Review Article, J Immunol Tech Infect Dis Vol: 14 Issue: 1
Vitiligo is a Breakdown of the Melanocyte-Immune Tolerance
Sherif S. Awad*
Department of Dermatology and Venereology, Minia University, Minia, Egypt
*Corresponding Author: Sherif S. Awad
Department of Dermatology and Venereology, Minia University, Minia, Egypt
E-mail: awadderma@mu.edu.eg
Received date: 28 May, 2024, Manuscript No. JIDIT-24-137378;
Editor assigned date: 30 May, 2024, PreQC No. JIDIT-24-137378 (PQ);
Reviewed date: 13 June, 2024, QC No. JIDIT-24-137378;
Revised date: 10 January, 2025, Manuscript No. JIDIT-24-137378 (R);
Published date: 17 January, 2025, DOI: 10.4172/2329-9541.1000397
Citation:Awad SS (2025) Vitiligo is a Breakdown of the Melanocyte-Immune Tolerance. J Immunol Tech Infect Dis 14:1.
Abstract
The human body tries to tolerate the pigment-producing cells through several mechanisms including; central deletion of melanocyte-specific T cells or peripheral induction of inhibition or anergy of the released cytotoxic T cells. The melanocytes also try to hide their antigenic components from the immune system. The breakdown of these mechanisms leads to the induction of the adaptive immunity and the production of unopposed melanocyte-specific cytotoxic T cells, destroying the melanocytes, leading to the development of depigmentation. Vitiligo will develop whenever tolerancejeopardizing factors outnumber or over-weigh the toleranceinducing factors.
Keywords: Vitiligo; Lymphocytes; Treg; Immunity; T Cells
Introduction
The pathogenesis of vitiligo remained a controversial subject for decades, but the immune theory is now providing the main answers to the vitiligo riddle, with cellular, rather than humoral immune reaction, functioning to destroy melanocytes [1].
For healthy pigmented skin, the body’s immune system should tolerate the melanocytes that normally inhabit the epidermis, but when a breakdown of this normal physiologic tolerance happens, vitiligo can evolve.
Melanocytes immune-privilege
The melanocytes possess particular immunogenic components involving the melanogenic enzymes and structural parts. These components include tyrosinase, MART-1, gp100, TRP-1, and TRP-2 [2]. These melanocytes, making up only 5% of the skin cell composition, exist within the epidermal compartment and lack direct access to the blood or lymphatic circulation. So, protein levels are far below the threshold required to ever accomplish successful priming of effector cytotoxic T lymphocyte responses [3]. Moreover, melanocytes are not exposing their antigenic materials as melanin is produced within melanosomes that are surrounded by the double membrane Pigment Globules (PG), which are released from the dendrites and captured by the keratinocytes where they are degraded [4]. Exposed melanogenic antigens are too limited under normal conditions to initiate an immune response. Further to that, melanocytes were proven to lack expression of MHC-I molecules, with further deficiency when melanocytes or their precursors are within pilosebaceous adnexal tissues. This deficiency of MHC-I molecules renders melanocytes more immune-privileged [5].
Literature Review
The central immune tolerance
The thymus has a negative selection mechanism which renders the developing T-cell repertoire nonreactive or tolerant to body cells with self Major Histocompatibility Complex (MHC) peptide ligands. The major mechanism of induction of self-tolerance is thought to be thymic clonal deletion. In this way, the thymus can delete all T cells with receptors specific to normal body tissues, including melanocytes [6].
The peripheral immune tolerance
It is crucial for the cellular immune response that naive T cells be primed and activated properly. At least two signals are required for the proper activation of T lymphocytes, one delivered by the T-cell receptor complex with antigen recognition and the other provided on engagement of costimulatory receptors, such as CD28. Activation of T cells in the absence of costimulatory molecule components results in an anergic state. Anergic clones of T cells due to failure or lack of CD28 or B7 are unable to function properly and participate in the induction of tolerance [7].
Several other factors were found to contribute to the process of T cell inhibition of activation or destruction and may include:
• Cytotoxic T-lymphocyte antigen 4 (CTLA-4), which inhibits CD28- dependent T cell activation, cell cycle progression, and IL-2 production. CTLA-4 inhibition is more pronounced after the initiation of T-cell activation [8].
• Programmed cell death-1 (PD-1), also called CD279, is a member of the CD28 family that is expressed on activated T cells and is considered an inhibitory molecule that participates in immune tolerance.
• T-cell immunoglobulin and mucin-domain containing-3 (Tim-3), which is a co-inhibitory receptor that is expressed on IFN-gproducing T cells and has been shown to suppress their responses upon interaction with their ligand(s) [9].
• Regulatory T cells (Treg) were first defined as CD4+CD25+ doublepositive cells with suppressive functions on immunological response (Alpdogan and van den Brink 2012). Treg cell-mediated suppression of human melanocyte-specific CD8+ T cells causes decreased proliferation, decreased cytokine production, decreased T cell receptor affinity and increased susceptibility to apoptosis. Treg cells can render self-reactive human CD8(+) T cells anergic [10]. Treg may act as cytotoxic T cells that express granzyme A after activation and can kill activated CD4+ and CD8+ T cells by a perforin-dependent mechanism.
• Fas/Fas-L is another important factor that enhances peripheral tolerance through induction of apoptosis of CTL [11].
• Lymphatic endothelial cells (LECs) induce peripheral tolerance by induction of high-level expression of PD-1 with inhibition of the high-affinity IL-2 receptor that is necessary for T(CD8) survival.
These factors help keep the melanocytes alive and functioning, yet in some situations other factors can antagonize this beneficial immune tolerance process, leading to the recognition of melanocytes as a possible threat, and subsequently initiating the T cytotoxic destructive process against the pigment cell [12].
The breakdown of tolerance
Vitiligo can evolve when tolerance is lost in the following circumstances;
• Loss of proper central deletion due to a thymic failure, which will produce lymphocytes with melanocyte-specific epitopes. Direct evidence has been provided that a large Melan-A/MART-1- specific CD8 T-cell pool is generated during thymic selection [13]. These Melan-A-specific T cells were able to lyse melanoma cells in vitro, indicating their cytotoxic reactivity. While an unusually large repertoire of CD8+ T cells specific for this antigen has been documented, the reasons for its generation have remained mysterious [14]. The existence of TRP-1 or TRP-2-specific CTL was not proven to induce a relevant breakdown of tolerance that can induce vitiligo [15].
• Expression of Cutaneous Lymphocyte associated Antigen (CLA) on activated T cells and their frequency correlated with the extent of depigmentation and disease activity. Through the expression of CLA, activated T cells can find their way to the skin where they show type 1-cytokine profiles and mediate melanocyte death [16].
• Melanocytes expressing MHC-II and melanosomal transmembrane proteins can be transported to the surface of melanocytes and processed as MHC class II antigens via the endocytic pathway [17].
Other stimulatory factors include
CD40 and CD40L as members of the Tumor Necrosis Factor (TNF)/ TNF-receptor family that are expressed on activated T cells and NK cells [17].
CD28 which is essential for T cell activation, proliferation and survival as T cells interact with APCs [18].
CD27 which by binding to its ligand (CD70), ensures lymphocyte survival, increased T-cell proliferation, and memory cell formation. Serum soluble sCD27 levels in vitiligo patients were found to be higher than that of control cases with a greater correlation with disease activity [19].
Inducible T cell co-stimulator ICOS.
Glucocorticoid-induced TNFR family-related receptor (GITR) is known for its ability to induce effector CD8 T cells.
• Treg cell failure is also possible and decreased Treg cell numbers and function have been reported in patients with several autoimmune diseases including vitiligo.
• PD-1 failure as vitiligo-like lesions developed after anti-PD-1 therapy.
• Lymphocytic Fas/Fas-L apoptosis failure can result, and functional polymorphism of the FAS gene was reported with a high vitiligo risk.
Discussion
Melanocytes developing new antigenic components
It is not always melanocyte-specific antigens that are targeted. Different antigen epitopes are expected to develop and could be triggered by infections, trauma, stress and mutations [20].
Trauma can modify antigenic epitopes or harm the cells enough to initiate innate immunity attracting NK cells and macrophages with subsequent initiation of related adaptive CTL immunity. Similarly, after oxidative stress, the accumulation of ROS in melanocytes leads to melanocyte damage and the production of autoantigens that cellular immunity will target. A positive correlation was observed between serum Macrophage Migration Inhibitory Factor (MIF) and the spreading and activity of the vitiligo.
Mutated melanocyte antigenic proteins are found in localized areas of segmental vitiligo and some cases of mosaic nonsegmental vitiligo, providing new patient-specific antigens that can initiate localized immunogenic response.
Viral infection was documented in many vitiligo cases and may initiate the immune response when infecting the melanocytes.
More recently, the microbiome was investigated in vitiligo cases and distinct microbiota composition with enrichment in Proteobacteria, Streptococcus and Mycoplasma was found. The disruption of the balance of the microbiome is thought to initiate autoimmune responses through molecular mimicry, epitope spreading, and bystander activation.
Cells developing oncogenic antigen epitopes existing in nevi can express antigenic epitopes like MAGE, possibly responsible for the cytotoxic reaction and the induction of depigmentation in halo nevi. On acquiring an oncogenic mutation, primary cells can enter Oncogene- Induced Senescence (OIS) characterized by proliferation arrest and secretion of proinflammatory mediators. OIS in melanocytes is accompanied by an antigen presentation phenotype, likely to promote activation of the adaptive immune system.
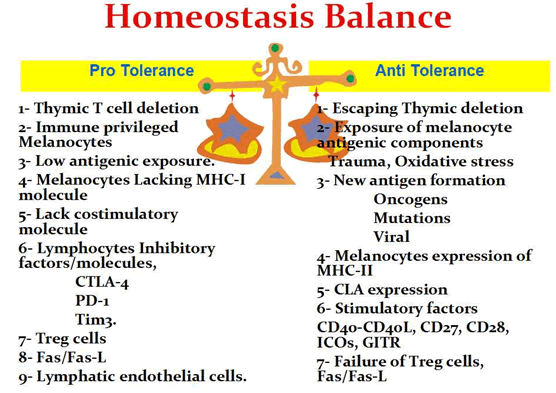
A brief enumeration of melanocyte pro-tolerance and anti-tolerance factors is illustrated in Figure 1.
Figure 1: Diagram showing a brief enumeration of pro-tolerance and anti-tolerance factors in an imaginary melanocyte homeostasis balance. A balanced level is required to avoid the risk of Vitiligo induction on one side or melanoma induction and spread on the other side.
Conclusion
In conclusion, the human body tries to tolerate the pigmentproducing cells through several mechanisms including; central deletion of melanocyte-specific T cells or peripheral induction of inhibition or energy of the released cytotoxic T cells. The melanocytes also try to hide their antigenic components from the immune system. The breakdown of these mechanisms leads to the immune-cytotoxic destruction of melanocytes and the development of depigmentation. Vitiligo can develop whenever tolerance-jeopardizing factors outnumber or over-weigh the tolerance-inducing factors.
References
- Alpdogan O, van den Brink MR (2012) Immune tolerance and transplantation. Semin Oncol 39: 629-642.
[Crossref] [Google Scholar] [PubMed]
- Andersen MH, Schrama D, Thor Straten P, Becker JC (2006) Cytotoxic T cells. J Invest Dermatol 126: 32-41.
[Crossref] [Google Scholar] [PubMed]
- Anderson MS, Su MA (2016) AIRE expands: new roles in immune tolerance and beyond. Nat Rev Immunol 16: 247-258.
[Crossref] [Google Scholar] [PubMed]
- Ando H, Niki Y, Ito M, Akiyama K, Matsui MS, et al. (2012). Melanosomes are transferred from melanocytes to keratinocytes through the processes of packaging, release, uptake, and dispersion. J Invest Dermatol 132: 1222-1229.
[Crossref] [Google Scholar] [PubMed]
- Basak PY, Adiloglu AK, Koc IG, Tas T, Akkaya VB, et al. (2008) Evaluation of activatory and inhibitory natural killer cell receptors in nonâ?ÂÂsegmental vitiligo: a flow cytometric study. J Eur Acad Dermatol Venereol 22: 970-976.
[Crossref] [Google Scholar] [PubMed]
- Ben Ahmed M, Zaraa I, Rekik R, Elbeldiâ?ÂÂFerchiou A, Kourda N, et al. (2012) Functional defects of peripheral regulatory T lymphocytes in patients with progressive vitiligo. Pigment Cell Melanoma Res 25: 99-109.
[Crossref] [Google Scholar] [PubMed]
- Boniface K, Seneschal J, Taïeb A, Merched A (2017) Vitiligo therapy: restoring immune privilege?. Exp Dermatol 26: 635-636.
[Crossref] [Google Scholar] [PubMed]
- Bzioueche H, Sjödin KS, West CE, Khemis A, Rocchi S, et al. (2021). Analysis of matched skin and gut microbiome of patients with vitiligo reveals deep skin dysbiosis: link with mitochondrial and immune changes. J Invest Dermatol 141: 2280-2290.
[Crossref] [Google Scholar] [PubMed]
- Côté AL, Zhang P, O'Sullivan JA, Jacobs VL, Clemis CR, et al. (2011) Stimulation of the glucocorticoid-induced TNF receptor family-related receptor on CD8 T cells induces protective and high-avidity T cell responses to tumor-specific antigens. J Immunol 186: 275-283.
[Crossref] [Google Scholar] [PubMed]
- Kawakami Y, Suzuki Y, Shofuda T, Kiniwa Y, Inozume T, et al. (2000) T cell immune responses against melanoma and melanocytes in cancer and autoimmunity. Pigment Cell Res 13: 163-169.
[Crossref] [Google Scholar] [PubMed]
- Khan U, Ghazanfar H. (2018) T Lymphocytes and Autoimmunity. Int Rev Cell Mol Biol 341: 125-168.
[Crossref] [Google Scholar] [PubMed]
- Kohil A, Abdalla W, Ibrahim WN, Al-Harbi KM, Al-Haidose A, et al. (2023) The Immunomodulatory Role of Microbiota in Rheumatic Heart Disease: What Do We Know and What Can We Learn from Other Rheumatic Diseases? Medicina (Kaunas) 59: 1629.
[Crossref] [Google Scholar] [PubMed]
- Lang KS, Caroli CC, Muhm A, Wernet D, Moris A, et al. (2001) HLA-A2 restricted, melanocyte-specific CD8(+) T lymphocytes detected in vitiligo patients are related to disease activity and are predominantly directed against MelanA/MART1. J Invest Dermatol 116: 891-897.
[Crossref] [Google Scholar] [PubMed]
- Le Poole IC, Luiten RM (2008) Autoimmune etiology of generalized vitiligo. Curr Dir Autoimmun 10: 227-243.
[Crossref] [Google Scholar] [PubMed]
- Li M, Sun D, Li C, Zhang Z, Gao L, et al. (2008) Functional polymorphisms of the FAS gene associated with risk of vitiligo in Chinese populations: a case-control analysis. J Invest Dermatol 128: 2820-2824.
[Crossref] [Google Scholar] [PubMed]
- Li Y, Teteloshvili N, Tan S, Rao S, Han A, et al. (2019) Humanized Mice Reveal New Insights into the Thymic Selection of Human Autoreactive CD8+ T Cells. Front Immunol 10: 63.
[Crossref] [Google Scholar] [PubMed]
- Lim WC, Olding M, Healy E, Millar TM (2018) Human Endothelial Cells Modulate CD4+ T Cell Populations and Enhance Regulatory T Cell Suppressive Capacity. Front Immunol 9: 565.
[Crossref] [Google Scholar] [PubMed]
- Moseley RP, Brown JI, Auld J (1997) An immunocytochemical study of MHC class I expression on human Langerhans cells and melanocytes. J Pathol 181: 419-425.
[Crossref] [Google Scholar] [PubMed]
- Rahimi A, Hossein-Nataj H, Hajheydari Z, Aryanian Z, Shayannia A, et al. (2019) Expression analysis of PD-1 and Tim-3 immune checkpoint receptors in patients with vitiligo; positive association with disease activity. Exp Dermatol 28: 674-681.
[Crossref] [Google Scholar] [PubMed]
- Rausch MP, Hastings KT (2012) GILT modulates CD4+ T-cell tolerance to the melanocyte differentiation antigen tyrosinase-related protein 1. J Invest Dermatol 132: 154-162.
[Crossref] [Google Scholar] [PubMed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi