Market Analysis, J Forensic Toxicol Pharmacol Vol: 8 Issue: 1
18th International Conference on Pharmacovigilence & Drug Safety Market Analysis at Pharmacovigilance 2020
Prof. Heidari
Professor, Chemistry, California South University, USA, E-mail: Researcher.@gmail.com
Keywords: Forensic Criminology, Forensic Death Investigation
The global pharmacovigilance market size was estimated at USD 4.31 billion in 2018 and is anticipated to witness a CAGR of 13.3% over the forecast period. Increasing incidence of Adverse Drug Reactions (ADRs) is expected to accelerate the demand for Pharmacovigilance (PV) services.
Growing prevalence of chronic diseases is another major contributor for the growth of the market. Treatment of these chronic diseases require uptake of combination of drugs resulting in ADR. PV services are used to curb this problem. According to the statistics published in the Journal of American Medical Association (JAMA), in 2014, ADR was one of the leading causes of mortality in U.S., resulting in more than 100,000 deaths every year.
Pharmacovigilance Market
The key factors propelling this market are increasing drug consumption and drug development rates, growing incidence rates of adverse drug reactions and drug toxicity, and increasing trend of outsourcing pharmacovigilance services. The increasing incidence of lifestyle diseases, such as diabetes, hypertension, and cardiac disorders, as a result of sedentary lifestyles, lack of physical activities, changing lifestyle patterns, and poor diets lead to increasing consumption of drugs, which, in turn, indicates the high demand for drug monitoring and further fuels the growth of the pharmacovigilance market. With the growing drug consumption, the need for the regular monitoring of drugs has also augmented, eventually boosting the pharmacovigilance market. Human infectious diseases are also on rise due to the changing climate, pervasive poverty, and increasing urbanization, which also surge the drug consumption and drive the drug development process. Furthermore, new drug developments need to get regulated and stimulate the overall pharmacovigilance market.
Scope of the Report
Pharmacovigilance is the science and activities relating to the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problems. The focus of pharmacovigilance is on ADR (adverse drug reactions) and drug toxicity. The pharmacovigilance market comprises of all types of adverse events reporting conducted during clinical trials in hospitals, pharmacies, and other healthcare sectors.
Key Market Trends:
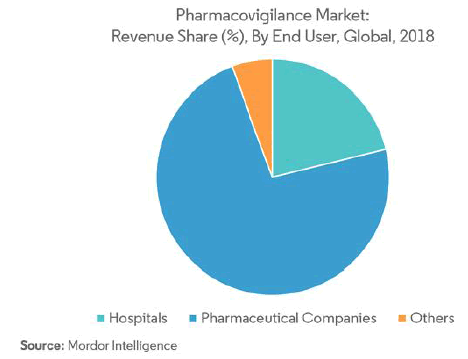
Pharmaceutical Companies are Expected to Hold the Highest Market Share in the End User Segment the end user segment of the pharmacovigilance market, pharmaceutical companies are believed to have the largest market size.
The role of pharmaceutical companies is to invest in the R&D of new compounds, have the commitment to bring a new drug to market to enhance the patients’ health and quality of life, strict governance to conduct clinical trials, product development activities as well as conduct relations with patients and healthcare professionals in accordance with ethical and legal principles. A major pharmaceutical company, such as Astra, has over 100 permanent, experienced staff in pharmacovigilance within its R&D organization in Sweden and the United Kingdom, and a similar number in local operating companies worldwide. This development has been driven by the increased recognition of the role of pharmacovigilance, the investigation, and marketing of a wider range of diverse medicinal products and more stringent and detailed regulatory requirements. Such developments that are occurring in the pharmaceutical companies are helping the pharmacovigilance market growth.
North America Dominates the Market and Expected to do Same in the Forecast Period:
North America currently dominates the market for pharmacovigilance and is expected to continue its stronghold for a few more years. Due to the shifting of high costs of in-house pharmacovigilance activities to CROs, the pharmacovigilance system in the United States is moving from a passive to a proactive role in the healthcare system. According to a 2017 publication in the Journal of American Medical Association, one out of three drugs in the United States may have the safety issues. Therefore, a need for modifying the current protocols for quick communication between healthcare providers and the FDA needs to be strengthened. Additionally, as more biosimilars would be available in the near future, accurately matching the adverse event is highly important. With that, the United States has a large market share of 56% in North America and is expected to register a growth rate of 12.3% over the forecast period.
The practice of monitoring the effects of medical drugs after they have been licensed for use, especially in order to identify and evaluate previously unreported adverse reactions. "the partnership hopes to develop diagnostic tools to improve pharmacovigilance"
The principle of international collaboration in the field of pharmacovigilance is the basis for the WHO Programme for International Drug Monitoring, through which over 150 member nations have systems in place that encourage healthcare personnel to record and report adverse effects of drugs in their patients. These reports are assessed locally and may lead to action within the country. Since 1978, the programme has been managed by the Uppsala Monitoring Centre to which member countries send their reports to be processed, evaluated and entered into an international database called VigiBase. Membership in the WHO Programme enables a country to know if similar reports are being made elsewhere. When there are several reports of adverse reactions to a particular drug, this process may lead to the detection of a signal, and an alert about a possible hazard communicated to member’s countries after detailed evaluation and expert review.
The "pharmerging", or emerging pharmaceutical market economies, which include Brazil, India, Russia, Argentina, Egypt, Indonesia, Mexico, Pakistan, Poland, Romania, South Africa, Thailand, Turkey, Ukraine and Vietnam, accrued one fifth of global 2011 pharmaceutical expenditures; in future, aggregated data for this set will include China as well.
China's economy is anticipated to pass Japan to become second in the ranking of individual countries' in pharmaceutical purchases by 2015, and so its PV regulation will become increasing important; China's regulation of PV is through its National Centre for Adverse Drug Reaction (ADR) Monitoring, under China's Ministry of Health.
As JE Sackman notes, as of April 2013 "there is no Latin American equivalent of the European Medicines Agency—no common body with the power to facilitate greater consistency across countries". For simplicity, and per sources, 17 smaller economies are discussed alongside the 4 pharmemerging large economies of Argentina, Brazil, Mexico and Venezuela—Bolivia, Chile, Colombia, Costa Rica, Cuba, Dominican Republic, Ecuador, El Salvador, Guatemala, Haiti, Honduras, Nicaragua, Panama, Paraguay, Peru, Suriname, and Uruguay. As of June 2012, 16 of this total of 21 countries have systems for immediate reporting and 9 have systems for periodic reporting of adverse events for on-market agents, while 10 and 8, respectively, have systems for immediate and periodic reporting of adverse events during clinical trials; most of these have PV requirements that rank as "high or medium...in line with international standards" (ibid.). The WHO's Pan American Network for Drug Regulatory Harmonization seeks to assist Latin American countries in develop harmonized PV regulations.
Some further PV regulatory examples from the pharmerging nations are as follows. In India, the PV regulatory authority is the Indian Pharmacopoeia Commission, with a National Coordination Centre under the Pharmacovigilance Program of India, in the Ministry of Health and Family Welfare. Scientists working on pharmacovigilance share their experiences, findings, innovative ideas and researches during the annual meeting of Society of Pharmacovigilance, India.[citation needed] In Egypt, PV is regulated by the Egyptian Pharmacovigilance Centre of the Egyptian Ministry of Health
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 
