Mini Review, J Nucl Ene Sci Power Generat Technol Vol: 12 Issue: 4
A Brief Explanation on Porous Stainless Steel Microspheres and Energy-Generating Nanotechnological Devices
Carla de Albuquerque Dias and Armindo Santos*
Nuclear Technology Development Center (CDTN/CNEN), Belo Horizonte 31270-901, Minas Gerais, Brazil
*Corresponding Author: Armindo Santos
Nuclear Technology Development Center (CDTN/CNEN)
Belo Horizonte 31270-901, Minas Gerais, Brazil
E-mail: santosa@cdtn.br
Received date: 23 July, 2023, Manuscript No. JNPGT-23-107784;
Editor assigned date: 25 July, 2023, PreQC No. JNPGT-23-107784 (PQ);
Reviewed date: 10 August, 2023, QC No. JNPGT-23-107784;
Revised date: 17 August, 2023, Manuscript No. JNPGT-23-107784(R);
Published date: 24 August, 2023 DOI: 10.4172/2325-9809.1000347.
Citation: Santos A and Dias CA (2023) A Brief Explanation on Porous Stainless Steel Microspheres and Energy-Generating Nanotechnological Devices. J Nucl Ene Sci Power Generat Technol 12:4.
Abstract
The concept of porous stainless steel microspheres was inspired by the intelligent design of functional structures present in nature and materialized with the help of the sol-gel technology. Porous metallic and/or ceramic microspheres can help in the development of conventional and advanced fuels, which can be used in technological devices (e.g., advanced nuclear reactors) for the generation of renewable energy, as renewable and safe sources of energy are required for the continuous civilizing evolution of the mankind. As a result of the above, some extra peculiarities of the synthesis and potential applications of the nanotechnological structure represented by the porous metallic microspheres are presented here.
Keywords
Advanced nuclear reactors; Porous metallic; Renewable energy
Introduction
Currently, the science and engineering seek to design, produce, and use structures, devices, and systems for practical use arising from the manipulation of atoms (0.1 nm-0.4 nm in diameter; and molecules, too) in order to aggregate them in nanometric dimensions ( 100 nm) and thus to produce nanostructured materials. The materialization of the intelligent design of these functional structures through nanotechnology has a simple reason: nanostructured materials have fundamental physical properties different from those of a single atom and bulk materials. Because of their low coordination number, atoms on a surface fuse much sooner than atoms in the bulk therefore, a nanostructured material will have a lower melting point because it has a large number of surface atoms. This and other characteristics of nanoparticles were exploited in the manufacture of the porous stainless steel microspheres and helped to enable the creation of the metal/fuel ceramic (UO2) interface without discontinuities during the sintering process of the SS-UO2 cermet pellets [1-6]. Some particularities in the synthesis of the porous stainless steel microspheres were omitted some of which are reported below extra details in the synthesis and practical applications of the porous stainless steel microspheres.
Literature review
The synthesis of nanostructured materials using wet chemical processes involves mixing components that are soluble or solubilisable in an aqueous and/or alcoholic medium, which, in the case of the stainless steel microspheres, are the compounds Fe(NO3)3.9H2O, Cr(NO3)3.9H2O, and Ni(NO3)2.6H2O. In these compounds, Fe, Cr, and Ni atoms are present in their ionic forms, mainly as Fe3+, Cr3+, and Ni2+. This ionic nature of Fe, Cr, and Ni atoms makes it possible to agglomerate them at room temperature and form nanometric structures. This nanostructuring is facilitated when interfering with the nucleation and growth of nanoparticles from the individual compounds of Fe, Cr, and Ni, which have been mixed at a molecular level. This interference can be produced with and without the use of false crystallization nuclei or organic Polyvinyl Alcohol (PVA), for example) and inorganic compounds, provided that the solubility of the ionic species of Fe, Cr, and Ni in the aqueous and/or alcoholic medium is altered, leading them to chemical precipitation. In the case of the stainless steel microspheres, the false nuclei are represented by the carbon black nanoparticles (depicted by CSolid), which will serve both as a pore template and as fuel in situ for the reactions in the subsequent oxide-metal reduction. The induction of agglomeration of metal ions to form nanomaterials takes place via hydrolysis and condensation reactions. As a result, there is a mixture of segregated nanoparticles and/or of them forming interfaces of the type Fe2O3.xH2O/Cr2O3.xH2O/NiO.xH2O/CSolid that will compose the nanostructured material. It is important to note that the creation of interfaces between these nanostructured materials can generate in situ a large amount of chemical energy in the form of heat and favor different thermally activated processes, particularly the oxide-metal reduction process. One cannot exclude the occurrence of Ni2+ and/or Cr3+ and/or Fe3+ chemisorption in the individual agglomerates formed (Fe2O3.xH2O, Cr2O3.xH2O and NiO.xH2O) during the chemical precipitation of the nanostructured material, even partially, which would lead to the formation of a typical solid solution of Fe, Cr, and Ni. The segregation of the produced nanoparticles can be attested by analytical techniques (Energy-Dispersive X-Ray Spectroscopy (EDS) analysis; and X-ray Diffraction Analysis (XRD)) of the resulting dry nanomaterial. The EDS analysis will show an elemental map of the “islands” of the Fe, Cr, and Ni elements and the CSolid compound, while the DRX analysis will display a spectrum with a characteristic amorphism halo, whose detailed analysis can reveal the formation of all metal oxides mentioned and the presence of the CSolid phase. Another important fact is that the composition of the nanostructured material may be contributing to the formation of eutectic metallic alloys, that is, a metallic alloy with a lower melting temperature than that of its individual constituents. These facts are important, because in the heat treatment applied subsequently; there will be a need to consider an optimal time for interdiffusion and homogenization of the elements to form the desired metallic alloy with or without pore closure, as well as without or with grain growth [7-9].
In the oxide-metal reduction, hydrogen (H2; supplied externally) and CO produced in situ via CSolid combustion at the Fe2O3.xH2O/CSolid, Cr2O3.xH2O/CSolid, and NiO.xH2O/CSolid interfaces are used as reducing agents as with CSolid, note that hydrogen has to be seen in the metal-oxide reduction of the microspheres as a fuel in situ. Because of this, there is an interesting connection between nanostructured materials and hydrogen, both of which are necessary for the production of renewable energy. The use of hydrogen as an environmentally friendly fuel, replacing fossil fuels, has been intensely researched. In this sense, hollow metallic spheres have emerged as one of the alternatives for applications involving hydrogen storage, because they have low density and high specific surface area. The relevant fact here is that nanostructured materials like these can optimize the thermodynamics and kinetics of hydrogen adsorption/ desorption, since they have an excellent capacity to store hydrogen on their surfaces and subsurfaces [10]. Although desired as an energy source and reducing agent, the use of hydrogen can form metal hydrides and hinder the interdiffusion of metallic elements and final homogenization of the metallic alloy [11]. On the other hand, the formation of metal hydrides is also an explored approach to store hydrogen and use it as fuel [12]. The formation of metal hydrides in real processes takes place at high temperatures (673K-873K) and at relatively high H2 pressures (0.1MPa-10MPa) due to the presence of a passivation layer of the types H2O and/or oxides, hydroxides, and compounds containing C and O of the metal or metals present, which can hinder the absorption and reaction mechanisms of hydrogen in the formation of metal hydrides. For the metal-hydrogen reaction to take place, the Hsub molecule must be transported to the gas-solid interface. Once there, it is necessary for the H2 molecule to be dissociated on the surface via chemisorption at special active sites. Next, the dissociated H atoms need to migrate to the active sites of the metal on the surface and in the bulk of the material, where they dissolve in the vicinity of the solid-gas interface. Grain boundaries, defects or other discontinuities in the bulk of the material are sites of nucleation of the metal hydride phase. In these locations, as soon as the concentration of atomic Hydrogen (H) in the metal exceeds the saturation concentration, a hydride phase is formed. It should be noted that most intermetallic hydrides formed are brittle. Furthermore, the resulting metallic hydride is less dense than the metallic alloy and this leads to the formation of cracks, which weakens the metallic alloy due to the presence of fracture in the hydride phase. This feature is exploited in metal alloy pulverizing processes. Fortunately, the reversibility of the metal hydride formation reaction also depends on the temperature and pressure of H2 to lead to its decomposition and allow the desorption of H2, the recombination of the metal atoms, and the homogenization of the metal alloy after its controlled annealing and cooling [13].
Discussion
It was interesting to realize that hydrogen could be being generated in situ in the heat treatment of the porous stainless steel microspheres via in situ CO and CO2 methanation reactions, even without an external source of H2. It is important to inform that in the heat treatment carried out on the microspheres there was: electrical energy being supplied; and in situ energy source represented by carbon (CSolid and PVA); an external energy source from hydrogen; and efficient metal catalysts (Fe0, Cr0, and Ni0 nanoparticles embedded in an oxide matrix). That is, the heat treatment on the microspheres was generating synthetic natural gas (a renewable fuel) inside the microspheres via CO and CO2 methanation reactions. This synthetic natural gas would predominantly contain CH4 (methane), which would also become an in situ source of H2 for oxide-metal reduction and metal hydrogenation reactions. Regarding the production of natural gas, it is well established knowledge that there are several processes to produce methane from CO2 and H2. Among them, thermochemical methanation is perhaps the most studied process, which is typically carried out in the temperature range 523K-623K and at 25 bars via hydrogenation of CO2 and CO (in this case, generated in situ). In this process, the H2 required for the thermochemical methanation reaction could additionally originate from the thermochemical decomposition of H2O molecules, which could initially be present in the calcined microspheres. Characteristically, the reactions involved in the methanation of CO2 and CO are highly exothermic. Thus, the reversible methanation reactions potentially taking place in the microspheres could be the following [14,15]:
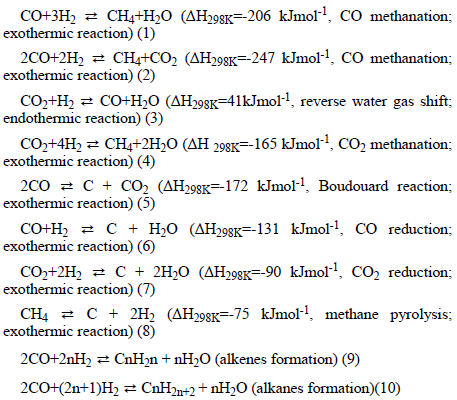
Considering only reactions (1) and (4), they could be responsible for an evolution of 2.03 (Equation 4) and 2.55 (Equation 1) kWh of heat per m3 of methane produced under normal temperature and pressure conditions, which could explain the increase in the nominal temperature in the thermal treatment of the spheres, which could exceed the melting temperature of the eutectic alloy formed in the microspheres [14]. The reactions in Equations (5-8) generate C molecules that are deposited on the surface of the phases, particularly those represented by Fe0, encapsulating them and decreasing their catalytic activity, besides, of course, justifying their residual presence in the formed metallic alloy [15].
Conclusion
The sol-gel technology used in the manufacture of the stainless steel microspheres proved to be a great approach to materialize the intelligent design of the functional structures of the nanostructured materials. Nanostructured materials have fundamental physical properties different from those of a single atom and bulk materials, as they have a large number of surface atoms. Such surface atoms have a low coordination number and, therefore, they fuse much earlier than the atoms in the bulk. In this way, a nanostructured material will have a lower melting point, which can facilitate the processing of nanometric structures at much lower nominal temperatures and, thus, avoid an exaggerated grain growth, as well as enable the maximization of the densification of a compacted solid.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi