Research Article, J Forensic Toxicol Pharmacol Vol: 6 Issue: 1
A Rapid Dilute and Shoot - Flow Injection MRM Method for the Quantification of EtG in Urine
Alagandula R and Guo B*
Department of Chemistry, Cleveland State University, 2121 Euclid Avenue, Cleveland, Ohio 44115, USA
*Corresponding Author : Guo B
SR 397, Department of Chemistry, 2121 Euclid Avenue, Cleveland, Ohio 44115, USA
Tel: 216-687-2471
Fax: 216-687-9298
E-mail: B.GUO@csuohio.edu
Received: June 26, 2017 Accepted: August 18, 2017 Published: August 25, 2017
Citation: Alagandula R, Guo B (2017) A Rapid Dilute and Shoot - Flow Injection MRM Method for the Quantification of EtG in Urine. J Forensic Toxicol Pharmacol 6:1. doi: 10.4172/2325-9841.1000154
Abstract
Quantification of ethyl glucuronide (EtG, a metabolite and biomarker of ethanol) in urine is of great interest in both forensic and clinical applications. Currently, a single run of LC-MS/MS can quantify the vast majority of drugs and abused substances included in the comprehensive urine drug testing (UDT) panel, but EtG must be quantified in a separate run because EtG is polar, limiting the throughput of current MS-based methods. In this study, we developed a simple and fast MRM method to quantify EtG in urine without LC separation. Briefly, a urine sample containing EtG is first diluted 20 times, followed by direct flow-injection of the diluted sample to the ESI source. Quantification of EtG was achieved by multiple reaction monitoring (MRM) operated in the negative mode with stable isotope labeled EtG as internal standard. The method was fully validated and has a dynamic range of 50-2000ng/mL (R2>0.998). The coefficients of variation and relative errors for interand intra-assay studies were in the range of 2.4-4.2%, respectively.In addition, a robustness test was performed with >1200 injections of urine samples and no significant deteriorations in sensitivity and specificity were observed. This new method has a total run time of only 2 min per sample, enabling analysis of >700 samples per day by one single mass spectrometer.
Keywords: Quantification; Ethyl glucuronide; Urine; Flow injection; Mmultiple reaction monitoring
Introduction
Alcohol abuse is a major concern for pain management due to the high risk of substance abuse in the pain management patient population. Hence, the level of alcohol in various patients is frequently monitored [1-4]. Ethyl glucuronide (EtG) is a long term and stable Phase II metabolite of ethanol and the presence of EtG in urine can be used to detect the intake of alcohol from hours to several days after the initial ingestion until after complete elimination of ethanol from the body. Due to its long half-life [5-8], EtG has been used as a biomarker of ethanol, one of the most abused substances.
In recent years, urine drug testing (UDT) has become a general clinical practice for monitoring compliance of medication, particularly pain management drugs. LC-MS/MS is emerging as the method of choice for UDT in many fields, including clinical and forensic toxicology, workplace drug testing, and doping control. Each year, there are millions of mass spectrometric-based UDTs performed. Among ~100 commonly prescribed pain management drugs and abused substances, the overwhelming majority of them can be quantified by a single LC-MS/MS run, in which they are first separated by a C18 column, followed by the MS/MS quantification operated in the positive ion mode [9-11].
However, EtG must be quantified separately. This is because it is polar, requiring the use of a different column (i.e. polar column). In addition, the negative ionization mode works best for EtG [12-20]. Performing a separate LC-MS/MS run just to quantify EtG significantly limits the throughput and increases the cost of UDT, becoming a major issue in UDT. Since LC separation is the most time-consuming part of a mass spectrometric-based procedure, we, in this study, developed and validated a fast dilute and shoot flowinjection (FI) MS/MS method without LC separation to quantify EtG in urine. In this method, a urine sample containing EtG is spiked with an internal standard (stable isotope labeled EtG), followed by dilution of the urine sample by 20 times to minimize the matrix effect. Then, the diluted urine sample is subjected to centrifugation to remove precipitates and solid particles. Thereafter, the supernatant of the diluted sample is directly injected (flow-injection) into the electrospray ionization (ESI) source of a mass spectrometer operated in a MRM mode for identification and quantification of EtG. We found that simple dilution for sample pretreatment along with good stable isotope labeled internal standards enabled the rapid, precise and robust quantification of EtG in urine without extensive prepurification (SPE or LLE) and LC separation.
Because the total run time of our method is only 2 minutes, a single MS/MS instrument can analyze >700 urine samples per day, increasing the throughput of MS-based UDT methods. To the best of our knowledge, this is the first FI-MS/MS method for the rapid and accurate quantification of EtG in urine without extensive sample purification and time-consuming LC separation. Detailed description of this method constitutes the focus of the present article.
Materials and Methods
Chemicals and reagents
Ethyl glucuronide (analyte) (EtG) and Ethyl glucuronide-DM5 (internal standard, IS) (EtG-D5) were purchased from Cerilliant Corporation (Round Rock, TX, USA). HPLC grade methanol and acetonitrile were purchased from Pharmco-Apper (Philadelphia, PA, USA). Deionized water was obtained from a Barnstead Nano pure water purification system from Thermo Scientific (Waltham, MA, USA). Ethanol free urine was donated by 10 different healthy volunteers and was verified not to contain ethanol before analysis.
Sample preparation
Urine Samples were prepared by mixing EtG and EtG-D5 (IS) at the concentrations of 50, 100, 200, 500, 1000, and 2000 ng/mL (EtG) and 500 ng/mL (IS), respectively, with 50 μL of human blank urine both pooled from 10 lots and individual lots as well. The spiked urine samples are then diluted 20 times with acetonitrile and then centrifuged at 13,000 g for 20 min. The supernatants were transferred into auto sampler vials and analyzed using FI-MS/MS.
Mass spectrometer
EtG in urine samples was analyzed using 5500 QTRAP triple quadrupole mass spectrometer (AB Sciex, Toronto, Canada) equipped with a Turbo V ion source operated in the ESI mode, interfaced with a HPLC system containing two LC-30 AD pumps, a DUG- 20A3R inline degasser, a SIL-30 AC auto sampler, a CBM- 20 A controller and a CTO-10AVP column oven (Shimadzu, Columbia, MD, USA) [21].
It is important to note that the HPLC system used here was just for flow-injecting samples from the sample vials to the ESI source and had no HPLC column. Ammonium acetate (0.5 mM) in 70% acetonitrile was used as a carrier solvent at a flow rate of 0.3 mL/min to inject and carry the sample to the MS and the same solvent was also used as a wash solvent, which was performed only after injecting the highest concentration sample (2000 ng/mL) at the end of each batch. 10 μL of each sample were injected with a total run time of 2 min per sample. All the MS and sample injection parameters were selected and controlled by Analyst software (version 1.6.1; AB Sciex, Toronto, Canada) [22].
MS/MS
For detection and quantification, the following ESI-MS/MS conditions were applied: (i) ESI source dependent parameters were optimized by direct infusion analysis: curtain gas (45), nebulization gas (Gas I) (28), heating gas (Gas II) (30), ion spray voltage (-4500 eV), temperature (500°C); and (ii) Analyte-dependent parameters were fine-tuned manually as follows: collision energy (35), cell exit potential (15), declustering potential (-100), entrance potential (15), and dwell time for each MRM transition (150 ms).
Both ESI source and analyte-dependent MS parameters were fine-tuned and optimized by direct infusion of EtG at 100 ng/mL in the negative ESI mode. Best ionization conditions for EtG ionization were selected based on selective combination of high ion intensity, low background noise in urine matrix and reproducible analyte peak area. The method was checked for cross-talk by injection of EtG, Q1 and Q3 were operated in unit resolution.
Stock and working solutions, calibrators, and quality control (QC) samples
Stock solutions of EtG and EtG-D5 (IS) were prepared at 50 μg/mL and 10 μg/mL from 1 mg/mL and 100 μg/mL main stock solutions, respectively. A set of EtG working solutions of 50, 100, 200, 500, 1000, and 2000 ng/mL spiked with 500 ng/mL EtG-D5 (IS) were prepared by serial dilution from the stock solutions in acetonitrile, respectively. Similarly, the pooled and individual urine calibrators (mixture of 10 lots of blank human urine) were prepared by spiking EtG at 50–2000 ng/mL and 500 ng/mL IS, followed by 20 times dilution and centrifugation. A full validation measurement was performed with QC samples at 50, 125, 445 and 1600 ng/mL, representing the lower limit of quantification (LLOQ), low QC (LQC), mid QC (MQC), and high QC (HQC), respectively, along with the calibrators (50-2000ng/ mL). The working solutions, QC and calibrators were then subjected to FI-MS/MS analysis. The calibration range was chosen to cover a broad range of the expected concentrations of EtG in the urine samples based on cut off value: 200ng/ml [1]. All the concentrations of EtG reported here are equivalent to the concentrations of EtG in urine without dilution.
Results and Discussion
Method development
Optimization of flow injection and selection of MRM transitions: We first optimized the MS/MS condition to identify and minimize any potential interference from urine matrix because our dilute-and-shoot FI-MS/MS method does not use LC to separate the interfering background from EtG and internal standard molecules. This was accomplished by individual screening of blank urine samples from 10 people without EtG under various MS parameters. MRM parameters were optimized with infusion, which lead to the selection of the optimized carrier solvent, buffer, and specific fragment ions to achieve the maximum sensitivity for both EtG and IS. Because EtG is a weak acid and polar molecule (pKa = 3.21 and log P = 1.51) [23], the negative ESI mode was selected. The IS used in this work was EtG-D5. The use of deuterated EtG as internal standard improved the quantitative accuracy, compensated for the matrix effect, and was essential to dilute-and-shoot based methods [24-27].
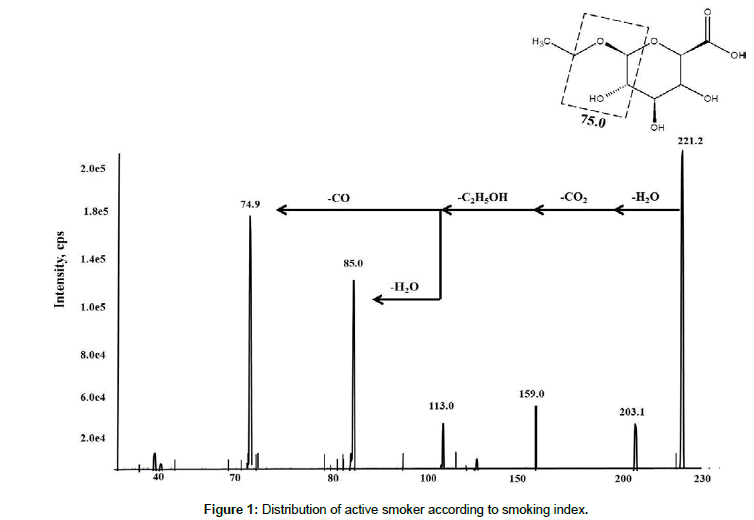
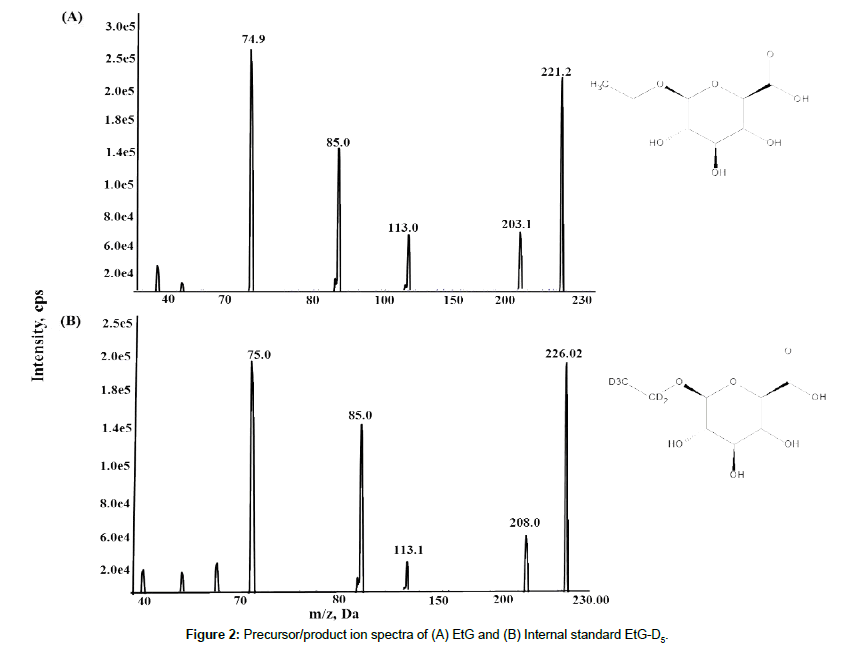
Fragmentation of EtG (deprotonated precursor ions) by collisioninduced dissociation (CID), with a collision energy of -40 eV and the dwell time at 300 ms, led to the identification of abundant product ions, at m/z 221.2 → 74.9 (quantitative) and m/z 221.2 → 85.0 (qualitative), and m/z 226.02→ 75.0 for IS (EtG-D5), respectively. The most abundant product ions of EtG and EtG-D5 (m/z 75 and 85) result from the fragmentation of glucuronic acid. The general fragmentation pattern of EtG is shown in Figure 1 and the product ion spectra for both EtG and IS are shown in Figure 2, respectively. The low abundance fragment ions may result from subsequent loss of water (m/z 203), loss of ethanol and CO2 (m/z 113), loss of water (85) and loss of CO (75.0). In this study, we did not use the less abundant transitions 221/113 and 221/159 for method validation, but they could be used as additional qualifiers for EtG identification [12,23,28].
The use of these fragmentation conditions led to the good signal to noise and minimized the interference from other molecules. Both quantitative and qualitative fragment ions were carefully evaluated during the method development and validation processes. A quantitative transition is used to calculate the concentration of EtG based on the quantifier ion, while a qualitative transition is used to confirm the specific identification of EtG based on the ratio of the qualifier ion to quantifier ion [29]. After optimization, we found that both EtG and IS were well detected at 0.7 minutes, allowing for both the qualitative and quantitative determination of EtG by MRM operated in negative ESI mode with a total run time of 2 minutes. The carrier solvent (0.5mM ammonium acetate in 70% acetonitrile) at a flow rate of 0.3 mL/min was used to inject samples, with 10μL sample volume. Software Analyst 1.5.1 (AB Sciex, Darmstadt, Germany) was used in data acquisition and analysis.
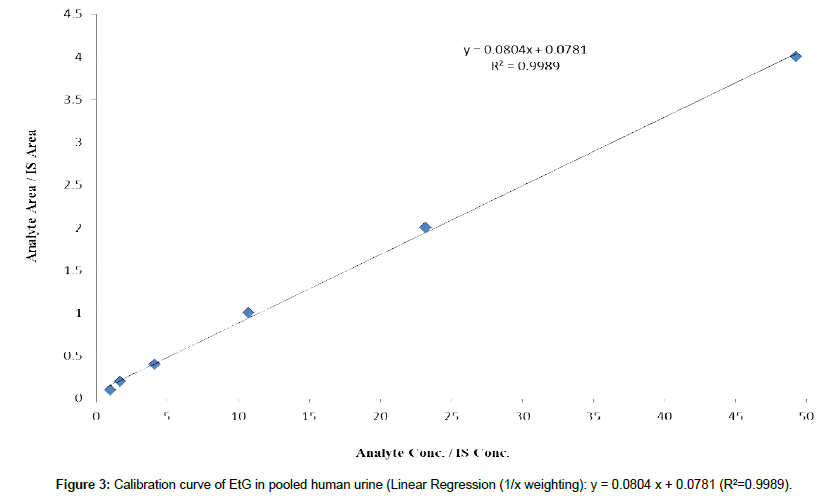
Cutoff and calibration range: Selecting an appropriate cutoff value allows an accurate measurement of EtG in urine. A too high cutoff value may produce false negative results, while a too low cutoff value can lead to false positive results. Based on current clinical practice, the cutoff value for EtG has been set at 100-200ng/ mL by many reference laboratories, and the same cutoff values were suggested by SAMSHA guidelines [1,30-33]. Hence we developed our calibration range from 50-2000 ng/mL (Figure 3), with the LLOQ (50 ng/mL) was set at 25% of the cutoff value of 200ng/mL. It is noted that the quantity of EtG listed below are the concentration of EtG in urine before 20x dilutions. EtG urine calibrators were prepared at the following 6 concentrations: 50 ng/mL, 100 ng/mL, 200 ng/mL (cutoff), 500 ng/mL, 1000 ng/mL and 2000 ng/mL, where the cutoff concentration in urine falls in the mid-point of our calibration range, allowing for the measurement of EtG at the concentrations both higher and lower than the cutoff value in urine samples.
Method validation
A full method validation was performed based on currently accepted FDA Bioanalytical Method guidelines [34]. The method was validated for linearity, precision, accuracy, selectivity, lower limit of quantification (LLOQ), matrix effect and sample stability parameters.
Linearity, selectivity, sensitivity and LLOQ: The calibration plots were evaluated by analyzing six replicates of spiked EtG pooled urine calibrators at 50, 100, 200, 500, 1000, and 2000 ng/mL, double blank and single blank (only IS). Excellent linearity was achieved with the mean correlation coefficient of R2 = 0.998 by plotting peak area ratio vs concentration using 1/X weighing factor. Standardized residual plots were plotted to evaluate the calibration curves to check for outliers for each calibrator during the course of method validation for five batches on five separate days (Inter assay). The standard deviation (SD) for the residuals was found to be in the range of 0.95– 2.7, which is acceptable (< ±3 SD).
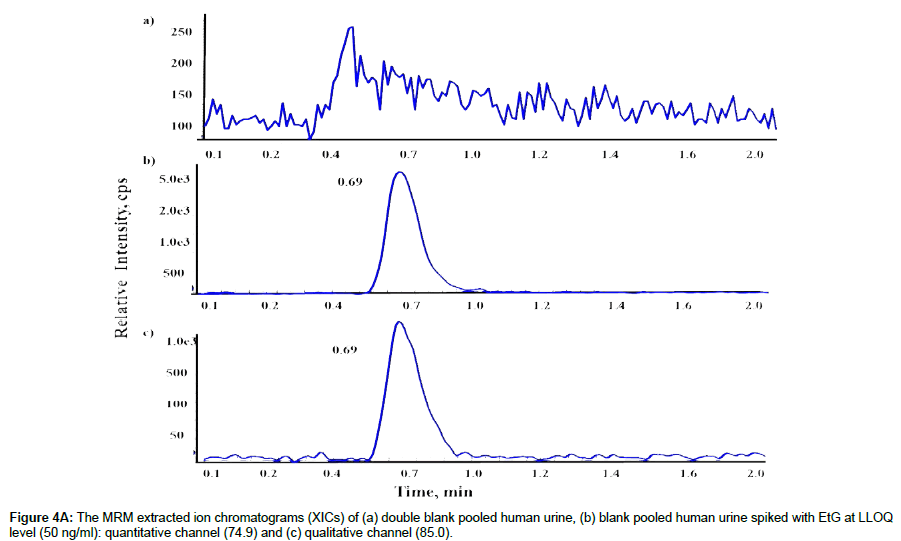
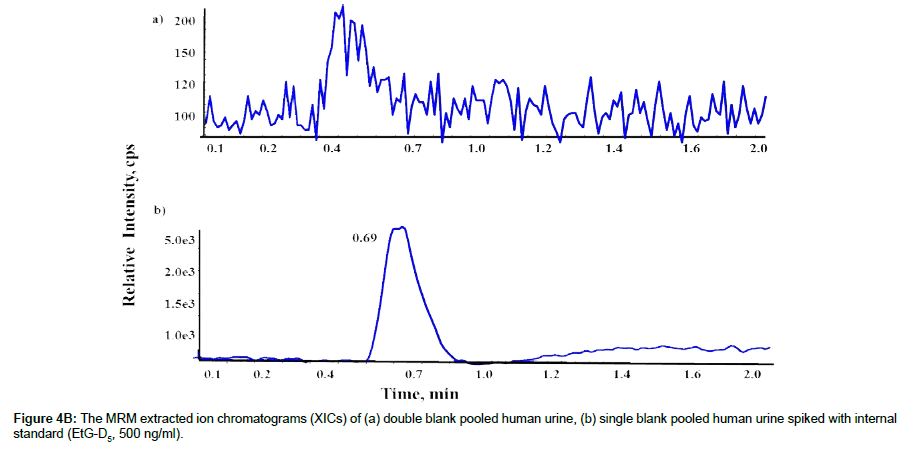
LLOQ and selectivity of the method were determined by evaluating 50ng/ml of EtG (LLOQ) in pooled and individual lots, blank urine, and double blank urine samples from 10 different sources to determine whether any endogenous compounds contribute for the significant background signals that might interfere with the detection of EtG or IS. The highest signal (peak) from EtG at LLOQ and IS at 500ng/mL were at 0.70 minutes after injecting samples. No significant interfering signals (peaks) were found during this time windows in any samples tested, indicating high selectivity of our method. The S/N ratio of the quantitative transition (74.9) and the qualitative transition (85.0) for EtG was 20 and 12 at LLOQ (50ng/mL) (Figure 4A), and the S/N ratio for IS for the transition at 85.0 was 50 at 500ng/ mL (Figure 4B). The coefficient of variation (%CV) and accuracy for LLOQ were <6.1 and <3.2%, respectively, meeting the requirement of FDA guidelines (Table 1).
| Spiked Concentration (ng/mL) |
Determined Concentration (ng/mL) | Accuracy (%RE) | Precision (%CV) |
|---|---|---|---|
| 50 | 5.07 ± 0.20 | 3.08% | .01% |
| 100 | 7.6 ± 0.21 | 2.40% | .86% |
| 200 | 95.6 ± 0.50 | 2.20% | .79% |
| 500 | 95.0 ± 0.01 | 1.01% | .79% |
| 1000 | 98.4 ± 0.11 | 0.16% | .32% |
| 2000 | 2001.0 ± 0.54 | 4.01% | 0.12% |
Table 1: Accuracy and precision of EtG calibration standards in pooled blank human urine (n=5).
Accuracy and precision: Accuracy of an analytical method describes the closeness of mean test results obtained by the method to the true value (concentration) of the analyte, and the deviation of the mean from true value serves as the measure of accuracy. The precision of an analytical method describes the closeness of individual measures of target analyte when the developed method is repeatedly applied to multiple aliquots of a pooled or single homogeneous biological matrix.
To assess the accuracy and precision of the method, the high, medium, and low quality control (QC) samples were prepared and analyzed. Software Valistat 2.0 was used to assess statistical variation, the accuracy (bias), as well as the intra-day and inter-day precision. Intra- and inter- assays were performed to assess precision and accuracy by measuring 5 replicates of 3 QC standards, LQC, MQC and HQC of 125, 450 and 1600 ng/mL, along with the calibrators (50-2000ng/mL) five times a day for five consecutive days. The QC concentrations selected were based on the cutoff of 200ng/mL. Both accuracy and precision determined were within the acceptance interval of ± 15% of the nominal values at all concentrations. The intraand inter- accuracy and precision for EtG at 3 QC levels was found to be less than 4.2 (%RE) and 2.4% (%RSD) respectively, meeting the acceptance criteria, indicating that the FI-MS/MS method developed is highly accurate and precise. Summarized results for intra-day and inter-day assay precision and accuracy are listed in Table 2.
| Intra-assay | Inter-assay | ||||||
|---|---|---|---|---|---|---|---|
| Spiked EtG (ng/mL) | Measured Mean ± SD (ng/mL) |
Precision (%RSD) | Accuracy (%RE) |
Measured Mean ± SD (ng/mL) |
Precision (%RSD) |
Accuracy (%RE) | |
| Low QC Mid QC High QC |
125 450 1600 |
123.06 ± 0.02 453.4 ± 0.07 1604.8 ± 0.26% |
2.33 0.66 1.59 |
1.12 3.78 0.31% |
122.02 ± 0.07 446.88 ± 0.47 1589.40 ± 0.1 |
2.0% 1.0% 0.66% |
2.39 4.19 1.39 |
Table 2: Inter- and intra-assay accuracy and precision of EtG in pooled blank human urine (n= 5).
Absolute and relative matrix effect: Matrix effects, both absolute and relative, were determined in triplicate at 3 QC concentrations (125, 450 and 1600 ng/mL) and LLOQ (50ng/mL) in both individual and pooled blank urine samples (10 different urines). The matrix effects were calculated by analyzing 10 individual blank urine lots to evaluate the degree of variation of ion suppression or enhancement among individual and pooled urine samples.
Absolute matrix effect was determined by comparing the peak area of EtG at 3 QC and LLOQ concentrations in 20x diluted blank urine samples (both pooled and individual lots) with the peak area of EtG in standard solutions at equivalent concentrations. The relative matrix effect was evaluated by comparing the peak area ratios of EtG/IS at 3 QC and LLOQ concentrations in 20x diluted blank urine (individual and pooled lots) with the peak area ration of EtG/IS at equivalent concentrations in standard solutions [35].
The absolute matrix effect was analyzed in triplicate at 3 QC and LLOQ concentrations for each individual urine (10 urines) and the % coefficient variation (%CV) ranged from 1.8-4.2% and the %CV for relative matrix effect ranged from 2.03-3.98%, respectively. For the pooled blank urine (mixture of 10 different blank urines), the % CV for absolute matrix effect was <0.2% and the relative matrix effect was < 1.6% at 3 QC and LLOQ concentrations. The variability of matrix effect in different urine samples was found to be <10% (%CV), which is within the acceptable limits (Table 3).
| Spiked EtG Concentration in pooled urine (ng/mL) | Absolute Matrix Effect (AME) ± SD (%) | %CV | Relative Matrix Effect (RME) ± SD (%) | %CV |
|---|---|---|---|---|
| 50 125 450 1600 |
91.5 ± 0.03 98.8 ± 0.12 97.07 ± 0.02 99.21 ± 0.01 |
1.19 1.07 0.53 0.20 |
96.68 ± 0.03 97.4 ± 0.12 98 ± 0.08 101.0 ± 0.02 |
3.76 3.8 2.7 1.06 |
Table 3: Absolute and relative matrix effect of EtG in the pooled blank human urine (n=3).
Sample stability: To evaluate the stability of EtG in diluted urine samples under the condition of our FI-MS/MS method, Low and high QC samples (at 125 and 1600ng/ml, n = 5) were exposed to following regimens: 6h at room temperature (bench-top stability), 2 months at -200C, and 3 freeze-thaw cycles within 3 days. The stability of EtG and IS in pooled blank human urine was evaluated after each storage condition by comparing the peak area after each storage condition to the peak area of the freshly prepared samples at equivalent concentrations. EtG and IS molecules were found to be stable with no significant loss or degradation in the presence of urine matrix in all the stability regimens studied (Table 4).
| Spiked Concentration(ng/mL) | Measured Concentration (ng/mL) Mean ± SD |
Stability (%Recovery) | ||
|---|---|---|---|---|
| Freeze thaw (3cycles) | Low High |
125 1600 |
124.2 ± 1.6 1595.3 ± 5.3 |
99.36 99.7 |
| Bench top(6hr) At room temp | Low High |
125 1600 |
122.2 ± 0.72 1594.7 ± 0.43 |
97.4 99.6 |
| 2 months at -20oc | Low High |
125 1600 |
122.0 ± 0.5 1587.2 ± 0.3 |
97.6 99.1 |
Table 4: EtG stability in pooled blank human urine (n=3).
Carryover and dilution integrity: Analyte carryover into a subsequent sample may lead to an inaccurate result. Hence, carryover must be evaluated during method validation. We performed the carryover analysis as part of method validation, where blank urine matrix samples (both individual and mixed urine samples) were analyzed immediately after completing the analysis of a urine sample containing the highest concentration of EtG (2000ng/mL). This analysis was carried out in triplicates. No significant signals (above the method’s LOD) were observed from any bland urine samples tested during the 2 minute analysis window, suggesting that our new method is free from carryover.
Dilution integrity is an important factor that must be determined during method validation when a quantitative method developed involves dilution. Hence, we next determined dilution efficiency. This was achieved by first spiking EtG to urine to create samples containing EtG at the concentration of 40ug/mL, 20 times higher than HLOQ (2ug/mL), followed by diluting the urine samples 20 times (i.e. the EtG concentration after 20 times dilution is 2ug/mL). We also created urine samples containing 2ug/mL without dilution. This was done in 6 replicates. Then, we compared the EtG peak area of the diluted samples with the EtG peak area of the undiluted samples to calculate precision and accuracy. We found that both the %CV and %RE were <10.5%.
Different dilution factors (i.e. 10, and 50 times) were also considered, but we selected a 20-fold dilution for this study. This selection was based on the consideration that 50 times dilution might create too dilute samples, increasing LLOQ, while 10 fold dilution may not be able to reduce the matrix effect enough to accurately quantify EtG in urine. Our study proved that a 20-fold dilution was adequate for our new FI-MS/MS method.
Interference from other drugs: As discussed earlier, current UDT detects 2 classes of analytes in the negative ESI mode. One is EtG and another is barbiturates. To determine whether co-presence of barbiturates in urine would interfere with the EtG quantification, we carried out an interference study by spiking butalbital, pentobarbital, secobarbital, phenobarbital and phenobarbital–D5 along with EtG and EtG-D5 (IS-500ng/mL) at 3 QC concentrations (125, 450 and 1600ng/mL) and LLOQ (50ng/mL) to pooled blank urine (a mixture of 10 different urines). This was done in in triplicates. We compared the quantification result in the presence of barbiturates with the result obtained in the absence of barbiturates. We found that the presence of these barbiturates drugs had no significant effect on the EtG quantification, indicating that our FI-MS/MS method can be used in the quantification of EtG in patients who may take other drugs.
Robustness: Since our FI-MS/MS method is developed for routine UDT performed in clinical labs, it must be reliable and robust. In other words, its performance (accuracy and precision) and selectivity should not decline and the result should be still reproducible even after over a large number of injections. Hence, we conducted a robustness study by assessing the performance of this method after over 1200 consecutive injections over a 3-days period. It was found that the peak areas for both EtG and IS were still highly reproducible even after over 1200 injections, suggesting that our FIMS/ MS method is robust even after being subjected to such a large number of injections.
Conclusion
We have developed a dilute-and-shoot FI-MS/MS method for quantification of EtG in urine. Since this method does not employ LC separation and has a total run time of 2 min per sample (injection), the throughput of one MS/MS instrument system could be as high as 720 samples per day, which is significantly higher than the throughput achieved by current LC-MS/MS methods.
Despite the presence of various urine matrix molecules, this method was able to accurately quantify EtG at 50 ng/mL (2-4 times lower than the current cutoff of 100-200ng/mL employed in clinical labs), and quantification was adversely affected even when this method does not employ LC separation and sample purification. The key to success of our FI-MS/MS method is the selection of proper MRM transitions, minimizing the effect of matrix molecules. We also demonstrated that our method is robust, evidenced by the fact that no significant deterioration in its performance was observed even after over 1200 injections, suggesting that our method is robust enough for routine UDT.
To the best of our knowledge, this is the first ultra-fast and robust ESI-MS/MS method for EtG quantification without LC separation and complex and costly sample preparation. Our validated method provides an excellent tool for monitoring alcohol abstinence and distinguishing “social” and “heavy alcohol abusers”. The validation results suggested that this method was accurate and precise, meeting the FDA requirements. Considering the importance and frequency of measuring the ethanol level in various populations, we expect that our method can be widely employed and could even replace current LC-MS/MS methods as the method of choice for EtG quantification.
The present study is part of our continue effort to develop diluteand- shoot flow-injection MS/MS (FI-MS/MS) method without LC separation to identify and quantify biomarkers in clinical specimens. Success of this study further suggests that dilute-and-shoot FI MS/ MS can be a general method for the biomarker quantification. Hence, we expect that the dilute-and-shoot FI-MS/MS concept can be employed to develop the simple and fast quantification method for other biomarkers.
Acknowledgement
We acknowledge Cleveland State University for financially supporting this research and also thank “The National Science Foundation Major Research Instrumentation Grant” (CHE-0923398) for supporting the requisition of the capital instrument: QTRAP 5500 mass spectrometer, a key part of our work.
References
- Albermann ME, Musshoff F, Madea B (2012) A High-Performance Liquid Chromatographicaphicnancially supporting Method for the Determination of Ethyl Glucuronide and Ethyl Sulfate in Urine Validated According to Forensic Guidelines. Journal of Chromatographic Science 50: 51-56.
- Foti RS, Fischer MB (2005) Assessment of UDP-glucuronosyltransferase catalyzed formation of ethyl glucuronide in human liver microsomes and recombinant UGTs. Forensic Sci Int 153: 109-116.
- Seidel S, Wurst FM, Alt A (2001) Ethyl glucuronide - a biological marker for recent alcohol consumption. Addict Biol 6: 205-212.
- United Nations Office on Drugs and Crime (UNODC) (2015) World drug report 2015. United Nations.
- Wurst FM, Kempter C, Metzger J, Seidl S, Alt A (2000) Ethyl glucuronide: a marker of recent alcohol consumption with clinical and forensic implications. Alcohol 20: 111-116.
- Palmer RB (2009) A review of the use of ethyl glucuronide as a marker for ethanol consumption in forensic and clinical medicine. Semin Diagn Pathol 26: 18-27.
- Schmitt G, Aderjan R, Keller T, Wu M (1995) Ethyl glucuronide: an unusual ethanol metabolite in humans. Synthesis, analytical data and determination in serum and urine. J Anal Toxicol 19: 91-94.
- Hoiseth G, Bernard JP, Karinen R, Johnsen L, Helander A, et al. (2007) A pharmacokinetic study of ethyl glucuronide in blood and urine: applications to forensic toxicology. Forensic Sci Int 172: 119-124.
- Feng J, Wang L, Dai I, Harmon T, Bernert JT (2007) Simultaneous determination of multiple drugs of abuse and relevant metabolites in urine by LC–MS-MS. J Anal Toxico 31: 359-368.
- Verplaetse R, Decabooter S, Cuypers E, Tytgat J (2013) Screening of urine and blood using limited sample preparation and information dependent acquisition with LC–MS/MS as alternative for immunoassays in forensic toxicology. J Forensic Toxicol Pharmacol 2: 2.
- Shin M, Ji D, Kang S, Yang W, Choi H, et al. (2013) Screening of multiple drugs of abuse and metabolites in urine using LC/MS/MS with polarity switching electrospray ionization. Arch Pharmaceut Res 37: 760-772.
- Janda I, Weinmann W, Kuehnle T, Lahode M, Alt A (2002) Determination of ethyl glucuronide in human hair by SPE and LC-MS/MS. For Sci Int 128: 59-65.
- Tarcomnicu T, Van Nuijs ALN, Aerts K, De Doncker M, Covaci A (2010) Ethyl glucuronide in meconium and hair by hydrophilic interaction liquid chromatography–tandem mass spectrometry. For Sci Int 196: 121-127.
- Weinmann W, Schaefer P, Thierauf A, Schreiber A, Wurst FM (2004) Confirmatory analysis of ethyl glucuronide in urine by liquid-chromatography/electrospray ionization/tandem mass spectrometry according to forensic guidelines. J Am Soc Mass Spectrom 15: 188-193.
- Granja J, Gamble T, Schreiber A, Ellis R and Sakuma T (2007) Confirmatory Analysis of Ethyl Glucuronide and Ethyl Sulfate in Urine by LC/MS/MS According to Forensic Guidelines. Therapeutic Drug Monitoring 29: 498.
- Lee R, Traynor A, LeCount J, Wood M (2014) Quantitative analysis of barbiturates in urine using UPLC/MS/MS. Waters Corporation.
- Xie X, Gao M, Kozak M. A simple method to analyze barbiturates in urine using a triple quadrupole mass spectrometer. ThermoFisher Scientific.
- Steinbauer E, Friel P, Fu R, Zhai A (2013) Analyze barbiturates in urine with Agilent 6430 LC/MS/MS and Poroshell 120 EC-C18. Agilent Technologies Inc.
- He X, Taylor A, Wang A (2017) Fast and simultaneous analysis of ethanol metabolites and barbiturates using the QTRAP® 4500 LC–MS/MS system. SCIEX. RUO-MKT-02-3698-A
- Lupo S (2016) “The Big Pain”: Development of pain-free methods for analyzing 231 multiclass drugs and metabolites by LC– MS/MS. RESTEK Lit. Cat.# CFAR2309-UNV.
- Voggu RR, Alagandula R, Zhou X, Su B, Zhong B, Guo B (2015) A rapid LC–MS/MS method for quantification of CSUOH0901, a novel antitumor agent, in rat plasma. Biomed chromatogr 29: 797-802.
- Alagandula R, Zhou X, Guo B (2016) A dilute-and-shoot flow-injection tandem mass spectrometry method for quantification of phenobarbital in urine. Rapid Commun Mass Spectrom 31: 39-46.
- Cabarcos P, Hassan HM, Taberneroa MJ, Scott KS (2013) Analysis of ethyl glucuronide in hair samples by liquid chromatography-electrospray ionization–tandem mass spectrometry (LC-ESI-MS/MS). J Appl Toxicol 33: 638–643.
- Pesce A, West C, West R, Latyshev S, Masters-Moore D, et al. (2014) Analytical considerations when monitoring pain medications by LC–MS/MS. J Anal Bioanal Tech S5: 003.
- Fletcher CM, Sleeman R (2016) Rapid identification of seized controlled substances and related compounds by tandem mass spectrometry without chromatography. Rapid Commun Mass Spectrom 30: 908-916.
- Tsai IL, Weng TI, Tseng YJ, Tan HKL, Sun HJ, Kuo CH (2013) Screening and confirmation of 62 drugs of abuse and metabolites in urine by ultra-high-performance liquid chromatography–quadrupole time-of-flight mass. J Anal Toxicol 37: 642-651.
- Cao Z, Kaleta E, Wang P (2015) Simultaneous quantitation of 78 drugs and metabolites in urine with a dilute-and-shoot LC– MS–MS assay. J Anal Toxicol 39: 335-346.
- Weinmann W, Svoboda M (1998) Fast Screening for Drugs of Abuse by Solid-Phase Extraction Combined with Flow-Injection Ion spray Tandem Mass Spectrometry. J Anal Toxicol 22: 319-328.
- Rivier L (2002) Criteria for the Identification of Compounds by Liquid Chromatography-Mass Spectrometry and Liquid Chromatography-Multiple Mass Spectrometry in Forensic Toxicology and Doping Analysis. Anal Chimica Acta 492: 69-82.
- Stephanson N, Dahl H, Helander A, Beck O (2002) Direct Quantification of Ethyl glucuronide in Clinical Urine Samples by Liquid Chromatography-Mass Spectrometry. Therap Drug Monitor 24: 645-651.
- Wurst FM, Kempter C, Seidl S, Alt A (1999) Ethylglucuronide- A Marker of Alcohol Consumption and a Relapse Marker with Clinical and Forensic Implications. Alcohol 34: 71-77.
- Jatlow P, O'Malley S (2010) Clinical (Non-forensic) Application of Ethylglucuronide Measurement: Are We Ready? Alcohol Clin Exp Res 34: 968-975.
- SAMHSA (2006) The role of biomarkers in the treatment of alcohol use disorders. Substance Abuse Treatment Advisor 5: 1-7.
- Biopharmaceutics Coordinating Committee (2001) Guidance for Industry, Bioanalytical Method Validation. Food and Drug Administration.
- Matuszewski BK, Constanzer ML, Chavez-Eng CM (2003) Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem 75: 3019-3030.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi