutations beyond H3F3A [1, 9-11].
Clinical features of GCTB at the time of presentation mainly depend on the size, location, and extent of destruction from the tumor. Pain is the most common symptom and is typically present for months. Acute or acute-on-chronic pain usually indicates a pathological fracture. Other associated symptoms that may or may not co-exist include local swelling, functional limitation, and restricted movement of the adjacent joints. Progressive localized back pain is the most common symptom in vertebral GCTB and is caused by the local destruction of the tumor. Neurological deficits and neurogenic claudication/sciatica may occur secondary to tumor expansion and spinal cord or nerve root compression. Pain and debility related to it could be severe if pathological fractures and spinal instability are present. Spinal instability warrants urgent surgical intervention aiming at resection and reconstruction [1-4, 12-15]. Diagnosis primarily depends on good physical examination, radiological studies (X-ray, CT scan, and MRI), and histopathological analysis, including cytogenetics (described above). Radiological findings reported in GCTB are described below (Table 2).
| Study | Radiological findings |
|---|---|
| X-ray | Presentations vary from a well-defined lesion with intact margin and cortex to an ill-defined aggressive lesion with bone destruction. Common findings include eccentric location, lytic lesions with or without pathological fractures, closed epiphyses, and extension to the subchondral bone. |
| CT | Detailed assessment of cortical bone destruction and tumor margins. Pathological fracture missed on X-ray. |
| MRI | Superior for evaluating intramedullary extension and soft tissue involvement. In vertebral GCTs, it will help assess epidural involvement and spinal cord compression. |
| Bone scan/PET | Useful for staging and detecting multifocal disease. |
Table 2: Radiological diagnosis of Giant cell tumor of bone (GCTB).
Grading the GCTB can aid in choosing the management and follow-up options. Numerous grading scales were previously described for GCT of bone based on histopathological, clinical, and radiographic findings, with limited use. The Campanacci classification system is the most popular one used (Table 3).
| Grade | Radiographic Features | Tumor margins | Cortical involvement | Soft tissue extension | Clinical Relevance |
|---|---|---|---|---|---|
| Grade I | Lucent, well-marginated; latent | Well-defined | Intact cortex | None | May be treated conservatively or with curettage alone. |
| Grade II | Active, well-marginated without a radiopaque rim; expanding | Well-defined, but without a sclerotic rim. | Cortex thinned, possibly bulging | Minimal or none | Requires extended curettage; may need adjuvants |
| Grade III | Ill-defined, indistinct; Aggressive | Poorly defined margins | Cortex destroyed | Obvious soft tissue extension/mass | Often requires wide resection due to high recurrence risk; needs adjuvants and frequent follow-ups |
Table 3: Campanacci’s Classification of Giant cell tumor of bone (GCTB).
| Management Option | Indications | Mechanism | Complications |
|---|---|---|---|
| Intralesional Curettage | The most common approach for accessible and resectable tumors in long bones | Surgical removal of the tumor via scraping | High recurrence rate (10–50%), may leave residual tumor |
| Curettage + Adjuvants | Standard for reducing recurrence post-curettage | Local cytotoxic or thermal destruction of residual cells | Local necrosis, tissue damage, chemical irritation, and increased risk of pathological fractures |
| High-speed burr drilling | After curettage to enhance tumor bed clearance | Mechanically removes microscopic residual tumor cells | Thermal damage to bone, iatrogenic fracture if aggressive |
| Phenol | Often used after curettage | Protein coagulation, chemical necrosis | Chemical burns, local toxicity if spilled |
| Hydrogen Peroxide | Alternative to phenol | Oxidative damage to residual tumor cells | Mild tissue irritation |
| Cryotherapy (Liquid Nitrogen) | Aggressive tumors, high recurrence risk | Freezing causes cell lysis and necrosis | Fracture risk due to bone weakening, local soft tissue injury |
| PMMA Bone Cement | Frequently used for structural support and thermal ablation | Exothermic polymerization destroys residual tumor cells | Thermal damage to surrounding tissues, future difficulty with revision surgery |
| Argon Beam Coagulation | Less common, specialized centers | Thermal coagulation of remaining tumor cells. Good functional outcome | Localized tissue injury, availability limited |
| Zoledronic Acid (Local) [19, 28-30] | Investigational use after curettage to decrease tumor size, recurrence and alleviate pain | Inhibits osteoclast activity, induces apoptosis in stromal cells | Limited clinical data on long-term use and safety; potential local irritation |
| Wide Resection | Extensive cortical destruction, joint invasion, recurrent or aggressive tumors, and anatomically challenging lesions | En bloc removal of tumor and surrounding bone | Joint dysfunction, functional impairment, increased risk of mechanical fractures, and need for reconstruction |
| Denosumab (RANKL inhibitor) | Unresectable, recurrent, and axial skeleton tumors and downstaging before surgery | Inhibits osteoclast-like giant cells, reduces tumor bulk | Osteonecrosis of the jaw, hypocalcemia, rebound growth on discontinuation, and unclear long-term safety |
| Radiotherapy | Inoperable cases (e.g., extensive tumors of the axial skeleton, mainly the spine) and diffuse pulmonary metastases | Local control via ionizing radiation | Risk of secondary sarcoma, radiation-induced fibrosis, and bone marrow suppression |
| Reconstruction (Graft/Prosthesis) | After wide resection, especially in weight-bearing joints (Knee, spine) | Restores structural integrity and joint function | Mechanical failure/ pseudoarthrosis of graft or prosthesis, infection, and the need for revision surgeries |
| Observation/Monitoring | Stable lesions or small asymptomatic pulmonary metastases | Non-interventional surveillance | Risk of delayed progression or destructive changes |
Table 4: Management Options of GCTB and Clinical Implications.
The management of GCTB involves a multidisciplinary approach aimed at eradicating the tumor while preserving limb function. Surgical excision remains the cornerstone of treatment. The preferred method is intralesional curettage, often combined with local adjuvants such as phenol, hydrogen peroxide, cryotherapy, or polymethylmethacrylate (PMMA) bone cement to reduce the risk of recurrence [1-3, 9, 10, 15-18]. For tumors with extensive cortical destruction, joint involvement, or in anatomically complex sites (e.g., pelvis, spine), wide resection may be necessary, sometimes followed by reconstruction using bone grafts or prostheses. Although effective in reducing recurrence, wide resection is associated with greater morbidity and functional loss. In cases that are unresectable, recurrent, or metastatic, or in patients who are deemed poor surgical candidates, denosumab, a RANKL inhibitor, is used to reduce tumor size and activity by inhibiting osteoclast-like giant cells [1-3, 9, 19-25]. While denosumab has shown promise in downstaging tumors and facilitating surgery, concerns exist about rebound tumor growth after discontinuation, and long-term use may be associated with complications such as osteonecrosis of the Jaw and atypical fractures [23-25]. Radiotherapy is generally avoided due to the risk of malignant transformation, but may be considered in select inoperable cases, and it decreases the local recurrence by 80% [9, 10, 26-28]. Radiation therapy (RT) is particularly beneficial in some cases of vertebral GCTB, where surgery is not possible due to the inaccessible location or deformity associated with wide resection. RT significantly improves long-term prognosis and functionality in these patients [26-28].
Metastatic disease is rarely seen in GCTB, occurs without malignant transformation, and does not directly influence the long-term prognosis. The lung is the most common site of metastasis, occurring in 1-4% of cases, typically encountered 1.5 to 2 years after the initial diagnosis. Metastasis occurs via hematogenous spread and is commonly encountered after surgical treatment or radiation therapy. Pulmonary lesions are mostly benign, sharing a similar pathological appearance to the primary tumor. Most lung lesions are resectable, while nonresectable lesions usually follow a benign course; adjuvant therapy with denosumab or radiation therapy could be considered if the metastatic disease burden is high or diffuse [8, 9, 26].
The prognosis of GCTB is generally favorable, as it is typically benign. As per most studies, 5-year survival rates exceed 90%, and 10-year survival is reported at over 85–90%. Significant morbidity is expected from the locally aggressive nature of the tumor, which is probably the reason for not having 100% survival rates. Almost all cases respond well to surgical resection combined with adjuvant therapy. However, they have a significant risk of local recurrence, with rates varying between 10% and 50%, largely depending on the extent and quality of surgical excision [1-3, 6, 19-22]. Intralesional curettage is often associated with higher recurrence rates than en bloc or wide resection. Other factors that increase recurrence rates include a higher Campanacci grade at diagnosis, soft tissue extension, larger tumor size, and tumor location (lower extremities and vertebral column). The higher recurrence rate in vertebral GCTs appears to be mainly related to the difficulty or inability to perform wide resections [1-3, 9, 10, 19-22]. Denosumab has shown promise in reducing the recurrence rates and has improved long-term outcomes in tumors that are partially resectable to unresectable. In vertebral GCTs, denosumab helped to preserve neurological function [23-25]. Malignant transformation into high-grade sarcoma is uncommon but significantly worsens the prognosis. Malignant transformation is particularly seen in patients who have had radiation therapy [1, 9, 26-28].
Regular follow-up is crucial for monitoring recurrences and complications. Follow-ups include clinical exams, local site radiographs, and chest imaging as indicated (X-ray is generally sufficient), primarily focusing on detecting local recurrence and pulmonary metastasis. Patients are monitored every 3 to 4 months during the first two years, every 6 months from years 2 to 5, and then annually thereafter. CT scans of the bone or lung are reserved for high-risk cases, and MRI may be employed in patients with suspected soft tissue extension. Closer surveillance is warranted for tumors with high-risk features such as axial skeleton involvement, prior recurrence, Campanacci Grade III lesions, or a history of treatment with denosumab or radiation therapy [1-3, 9, 10, 19-22, 28].
Here, we present a rare case of a 20-year-old male with an osseous giant cell tumor in the thoracic vertebra at diagnosis, treated with resection and reconstructive surgery using an expandable cage graft, and had an aggressive recurrence and evidence of pulmonary metastatic disease within 3 months of initial tumor debulking, requiring re-section and exchange of cage graft and denosumab therapy. Through this case report, we would like to reiterate that vertebral GCTBs carry a higher risk of local recurrence and a significant risk of pulmonary metastases compared to the GCTBs of long bones. Prompt diagnosis, multidisciplinary management, and appropriate close follow-up are crucial to preserving spinal stability and neurological function.
Conclusion
Vertebral GCT is an uncommon bone tumor in a 20-year-old male. Recurrence of GCTB after local excision typically occurs 1-2 years following the surgery, while pulmonary metastasis is usually observed 1.5-2 years after the initial diagnosis. Aggressive local recurrence and extensive pulmonary metastasis within 3 months after initial tumor debulking render this case an exceptionally rare scenario. Through this case report, we would like to emphasize that vertebral GCTB carries a higher risk of local recurrence and a significant risk of pulmonary metastases compared to the other types of GCTB. Early diagnosis, proper surgical planning, and judicious use of adjuvant therapies are crucial for improving outcomes and minimizing morbidity. Future studies are necessary to refine treatment protocols and explore novel therapeutic avenues.
Conflict of Interest
The authors declare that they do not have any conflict of interest.
Financial Disclosure
The authors declare that they do not have a financial relationship with any commercial entity that has an interest in the subject of this manuscript.
Informed Consent
No patient identifiers or pictures of the patient are used in this manuscript. Verbal consent is obtained from the patient.
Author Contributions
All the authors participated actively in this case report. They were actively involved in writing different article sections, helping with images/ references, or revising the article before submission.
M Idamakanti: Started the article, actively wrote and revised different sections of the article, gathered images, conducted literature review, and added references.
RI Bijjam: Identified the rarity of the case report. Contributed to different sections of the article and helped with proofreading (grammar) and revising the article.
A Bakhirev: Provided histopathology slides and input on pathogenesis and immunocytochemistry.
E Binder: Actively reviewed, edited, and revised the article with current guidelines and conducted literature review, as the consulting Oncologist.
Data Availability
The authors declare that data supporting the findings of this case report are available within the article and its supplementary files.
REFERENCES
- Hosseinzadeh S, Tiwari V, De Jesus O (2024) Giant Cell Tumor (Osteoclastoma).Treasure Island (FL): StatPearls Publishing: 32644655.
- Sobti A, Agrawal P, Agarwala S, Agarwal M (2016) Giant Cell Tumor of Bone - An Overview. Arch Bone Jt Surg 4:2-9.
- Montgomery C, Couch C, Emory CL, Nicholas R (2019) Giant Cell Tumor of Bone: Review of Current Literature, Evaluation, and Treatment Options. J Knee Surg 32:331-336.
- Goldenberg RR, Campbell CJ, Bonfiglio M (1970) Giant-cell tumor of bone. An analysis of two hundred and eighteen cases. J Bone Joint Surg Am 52: 619-64.
- McCarthy EF, Weber KL (2009) Giant cell tumor of bone in elderly patients: a study of ten patients. Iowa Orthop J 29: 79-82.
- Lausten GS, Jensen PK, Schiødt T, Lund B (1996) Local recurrences in giant cell tumour of bone. Long-term follow up of 31 cases. Int Orthop 20: 172-176.
- McDonald DJ, Sim FH, McLeod RA, Dahlin DC (1986) Giant-cell tumor of bone. J Bone Joint Surg Am 68: 235-42.
- Muheremu A, Niu X (2014) Pulmonary metastasis of giant cell tumor of bones. World J Surg Oncol 12: 261.
- Chakarun CJ, Forrester DM, Gottsegen CJ, Patel DB, White EA, et al. (2013) Giant cell tumor of bone: review, mimics, and new developments in treatment. Radiographics 33: 197-211.
- Jha Y, Chaudhary K (2023) Giant Cell Tumour of Bone: A Comprehensive Review of Pathogenesis, Diagnosis, and Treatment. Cureus 15: e46945.
- Noh BJ, Park YK (2018) Giant cell tumor of bone: updated molecular pathogenesis and tumor biology. Hum Pathol 81:1-8.
- Cowan RW, Singh G (2013) Giant cell tumor of bone: a basic science perspective. Bone 52: 238-246.
- Thomas DM, Skubitz KM (2009) Giant cell tumour of bone. Curr Opin Oncol 21: 338-44.
- Raskin KA, Schwab JH, Mankin HJ, Springfield DS, Hornicek FJ (2013) Giant cell tumor of bone. J Am Acad Orthop Surg 21: 118-126.
- Asu Mallick A, Chawla SP (2021) Giant Cell Tumor of Bone: An Update. Curr Oncol Rep 23: 51.
- Turcotte RE (2006) Giant cell tumor of bone. Orthop Clin North Am 37: 35-51.
- Mavrogenis AF, Igoumenou VG, Megaloikonomos PD, Panagopoulos GN, Papagelopoulos PJ, et al (2017) Giant cell tumor of bone revisited. SICOT J. 3: 54.
- Campanacci M, Baldini N, Boriani S, Sudanese A (1987) Giant-cell tumor of bone. J Bone Joint Surg Am 69: 106-114.
- Tsukamoto S, Mavrogenis AF, Kido A, Errani C (2021) Current Concepts in the Treatment of Giant Cell Tumors of Bone. Cancers (Basel) 13: 3647.
- Van der Heijden L, Dijkstra PD, Van de Sande MA, Kroep JR, Nout RA, et al. (2014) The clinical approach toward giant cell tumor of bone. Oncologist 19: 550-561.
- Skubitz KM (2014) Giant cell tumor of bone: current treatment options. Curr Treat Options Oncol 15: 507-518.
- Van der Heijden L, Lipplaa A, Van Langevelde K, Bovée JVMG, Van de Sande MAJ, et al (2022) Updated concepts in treatment of giant cell tumor of bone. Curr Opin Oncol 34: 371-378.
- Nagano A, Urakawa H, Tanaka K, Ozaki T (2022) Current management of giant-cell tumor of bone in the denosumab era. Jpn J Clin Oncol 52: 411-416.
- Li H, Gao J, Gao Y, Lin N, Zheng M, Ye Z (2020) Denosumab in Giant Cell Tumor of Bone: Current Status and Pitfalls. Front Oncol 10: 580605.
- Borkowska AM, Szumera-Ciećkiewicz A, Szostakowski B, Pieńkowski A, Rutkowski PL (2022) Denosumab in Giant Cell Tumor of Bone: Multidisciplinary Medical Management Based on Pathophysiological Mechanisms and Real-World Evidence. Cancers (Basel) 14: 2290.
- Shi W, Indelicato DJ, Reith J, Smith KB, Morris CG, et al (2013) Radiotherapy in the management of giant cell tumor of bone. Am J Clin Oncol 36: 505-508.
- Ma Y, Xu W, Yin H, Huang Q, Liu T, et al. (2015) Therapeutic radiotherapy for giant cell tumor of the spine: a systemic review. Eur Spine J 24: 1754-1760.
- Ng VY, Davidson DJ, Kim EY, Pollack SM, Conrad Iii EU, et al (2014) The multidisciplinary management of giant cell tumor of bone. Expert Rev Anticancer Ther. 14: 783-790.
- Nishisho T, Hanaoka N, Miyagi R, Sakai T, Toki S, et al (2015) Local administration of zoledronic acid for giant cell tumor of bone. Orthopedics 38: e25-30.
- Kumar A, Sinha S, Haider Y, Jameel J, Kumar S (2021) Role of Zoledronic Acid Supplementation in Reducing Post-Surgical Recurrence of Giant Cell Tumor of Bone: A Meta-Analysis of Comparative Studies. Cureus 13: e16742.
Google Scholar, Cross ref, PubMed
Google Scholar, Cross ref, PubMed
Google Scholar, Cross ref, PubMed
Google Scholar, Cross ref, PubMed, PubMed
Google Scholar, Cross ref, PubMed
Google Scholar, Cross ref, PubMed
Google scholar, Cross ref, PubMed
Google scholar, Cross ref, PubMed
Google scholar, Cross ref, PubMed
Google Scholar, Cross ref, PubMed
Google scholar, Cross ref, PubMed
Google scholar, Cross ref, PubMed
Google scholar, Cross ref, PubMed
Google scholar, Cross ref, PubMed
Google scholar, Cross ref, PubMed
Google scholar, Cross ref, PubMed
Google scholar, Cross ref, PubMed
Google scholar, Cross ref, PubMed
Google scholar, Cross ref, PubMed
Google scholar, Cross ref, PubMed
Google scholar, Cross ref, PubMed
Google scholar, Cross ref, PubMed
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi