Research Article, J Forensic Toxicol Pharmacol Vol: 11 Issue: 2
Development and Validation of Ase Extraction Compared to Standard Spe Technique in Forensic Toxicology: A Study on Whole Blood Samples for Psychoactive Drugs, Antagonists, Medications, and Anesthetic
Domenico Di Candia1, Fabrizio Galbiati2, Michele Boracchi1, Gaia Giordano1* and Riccardo Zoja1
1Section of Forensic Medicine, Department of Biomedical Sciences for Health, University of Milan, Milan, Italy
2Thermo Fisher Scientific (Schweiz) AG, Reinach, Switzerland
*Corresponding Author:Gaia Giordano
Section of Forensic Medicine, Department of Biomedical Sciences for Health, University of Milan, Milan, Italy
Tel:+39 3337180997
E-mail: gaia.giordano@unimi.it
Received date: 01 March, 2022, Manuscript No. JFTP- 22-49987;
Editor assigned date: 03 March, 2022, PreQC No. JFTP- 22-49987 (PQ);
Reviewed date: 17 March, 2022, QC No JFTP- 21-49987;
Revised date: 22 March, 2022, Manuscript No. JFTP- 22-49987 (R);
Published date: 29 March, 2022, DOI: 10.4172/2325-9841.1000122
Citation: Giordano G , Candia D.DI, Galbiati F, Boracchi M, Zoja R, et al (2022) Development and Validation of Ase Extraction Compared to Standard Spe Technique in Forensic Toxicology: A Study on Whole Blood Samples for Psychoactive Drugs, Antagonists, Medications, and Anesthetics. J Forensic Toxicol Pharmacol 11:1.
Keywords: Accelerated Solvent Extraction (ASE); validation of , ASE for forensic toxicology; whole blood samples; Toxicological , analyses; Forensic Toxicology; Comparison with SPE technique; , Validation of ASE with human biological samples;
Keywords
Accelerated Solvent Extraction (ASE); validation of ASE for forensic toxicology; whole blood samples; Toxicological analyses; Forensic Toxicology; Comparison with SPE technique; Validation of ASE with human biological samples;
Introduction
Sample extraction procedures are often considered as a bottle-neck step in analytical methods. Many techniques have been used with the purpose of achieving greater efficiency, a reduction of the time spent for the extraction and of the amount of solvent used. The Solid-Phase Extraction (SPE) is still considered as a reliable extractive technique due to its many advantages compared to the other traditional methods, such as liquid/liquid extraction [1], reducing the use of great amounts of solvent, the operation time/procedure steps, and others [2]. Among the various fields in which SPE is applied, the forensic-toxicological field still takes great advantages and is therefore widely used in forensic laboratories [3].
Solid-phase extraction is a well-known and tested method of preparation for samples involved in several scientific fields and represents a fundamental column in toxicological analyses. This type of extraction is based on a principle similar to the partition chromatography, in which several stationary phases were developed for more targeted extractions [4].
Different matrices can be collected for toxicological analysis (blood, urine, bile, hair, etc.) and required a thoughtful preparation of the specimens influencing the outcome and the reproducibility of the analysis. Over the years, SPE has become a performant method in the extraction of molecules and nowadays is used as a standard procedure in forensic field in many laboratories around the world. It allows to efficiently purify and concentrate the samples before performing a liquid or gas chromatographic analysis. Indeed, its impact on the quality of the analysis is greater and it allows concentrating the substances enabling the detection and dosing in a biological matrix excluding endogenous substances. In some cases, it allows also to target the extraction of several compounds permitting the detection of substances that can be considered difficult to extract and would not be detectable with standard procedures. However, this technique requires a fair amount of time, is operator-dependent, has high costs and is subjected to possible human mistakes that could invalidate the extraction [5].
The Accelerated-Solvent Extraction (ASE) is an alternative automatized procedure for the extraction and purification of substances from biological matrices, commonly used in non-forensic laboratories treating, mostly, animal and vegetable samples [3,5,6]. ASE uses operator-independent protocol, decreasing the bias due to operator-dependent procedures, process a highest number of samples in sequence, it improves the extractive processes, reduces the time required for multiple extractions and increase the samples throughputs. This technique uses organic solvents at variable pressures and temperatures above the boiling point, creating a time-saving procedure with low consumption of solvents [7-11]. With this procedure, a sample is enclosed in a sample cartridge that is filled with an extraction fluid and used to statically extract the sample under elevated temperature (50°C-200°C) and pressure (500 psi-3000 psi) conditions in a short time period (15-25 minutes). The compressed gas is used to purge the sample extract from the cell into a collection vessel.
Many studies have validated the ASE extraction method in animal [12-19] and botanical [7,8,20-28] field. However, there is a paucity of studies regarding the ASE extraction validation on human biological samples, limited to the meconium [29,30], bone matrix [31,32] and blood samples [33,34], searching for cocaine, dioxins, fatty acids and nicotine and cotinine.
For this reason, the purpose of this study was to investigate, under a validation method from qualitative and quantitative point-of-view, whether the accelerated-solvent extraction technique can be used in substitution of the operator-dependent solid-phase extraction in forensic toxicological field. Its reliability is tested on a large number of substances and on whole blood matrix commonly used in forensic toxicological analysis.
Material and Methods
Instruments involved
A standard 12-port vacuum manifold and Bond Elut™ Certify cartridges 130 mg (Agilent) were used for SPE procedure. Whereas the pressurized fluid extractions were carried out with a Thermo Scientific™ Dionex™ ASE 350™ Accelerated Solvent Extractor equipped with 10 mL stainless steel extraction cells. The extracts were collected in 30 mL vials and evaporated to dryness under reduced pressure. The samples were analyzed with a thermo scientific™ TSQ fortis™ II triple-quadrupole mass spectrometer.
Sample collection
Samples of femoral blood were collected from different cadavers during the autopsy examination at the Bureau of Legal Medicine of Milan. All blood matrices were collected using sterilized syringes, placed in sealed vials and stored in a -20°C refreeze environment until the analysis time. The whole blood was stabilized with sodium fluoride and potassium oxalate.
All blank whole blood samples collected for this study were previously analyzed to evaluate the absence of molecules of toxicological interest.
Chemicals and reagents
All the standards molecules (psychoactive drugs, antagonists, medications and anesthetics) involved in this study and the internal standard SKF 525-A (Proadifen hydrochloride, analytical standard, >95%, 100 mg) as well, were purchased from Sigma-Aldrich and stored at -20°C.
Morphine, codeine, amphetamine, methamphetamine, MDA, MDMA, MDEA, benzoylecgonine, methadone, naloxone, naltrexone, diazepam, flurazepam, bromazepam, midazolam, phenobarbital, thiopental, carbamazepine, haloperidol, clozapine, fentanyl and propofol (each 1 mg/mL in methanol); 6-MAM, cocaine, LSD and olanzapine (each 1 mg/mL in acetonitrile); ketamine hydrochloride, sertraline hydrochloride, chlorpromazine hydrochloride (each 1 mg/mL in methanol, as free base); citalopram hydrobromide (1 mg/mL in methanol, as free base); EDDP perchlorate (1 mg/mL in methanol, as pyrrolinium); quetiapine fumarate (1 mg/mL in methanol, as free base); delorazepam (100 µg/mL in acetonitrile); remifentanil hydrochloride (100 µg/mL in methanol, as free base); promazine hydrochloride (VETRANAL ®, analytical standard, 250 mg); flumazenil (>99%, HPLC, solid, 25 mg).
Working solutions of each molecule and internal standard were prepared at 10 µg/mL or 5 µg/mL, starting from the standard solutions, and stored at -20°C until use.
Solvents used in the extraction processes were purchased by Sigma-Aldrich (methanol, hydrochloric acid and chloroform), VWR chemicals (acetone, ethyl acetate, dichloromethane, isopropanol and N-hexane). Buffer solution pH 6.88 was purchased from PanReac AppliChem ITW Reagents. Diatomaceous Earth and ASE cellulose filter Restek 20 mm were purchased from thermo fisher scientific (Waltham, MA, USA).
Classification of molecules under investigation
The molecules under investigation were divided into clusters. The psychoactive drugs comprise from cluster 1 to cluster 5. cluster 1: Morphine, 6-MAM, codeine, ketamine; cluster 2: Amphetamine, methamphetamine, MDA, MDMA, MDEA; cluster 3: Cocaine, benzoylecgonine; cluster 4: Methadone, EDDP; cluster 5: LSD. Antagonistic drugs (cluster 6) were composed of naloxone, naltrexone, and flumazenil. Medication (from cluster 7 to 9): Cluster 7, composed of benzodiazepines (diazepam, flurazepam, bromazepam, delorazepam, midazolam); cluster 8 consisted of barbiturates (phenobarbital and thiopental); cluster 9 constituted of antipsychotics/neuroleptics (carbamazepine, citalopram, sertraline, chlorpromazine, promazine, haloperidol, clozapine, olanzapine, and quetiapine). Then, for the anesthetic group, that formed cluster 10, fentanyl, remifentanil and propofol were chosen.
Sample preparation for SPE extraction
According to the standard procedure, 100 ng of Internal Standard SKF 525-A (Proadifen hydrochloride) and appropriate concentrations of each compound, obtained from the working solutions previously prepared and correctly stored, were added to 0.5 mL of each whole blood samples. The samples were successively diluted to 5 mL using a pH 6.88 phosphate buffer solution. The solutions obtained were agitated on a vortex mixer (Heidolph, REAX top), then placed on a rotating wheel (Falc F205) for 30 minutes and then centrifuged for 10 minutes at 3500 rpm (Thermo Scientific, Heraeus Biofuge primo centrifuge).
Sample preparation for ASE extraction
Whole blood samples (0.5 mL per sample) were spiked with 100 ng of internal standard SKF 525-A (Proadifen hydrochloride) and appropriate concentrations of each compound under investigation. Then, the specimens were properly mixed on a vortex mixer (Heidolph, REAX top).
Extraction procedure for SPE extraction
The solutions obtained were loaded on the Bond Elut™ certify cartridges 130 mg (Agilent) previously conditioned with 2 mL of methanol and 2 mL of pH 6.88 phosphate buffer. After the wash out with 2 mL of pH 6.88 phosphate buffer, 1.5 mL of hydrochloric acid 0.01 M and 0.3 mL of methanol, the cartridges were left to dry for 30 minutes at reduced pressure. As a last step, the cartridges were eluted with 2 mL of a mixture of chloroform and acetone 1:1 to obtain an acid/neutral extract. Those molecules that yield better with a basic extraction were extracted with 1 mL of ethyl acetate at 2% of ammonia followed by 1 mL of a mixture composed by dichloromethane-isopropanol in a rate of 8:2 at 2% of ammonia [35].
The eluates obtained were let dry in a vacuum rotary evaporator (Thermo Scientific, Savant SpeedVac Concentrator), then restored with 100 µl of methanol and 2 µl of these final solutions were analyzed via a thermo scientific™ TSQ Fortis™ II triple-quadrupole mass spectrometer.
Extraction procedure for ASE extraction
The specimens, properly mixed on a vortex mixer, were then poured in 10 mL stainless-steel cells, provided with cellulose filters Restek 20 mm on the bottom of the cell and previously filled with Dionex™ ASE™ Prep DE (Diatomaceous Earth). The cells, tightly sealed, were placed on the ASE 350 Accelerated-Solvent Extractor. The condition protocol of extraction in use was the extraction solvent n-hexane: Acetone (4:1), temperature 80°C, pressure 1500 psi, heat-up time and static time 5 minutes each, flush volume 60%, purge time 100 seconds and static cycle 1. The total extraction time is 12 minutes per sample (a total of 24 cells could be load together on ASE extractor and being extracted autonomously by the instrument in about 5 hours) with the use of 10 mL of solvent per sample. The eluates, collected in glass vials, were let dry in a vacuum rotary evaporator (Thermo Scientific, Savant SpeedVac Concentrator), then restored with 100 µl of methanol and 2 µl of these final solutions were analyzed via a Thermo Scientific™ TSQ Fortis™ II Triple-Quadrupole Mass Spectrometer.
Calibration curves
Calibration curves were prepared for each substance with 6 non-zero calibration points replicated for 5 runs for both ASE extraction and SPE extraction. Calibration curves for each molecule were developed starting from working solutions with the same ranges: 10-25-50-100-200-400 ng/mL. Calibration model and carryover of each substance for ASE extraction are explained in detailed in method validation section.
HPLC-MS/MS conditions
The liquid chromatography was performed using a Thermo Scientific™ TSQ Fortis™ II Triple-Quadrupole Mass Spectrometer (Thermo Scientific, San Jose, CA, USA) coupled with a HPLC system constituted by a Surveyor MS quaternary pump with degasser, surveyor AS auto-sampler, oven with Rheodyne valve and a 20 µL loop. The column used was Thermo Scientific HyperSil Gold 50 × 2.1 mm con particle size 1.9 µm reverse phase which was maintained at 35°C and eluted at a constant flow rate of 0.400 mL/min.
Solvent A used for analysis (ammonium formate 20 mM 0.1% formic acid) and B (MeOH) represented the mobile phase utilized for the gradient. Solvent A and B were 10% and 90% at 0.00 minutes × 1.00 minutes, respectively. Solvent A was increased 95% at 4.00 minutes, held to 3.00 minutes then decreased to 10% at 9.00 minutes and held at 10% to 15.00 minutes.
Mass spectrometry was performed using a Thermo Scientific™ TSQ Fortis™ II Triple-Quadrupole Mass Spectrometer (Thermo Scientific, San Jose, CA, U.S.A.) equipped with a Heated Electrospray Ionization Source (HESI). Capillary temperature and vaporization temperature were set at 330°C and 280°C while electrospray tension was set at 3.50 kV with a positive mode. Complete scanning acquisition was combined with a (DIA) Data Independent Acquisition mode providing MS2 spectrum for confirmation response according to an inclusion list.
The positive ion spray voltage was 3,500 V, the sheath gas was 45 Arb, the aux gas 20 Arb and the sweep gas 10 Arb. Q1 resolution was 0.4 FWHM and Q3 resolution was 0.7 FWMH. The CID gas was set 1.5 mTorr. Full Scan (FS) acquisition was combined with a DIA protocol providing MS/MS spectrum for confirmation response according to the inclusion list. Resolution power of the FS was set at 70.000 FWHM. The mass range was set to 50-650. Automatic Gain Control (AGC) was set at 1 × 10-6 and maximum injection time was set at 200 ms. The DIA segment operated with positive mode at 35.000 FWHM and the AGC target was set at 5 × 10-4 with a maximum injection time of 100 ms. The quadrupole filtered precursor ions with an isolation range of 2 m/z. Fragmentation of the precursors was optimized with a Normalized Collision Energy in 3 steps (NCE) (10-40-60 eV) (Table 1).
| Molecules | Precursor ion (m/z) | Product (m/z) | Product (m/z) | Product (m/z) |
|---|---|---|---|---|
| Morphine | 286.2 | 165 | 152.9 | 201.1 |
| 6-MAM | 328.2 | 165 | 210.9 | 193 |
| Codeine | 300.5 | 165 | 215 | 199 |
| Ketamine | 238.2 | 124.9 | 219.9 | 206.9 |
| Amphetamine | 136.5 | 91 | 119 | 65 |
| Methamphetamine | 150.5 | 91 | 119 | 65 |
| MDA | 180.5 | 163 | 135 | 133 |
| MDMA | 194.5 | 163 | 135 | 105 |
| MDEA | 208.5 | 163 | 135 | 105.1 |
| Cocaine | 304.2 | 182 | 82 | 105 |
| Benzoylecgonine | 290 | 168 | 105 | 77 |
| Methadone | 310.4 | 265 | 105 | 218.9 |
| EDDP | 278.4 | 234 | 249 | 219 |
| LSD | 324.2 | 223 | 207.9 | 281 |
| Naloxone | 328.5 | 310.1 | 212.1 | 268.2 |
| Naltrexone | 342.2 | 324.2 | 306 | 57 |
| Flumazenil | 304.1 | 257.9 | 217 | 228.7 |
| Diazepam | 285.1 | 193 | 154 | 222.2 |
| Flurazepam | 388.2 | 315 | 100.1 | 72.1 |
| Bromazepam | 316 | 182 | 208.9 | 287.9 |
| Delorazepam | 305.2 | 183 | 91.1 | 182.2 |
| Midazolam | 326.3 | 290.9 | 249 | 222.9 |
| Phenobarbital | 217.1 | 117.1 | 91 | 79 |
| Thiopental | 265.1 | 195 | 248.1 | 246.7 |
| Carbamazepine | 237.1 | 194.1 | 103 | 166.1 |
| Citalopram | 325.2 | 109 | 262 | 233.9 |
| Sertraline | 306.1 | 158.8 | 274.9 | 122.9 |
| Chlorpromazine | 319.3 | 86.1 | 57.9 | 245.8 |
| Promazine | 285.4 | 86.1 | 180 | 211.9 |
| Haloperidol | 376.2 | 123 | 165 | 95 |
| Clozapine | 327.4 | 269.9 | 192 | 226.9 |
| Olanzapine | 313.4 | 255.9 | 197.9 | 212.8 |
| Quetiapine | 384.4 | 253 | 221.1 | 279.1 |
| Fentanyl | 337.3 | 188.1 | 105 | 132.1 |
| Remifentanil | 377.2 | 345.2 | 291.2 | 87 |
| Propofol | 179.1 | 137 | 95 | 119.1 |
Table 1: Precursor ion and products of each molecule.
Method validation for ASE extraction: Validation plan
Evaluation of method performance including bias, calibration model, carryover, interference studies, ionization suppression/enhancement, Limit of Detection (LOD), Limit of Quantification (LOQ), precision and processed sample stability were performed according to the standard practices for method validation in forensic toxicology [36]. The acceptance criteria of method validation are summarized in Table 2.
| Parameter | Acceptance criteria |
|---|---|
| Bias | <20 % |
| Precision | % CV<20%; |
| Calibration model | Linear model desired: 10-400 ng/mL per molecule |
| Carryover | Carryover after the highest calibrator samples does not exceed the 10% of the lowest calibrator signal |
| Interference studies | No interfering signal from matrix, internal standard, common prescription medication or drug of abuse |
| Ionization suppression/enhancement | <25% suppression/enhancement and <20% of %CV |
| Limit of Detection (LOD) | Decision point procedure (5 ng/mL) |
| Lower Limit of Quantification (LLOQ) | Lowest non-zero calibrator procedure; bias <20% and precision <20% |
| Sample stability | Replicates over 78 hours at room temperature |
Table 2: Validation parameters to be assessed for ASE extraction.
Bias and precision
Bias and precision were calculated in pooled fortified matrix of whole blood samples using 3 separate samples at three different concentration pools (low, medium and high) over five different runs. The bias obtained for all the molecules was lower ± 20% at each concentration. Within-run precision and between-run precision were calculated by ANOVA Single Factor Calculations approach for all the molecules investigated in this study with a coefficient of variation (%CV) lower than ± 20%. The calculations obtained for morphine are reported as an example in this paragraph (Tables 3 to 8). Bias of all the molecules was below 8.3% and above -2.2%. Within-run precision for all the molecule was below 12.64%, whereas between-run precision <19.10%.
| Low (30 ng/ml) | Run 1 | Run 2 | Run 3 | Run 4 | Run 5 |
|---|---|---|---|---|---|
| Rep 1 | 30 | 35 | 34 | 28 | 29 |
| Rep 2 | 26 | 28 | 31 | 32 | 27 |
| Rep 3 | 31 | 29 | 30 | 32 | 26 |
| Medium (150 ng/ml) | Run 1 | Run 2 | Run 3 | Run 4 | Run 5 |
| Rep 1 | 141 | 153 | 149 | 150 | 147 |
| Rep 2 | 143 | 148 | 152 | 140 | 156 |
| Rep 3 | 140 | 142 | 139 | 150 | 151 |
| High (300 ng/ml) | Run 1 | Run 2 | Run 3 | Run 4 | Run 5 |
| Rep 1 | 301 | 310 | 299 | 298 | 303 |
| Rep 2 | 300 | 296 | 306 | 308 | 300 |
| Rep 3 | 298 | 300 | 302 | 297 | 295 |
Table 3: Quantitative results (ng/mL) of bias and precision runs for morphine.
| Parameters | Calculated mean | Bias |
|---|---|---|
| Low (30 ng/ml) | 29.8 | -0.70% |
| Medium (150 ng/ml) | 146.7 | -2.20% |
| High (300 ng/ml) | 300.8 | 0.30% |
Table 4: Mean concentrations (ng/mL) for bias calculations of morphine.
| Summary | ||||||
|---|---|---|---|---|---|---|
| Groups | Count | Sum | Average | Variance | ||
| Run 1 | 3 | 87 | 29 | 7 | ||
| Run 2 | 3 | 92 | 30.6666667 | 14.3333333 | ||
| Run 3 | 3 | 95 | 31.6666667 | 4.33333333 | ||
| Run 4 | 3 | 92 | 30.6666667 | 5.33333333 | ||
| Run 5 | 3 | 82 | 27.3333333 | 2.33333333 | ||
| ANOVA | ||||||
| Source of variance | SS | df | MS | F | P-value | F crit |
| Between groups | 35.0666667 | 4 | 8.76666667 | 1.315 | 0.329115501 | 3.47804969 |
| Within groups | 66.6666667 | 10 | 6.66666667 | |||
| Total | 101.733333 | 14 | ||||
Table 5: ANOVA single factor calculations for 30 ng/ml sample of morphine.
| Summary | ||||||
|---|---|---|---|---|---|---|
| Groups | Count | Sum | Average | Variance | ||
| Run 1 | 3 | 424 | 141.333333 | 2.33333333 | ||
| Run 2 | 3 | 443 | 147.666667 | 30.3333333 | ||
| Run 3 | 3 | 440 | 146.666667 | 46.3333333 | ||
| Run 4 | 3 | 440 | 146.666667 | 33.3333333 | ||
| Run 5 | 3 | 454 | 151.333333 | 20.3333333 | ||
| ANOVA | ||||||
| Source of variance | SS | df | MS | F | P-value | F crit |
| Between groups | 153.6 | 4 | 38.4 | 1.44723618 | 0.288745941 | 3.47804969 |
| Within groups | 265.333333 | 10 | 26.5333333 | |||
| Total | 418.933333 | 14 | ||||
Table 6: ANOVA single factor calculations for 150 ng/ml sample of morphine.
| Summary | ||||||
|---|---|---|---|---|---|---|
| Groups | Count | Sum | Average | Variance | ||
| Run 1 | 3 | 899 | 299.666667 | 2.33333333 | ||
| Run 2 | 3 | 906 | 302 | 52 | ||
| Run 3 | 3 | 907 | 302.333333 | 12.3333333 | ||
| Run 4 | 3 | 903 | 301 | 37 | ||
| Run 5 | 3 | 898 | 299.333333 | 16.3333333 | ||
| ANOVA | ||||||
| Source of variance | SS | df | MS | F | P-value | F crit |
| Between groups | 21.7333333 | 4 | 5.43333333 | 0.22638889 | 0.917430067 | 3.47804969 |
| Within groups | 240 | 10 | 24 | |||
| Total | 261.733333 | 14 | ||||
Table 7: ANOVA single factor calculations for 300 ng/ml sample of morphine.
| Between-run precision % CV | Within-run precision % CV | |
|---|---|---|
| 30 ng/mL | 16.36 | 8.64 |
| 150 ng/mL | 12.88 | 3.51 |
| 300 ng/mL | 2.56 | 1.62 |
Table 8: Between-run precision and within-run precision for each concentration of morphine.
Calibration model and carry-over
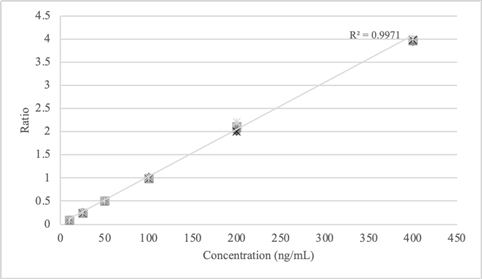
Linear calibration model was developed for all the molecules investigated in this study including a working range of 10-400 ng/mL. The calibration samples were prepared in blank whole blood (previously analyzed) at concentration of 10, 25, 50, 100, 200 and 400 ng/mL. Each calibrator was analyzed once per run in five separate runs (Table 9). All the data obtained from the 5 runs were combined into a single calibration curve. The origin was not included as calibration point. The coefficient of determination (r2) for linear calibration model was calculated ≥ 0.99 for each molecule. It is reported as an example the calibration model developed for morphine (Table 9 and Figure 1).
| Conc ng/mL | Run 1 | Run 2 | Run 3 | Run 4 | Run 5 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak Area | Ratio | Peak Area | Ratio | Peak Area | Ratio | Peak Area | Ratio | Peak Area | Ratio | ||||||
| Drug | Int. Std | Drug | Int. Std | Drug | Int. Std | Drug | Int. Std | Drug | Int. Std | ||||||
| 10 | 49282 | 554757 | 0.08883529 | 50872 | 559744 | 0.0908844 | 48652 | 550362 | 0.0884 | 49249 | 554870 | 0.08875773 | 49183 | 559782 | 0.08786099 |
| 25 | 140862 | 550186 | 0.25602614 | 137691 | 553097 | 0.24894548 | 143827 | 559626 | 0.25700557 | 138974 | 558272 | 0.248936 | 142160 | 557693 | 0.25490727 |
| 50 | 278734 | 555725 | 0.50156822 | 279391 | 554609 | 0.50376211 | 277268 | 552961 | 0.50142415 | 281738 | 555765 | 0.50693728 | 279784 | 551232 | 0.50756124 |
| 100 | 560918 | 551263 | 1.01751433 | 555632 | 557393 | 0.99684065 | 557844 | 552137 | 1.0103362 | 552013 | 555880 | 0.99304346 | 560991 | 554577 | 1.01156557 |
| 200 | 1115850 | 558452 | 1.99811264 | 1185014 | 559150 | 2.11931324 | 1146677 | 554081 | 2.0695115 | 1109963 | 555787 | 1.99710141 | 1217309 | 550313 | 2.21203024 |
| 400 | 2200943 | 557562 | 3.94744082 | 2210197 | 556905 | 3.96871459 | 2210658 | 556439 | 3.97286675 | 2217273 | 555911 | 3.98853953 | 2214102 | 551976 | 4.01122875 |
Table 9: Calibration curve data for morphine.
The carryover effect was investigated by injecting in triplicates an extracted blank sample of whole blood after each calibration point with the highest concentration. It was noted that the carryover was not present for any drugs or the internal standard in any of the extracted blank samples.
Interference studies
Eleven different sources of blank whole blood were analyzed to evaluate matrix interferences. The blank matrices were extracted without the addition of internal standard and analyzed. No interferences at the retention time of the molecules under investigation were observed after the analyses of the blank whole blood. Then, one matrix was randomly selected, spiked with the internal standard, extracted with the new method, and analyzed to demonstrate the absence of interferences at the retention time of the molecules under investigation by the internal standard. Another blank matrix sample was spiked with molecules (divided in clusters previously described in paragraph 2.4: Classification of molecules under investigation) at 400 ng/mL and analyzed without the internal standard to evaluate if unlabeled analyte ions interfere with the signal of the internal standard and if some molecules could interfere with others. No interferences were observed between the analytes and internal standard.
Ionization suppression/enhancement
As the instrumental portion of the method involves LC-MS/MS system, the validation procedure needed to conduct the ionization suppression/enhancement. The post-column extraction approach was chosen to perform the ionization suppression/enhancement procedure and two sets of samples were involved for this experiment. The first set consists in standards prepared in mobile phase at 30 and 300 ng/mL of the molecules under investigations and with 100 ng/mL of internal standard, not extracted, but simply injected six times each.
The second set consists in ten blank samples of whole blood, collected from an independent source of blank whole blood previously analyzed, the same ten blank matrices used for interference studies (in interference studies the matrices involved were eleven). Each blank matrix was extracted in duplicate and then fortified with 30 and 300 ng/mL with the cluster of molecules under investigation and with 100 ng/mL of the internal standard. Each concentration set sample was injected one time. Here, it is reported the example of morphine (Tables 10,11).
| 30 ng/mL | 300 ng/mL | |||
|---|---|---|---|---|
| Drug | Int. Std | Drug | Int. Std. | |
| Set 1 | 169685 | 550608 | 1559061 | 556055 |
| Set 2 | 170823 | 555970 | 1592552 | 548171 |
Table 10: Average peaks area from suppression/enhancement experiments of morphine.
| % Ionization suppression/enhancement | %CV | |
|---|---|---|
| 30 ng/mL | 0.67 | 0.53 |
| 300 ng/mL | 2.15 | 1.5 |
Table 11: Calculation of ionization suppression/enhancement and coefficient of variation on each concentration of morphine.
All the data obtained for the ionization suppression/enhancement do not exceed the 25% (precisely do not exceed 5.73% and -5.12%) and the %CV calculated for each substance was ≤ 3.94%. However, even if the ionization suppression was noted in some molecules, the ionization suppression/enhancement calculated for the internal standard in all sets never exceeds -1.59% and 1.49% (data not shown).
Limit of Detection (LOD): Decision point concentration approach
The LOD was defined using the decision point method. A concentration of 5 ng/mL was administratively defined for all the molecules selected for this study. Three different blank matrix sources were fortified with the analytes (divided per cluster, therefore a total of 30 blank matrices were collected for LOD validation) at 5 ng/mL and analyzed per three runs. The identification criteria of the substances were met (retention time, peak shape, and mass spectral ion ratios) for all the molecules and replicates analyzed.
Lower Limit of Quantification (LLOQ): Lowest non-zero calibrator approach
LLOQ was performed using the lower non-zero calibrator approach. Three different matrix sources per molecule were fortified with the analytes at 10 ng/mL and were analyzed over three runs demonstrating that all detection, identification, bias and precision criteria were met. In this paragraph are reported the results obtained for morphine (Table 12). Bias and precision of all the molecules were <15.6% and <9%, respectively.
| Run 1 (ng/mL) | Run 2 (ng/mL) | Run 3 (ng/mL) | |
|---|---|---|---|
| Matrix 1 | 10 | 11 | 10 |
| Matrix 2 | 11 | 12 | 12 |
| Matrix 3 | 11 | 11 | 12 |
Table 12: Data of LLOQ for morphine: Three different sources of matrix (matrix 1, matrix 2, matrix 3); three different runs (run 1, run 2, run 3); bias and precision of all the concentrations obtained.
Samples stability test
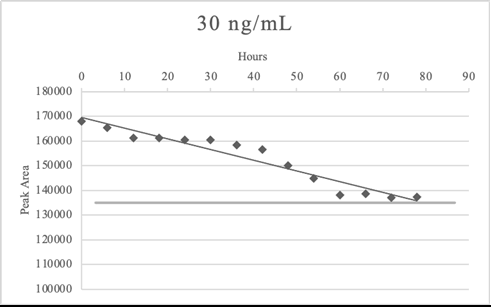
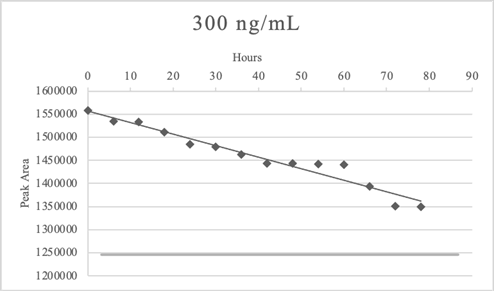
In laboratory the samples are analyzed in batches, however we recognized that some atypical events could arise (e.g. loss of power). Therefore, samples stability studies were performed to evaluate loss of analytes in processed samples at low and high concentration. After the sample extraction, the elution was divided into 14 vials for instrumental analyses. The first vial was analyzed immediately in triplicates, while the 13 remaining vials were left at room temperature (considering the possibility of loss of power) and analyzed after 6 hours each in triplicates. The last vial was analyzed in triplicates 78 hours after the extraction procedure.
Table 13 and Figures 2,3 reported the example of sample stability test for morphine. Morphine remained stable at both concentrations in the time frame considered.
| Average Peak Area | ||||
|---|---|---|---|---|
| Time (hours) | 30 ng/mL | 300 ng/mL | ||
| Drug | Int. Std. | Drug | Int. Std | |
| 0 | 167946 | 559732 | 1557473 | 559208 |
| 6 | 165279 | 558465 | 1533884 | 559116 |
| 12 | 161287 | 558224 | 1532493 | 558159 |
| 18 | 161205 | 559909 | 1511495 | 558351 |
| 24 | 160538 | 558627 | 1484445 | 558430 |
| 30 | 160508 | 559318 | 1478988 | 558024 |
| 36 | 158439 | 558279 | 1463321 | 557166 |
| 42 | 156476 | 558112 | 1443378 | 558539 |
| 48 | 150104 | 559608 | 1443250 | 558594 |
| 54 | 144847 | 558677 | 1441848 | 556962 |
| 60 | 138083 | 559356 | 1441024 | 557308 |
| 66 | 138707 | 558715 | 1394247 | 557591 |
| 72 | 137135 | 558655 | 1350941 | 557453 |
| 78 | 137329 | 557284 | 1349018 | 555321 |
Table 13: Average Peak Areas for processed sample stability tests of morphine.
In some cases, the analytes slightly exceed the bias set at ± 20%, indeed in LSD stability test the last vial of 30 ng/mL (78 hours after the extraction) was less than 20% of the time zero average signal. Flumazenil and clozapine exceeded the bias criteria at 78 hours after the extraction in both concentrations. Bromazepam 300 ng/mL did not meet the criteria of bias after 72 hours.
Documentation of Results of ASE Validation Method
The results of ASE extraction validation are reported in Table 14. All the criteria of acceptance are met making evidence that the method was efficiently validated to analyze whole blood for psychoactive drugs, antagonists, medications, and anesthetics.
| Parameter | Acceptance criteria | Result |
|---|---|---|
| Bias | <20% | -2.2% to 8.3% |
| Precision | % CV <20% | Between-run precision: <19.10% |
| Within-run precision: <12.64% | ||
| Calibration model | Linear model desired: 10-400 ng/mL per molecule | 10-400 ng/mL (linear model) R2 ³ 0.99 |
| Carryover | Carryover after the highest calibrator samples does not exceed the 10% of the lowest calibrator signal | No carryover was observed |
| Interference studies | No interfering signal from matrix, internal standard, common prescription medication or drug of abuse | No interferences were observed |
| Ionization suppression/enhancement | <25% suppression/enhancement and <20% of %CV | -5.12% to 5.73%; £ 3.94% CV |
| Limit of Detection | Decision point procedure (5 ng/mL) | 5 ng/mL (Decision point procedure) |
| Lower Limit of Quantification | Lowest non-zero calibrator procedure; bias <20% and precision <20 % | 10 ng/mL (Lowest Non-Zero calibrator approach) |
| Sample stability | Replicates over 78 hours at room temperature | LSD at 30 ng/mL until 72 hours |
| Flumazenil and clozapine (at both concentrations) until 72 hours | ||
| Bromazepam at 300 ng/mL until 66 hours | ||
| More than 78 hours for the remaining molecules |
Table 14: Summary of validation results.
Comparison between Ase Extraction Technique and Spe Extraction Technique
Relative and absolute recovery of samples
To compare the new ASE technique with the standardized SPE technique, we decided to perform relative and absolute recovery tests of whole blood matrix with both techniques following the some international guidelines [37]. To compare the extractive method, blank whole blood matrix was spiked with appropriate concentration of molecules (low, medium and high concentration for absolute recovery; low and high concentration for relative recovery) and of internal standard. The spiked samples were divided in two different samples to be extracted with the new method (ASE extraction) and the standardized method (SPE extraction).
Relative recovery represents the matrix effect (ionization suppression/enhancement), therefore for the ASE extraction were considered the data obtained for ionization suppression/enhancement reported in Tables 10,11 and summarized together with the results of SPE extraction relative recovery in Table 15. The relative recovery was calculated for SPE extraction too with the same procedure used for ASE extraction (Table 15).
| ASE extraction | SPE extraction | |||||||
|---|---|---|---|---|---|---|---|---|
| 30 ng/mL | 300 ng/mL | 30 ng/mL | 300 ng/mL | |||||
| Molecules | Ion. Suppr./enhance. % | % CV | Ion. Suppr./enhance. % | % CV | Ion. Suppr./enhance. % | % CV | Ion. Suppr./enhance. % | % CV |
| Morphine | 0.67 | 0.53 | 2.15 | 1.5 | -0.1 | 0.49 | -2.54 | 1.66 |
| 6-MAM | 0.15 | 0.11 | 4.1 | 2.83 | 0.66 | 0.51 | 2.19 | 4 |
| Codeine | 1.66 | 1.16 | 1.95 | 1.36 | 1.22 | 1.53 | 1.5 | 0.71 |
| Ketamine | 5.73 | 3.94 | 3.15 | 2.19 | 3.57 | 1.8 | 4 | 1.73 |
| Amphetamine | -0.19 | 0.13 | -2.97 | 2.13 | -0.81 | 0.93 | -1.63 | 1.2 |
| Methamphetamine | 1.56 | 0.05 | 1.09 | 0.04 | 1.76 | 0.18 | 1.04 | 0.99 |
| MDA | -0.05 | 0.04 | -5.12 | 3.71 | -0.83 | 2.97 | -3.69 | 4.29 |
| MDMA | 2.13 | 1.49 | 0.33 | 0.23 | 2.75 | 1.9 | 0.67 | 0.58 |
| MDEA | -1.5 | 1.07 | 0.05 | 0.04 | -1.9 | 1 | 0.9 | 0.31 |
| Cocaine | 4.33 | 3 | 0.06 | 0.04 | 3.22 | 3.63 | 0.4 | 1 |
| Benzoylecgonine | 2.86 | 1.99 | -0.28 | 0.2 | 2.54 | 1.39 | -0.21 | 0.31 |
| Methadone | 3.04 | 2.12 | 2.22 | 1.55 | 3.54 | 2.78 | 2.45 | 1.15 |
| EDDP | -4.72 | 3.42 | -4.1 | 2.96 | -4.83 | 3.49 | -2.27 | 3.56 |
| LSD | 0.79 | 0.56 | 0.8 | 0.56 | 0.29 | 0.87 | -0.8 | 1.03 |
| Naloxone | 0.92 | 0.26 | 0.64 | 0.19 | 0.26 | 0.94 | 0.36 | 0.39 |
| Naltrexone | -0.69 | 0.49 | -1.24 | 0.88 | -0.5 | 0.66 | -0.75 | 1.36 |
| Flumazenil | 1.44 | 1.01 | 1.23 | 0.87 | 1.13 | 0.77 | 0.3 | 1.57 |
| Diazepam | 1.54 | 1.09 | 0.13 | 0.1 | 2.88 | 1.6 | 0.1 | 0.74 |
| Flurazepam | 0.55 | 0.39 | 2.33 | 1.63 | 0.5 | 0.18 | 3.65 | 3.15 |
| Bromazepam | 1.32 | 0.93 | 0.15 | 0.11 | 1.62 | 0.37 | 0.59 | 0.41 |
| Delorazepam | -0.1 | 0.08 | 0.05 | 0.04 | -0.25 | 0.29 | -0.64 | 0.22 |
| Midazolam | 7.83 | 5.29 | 3.63 | 2.52 | 7.39 | 4.92 | 3.06 | 3.96 |
| Phenobarbital | -1.4 | 1 | -0.07 | 0.05 | -0.77 | 0.84 | 0.67 | 1.04 |
| Thiopental | 0.64 | 0.45 | 2.8 | 1.95 | -0.28 | 0.55 | 1.2 | 1.88 |
| Carbamazepine | 2.96 | 2.1 | 0.46 | 0.32 | 2.37 | 1.52 | 0.85 | 0.33 |
| Citalopram | 1.1 | 0.76 | 1.96 | 1.37 | 1.49 | 0.77 | 1.07 | 1.93 |
| Sertraline | 1 | 0.71 | 3.5 | 2.41 | 1.72 | 0.77 | 3.59 | 3.34 |
| Chlorpromazine | 0.88 | 0.62 | -0.18 | 0.13 | -1.16 | 1.25 | 0.32 | 0.47 |
| Promazine | 5.18 | 3.57 | 1.71 | 2.39 | 5.96 | 3.93 | 1.97 | 2.95 |
| Haloperidol | 0.61 | 0.43 | 0.47 | 0.34 | -0.55 | 1.03 | 1.86 | 0.24 |
| Clozapine | 2.97 | 2.07 | 0.25 | 0.18 | 2.22 | 2.04 | -0.99 | 0.12 |
| Olanzapine | 1.27 | 0.89 | -0.09 | 0.06 | 1.42 | 0.16 | 1 | 2.93 |
| Quetiapine | 3.52 | 2.45 | 0.96 | 0.67 | 3.03 | 2.35 | 0.34 | 0.61 |
| Fentanyl | 0.62 | 0.44 | -0.18 | 0.13 | 0.78 | 1.83 | -0.27 | 0.07 |
| Remifentanil | 1.36 | 0.95 | 0.63 | 0.45 | 1.17 | 0.99 | 0.8 | 0.43 |
| Propofol | 0.66 | 0.46 | 0.12 | 0.08 | 0.56 | 0.51 | 0.47 | 0.27 |
Table 15: Relative recovery tests of ASE extraction and SPE extraction at 30 ng/mL and 300 ng/mL per substance.
Absolute recovery of low, medium and high concentration for ASE extraction were calculated for both ASE extraction and SPE extraction by comparing analytes response obtained in extracted samples with analyte response at the same concentration put in vial with mobile phase. The samples fortified at 3 levels of concentration (low, medium and high) used for bias and precision ASE extraction validation were used also for absolute recovery test and compare with analyte response at the same concentration put in vial with mobile phase (Table 16).
| ASE extraction | SPE extraction | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30 ng/mL | 150 ng/mL | 300 ng/mL | 30 ng/mL | 150 ng/mL | 300 ng/mL | |||||||
| Molecules | Recovery % | % CV | Recovery % | % CV | Recovery % | % CV | Recovery % | %CV | Recovery % | % CV | Recovery % | % CV |
| Morphine | 98 | 13.57 | 94 | 5.63 | 101 | 1.28 | 99 | 16.77 | 105 | 5.59 | 106 | 3.67 |
| 6-MAM | 96 | 5.72 | 96 | 6.99 | 99 | 1.97 | 98 | 18.64 | 99 | 2.41 | 104 | 10.26 |
| Codeine | 91 | 7.69 | 98 | 1.16 | 102 | 2.54 | 87 | 7.34 | 94 | 1.77 | 103 | 14.83 |
| Ketamine | 91 | 17.74 | 100 | 2.56 | 91 | 2.9 | 102 | 18.47 | 97 | 3.21 | 107 | 17.76 |
| Amphetamine | 93 | 16.52 | 102 | 6.48 | 95 | 18.95 | 95 | 7.53 | 99 | 8.41 | 97 | 6.47 |
| Methamphetamine | 99 | 10.89 | 100 | 8.08 | 102 | 5.59 | 89 | 16.47 | 90 | 9.79 | 87 | 10.41 |
| MDA | 97 | 7.42 | 94 | 6.63 | 101 | 2.11 | 80 | 10.77 | 81 | 7.69 | 83 | 11.42 |
| MDMA | 86 | 8.13 | 101 | 4.58 | 107 | 11.2 | 99 | 12.48 | 103 | 6.68 | 102 | 4.52 |
| MDEA | 95 | 15.15 | 93 | 15.59 | 103 | 2.35 | 92 | 17.4 | 92 | 14.62 | 100 | 5.57 |
| Cocaine | 91 | 13.74 | 93 | 5.47 | 89 | 1.83 | 99 | 4.46 | 100 | 4.41 | 98 | 1.06 |
| Benzoylecgonine | 92 | 15.46 | 90 | 9.57 | 92 | 9.68 | 101 | 8.89 | 100 | 2.19 | 96 | 3.71 |
| Methadone | 82 | 9.51 | 94 | 12.67 | 87 | 16.56 | 87 | 3.87 | 92 | 4.95 | 91 | 17.54 |
| EDDP | 98 | 8.74 | 89 | 2.63 | 101 | 1.6 | 94 | 5.81 | 91 | 3.11 | 97 | 2.56 |
| LSD | 85 | 13.27 | 92 | 14.48 | 89 | 10.81 | 85 | 8.44 | 99 | 12.5 | 93 | 6.36 |
| Naloxone | 96 | 15.38 | 94 | 18.31 | 89 | 0.52 | 99 | 13.55 | 83 | 1.57 | 92 | 9.01 |
| Naltrexone | 96 | 4.34 | 90 | 11.61 | 102 | 4.39 | 97 | 19.59 | 91 | 12.25 | 101 | 5.41 |
| Flumazenil | 81 | 10.39 | 82 | 4.67 | 81 | 9.15 | 82 | 8.62 | 83 | 15.32 | 82 | 17.11 |
| Diazepam | 99 | 3.35 | 98 | 2.8 | 99 | 1.04 | 97 | 3.77 | 95 | 2.53 | 100 | 1.71 |
| Flurazepam | 103 | 9.89 | 106 | 10.46 | 101 | 13.7 | 101 | 7.39 | 102 | 6.8 | 99 | 8.81 |
| Bromazepam | 89 | 12.34 | 88 | 7.33 | 90 | 14.93 | 91 | 12.98 | 87 | 7.84 | 89 | 19.59 |
| Delorazepam | 95 | 13.68 | 94 | 7.26 | 102 | 12.89 | 96 | 10.94 | 96 | 6.3 | 104 | 14.16 |
| Midazolam | 99 | 14.57 | 94 | 6.03 | 87 | 2.77 | 92 | 10.96 | 91 | 5.51 | 88 | 3.59 |
| Phenobarbital | 82 | 5.78 | 80 | 3.04 | 83 | 5.38 | 84 | 5.06 | 82 | 3.77 | 86 | 6.47 |
| Thiopental | 88 | 12.43 | 86 | 6.06 | 82 | 1.75 | 83 | 11.97 | 87 | 7.63 | 91 | 1.4 |
| Carbamazepine | 96 | 16.05 | 102 | 5.66 | 105 | 4.14 | 98 | 19.97 | 107 | 5.57 | 106 | 5.51 |
| Citalopram | 96 | 17.44 | 85 | 3.87 | 96 | 1.67 | 95 | 10.43 | 84 | 6.1 | 93 | 1.27 |
| Sertraline | 100 | 18.76 | 100 | 12.12 | 96 | 4.81 | 99 | 9.5 | 98 | 6.16 | 96 | 5.32 |
| Chlorpromazine | 82 | 19.29 | 97 | 1.17 | 93 | 17.26 | 85 | 18.73 | 96 | 0.56 | 91 | 10.9 |
| Promazine | 83 | 14.61 | 91 | 1.48 | 90 | 0.83 | 86 | 14.32 | 89 | 2.21 | 88 | 1.05 |
| Haloperidol | 97 | 15.84 | 99 | 5.45 | 100 | 9.95 | 96 | 13.61 | 98 | 4.52 | 191 | 10.75 |
| Clozapine | 86 | 11.05 | 87 | 3.75 | 103 | 10.37 | 87 | 13.1 | 86 | 4.68 | 101 | 11.23 |
| Olanzapine | 99 | 6.77 | 97 | 3.15 | 98 | 2.26 | 88 | 15.91 | 86 | 15.14 | 89 | 14.15 |
| Quetiapine | 89 | 10.78 | 95 | 13.39 | 99 | 13.94 | 87 | 10.67 | 96 | 13.02 | 98 | 12.97 |
| Fentanyl | 81 | 8.76 | 84 | 16.1 | 87 | 4.08 | 83 | 9.26 | 85 | 16.19 | 89 | 2.9 |
| Remifentanil | 91 | 8.57 | 97 | 3.25 | 94 | 1.1 | 92 | 7.41 | 96 | 4.99 | 95 | 1.44 |
| Propofol | 92 | 8.79 | 95 | 10.33 | 99 | 11.72 | 93 | 7.5 | 96 | 10.76 | 100 | 10.18 |
Table 16: Absolute recovery tests of ASE extraction and SPE extraction at 30 ng/mL, 150 ng/mL and 300 ng/mL per substance.
The ionization suppression/enhancement for ASE extraction ranges from -5.12% to 5.73% with value of %CV below 3.94% (Table 15), whereas the ionization suppression/enhancement for SPE extraction was below 7.39% and above -4.83% with %CV<4.92%. Absolute recovery for both the extractive method was between 80%-107% of all the molecules with %CV below 19.97%.
Discussion
In this work, ASE extraction technique was validated as new method of extraction obtaining results that met the criteria of standard practices for method validation in forensic toxicology [36] (Table 14). Then, the new validated method was compared with the standardized SPE extraction. Moreover, the analytical results obtained by the comparison of these two methods underlined the efficiency of the ASE extraction when compared to SPE extraction. Recovery tests show that most of the molecules of interest can be equally extracted with both methods with satisfactory results, whereas some molecules have better recovery in ASE extraction: Methamphetamine, MDA and olanzapine, for example, have better recovery results with ASE technique with lower %CV in respect with SPE technique (Table 16). On the other hand, cocaine and benzoylecgonine have a greater affinity for SPE extraction with lower %CV (Table 16). Other molecules analyzed have demonstrated worse recovery values in both methods (e.g. flumazenil and phenobarbital) (Table 16). However, even some differences are present; the results obtained from both extractive methods are superimposable. Chromatographic profiles indicate ASE generated extracts nearly identical in composition to those generated by conventional techniques.
The ASE extraction was validated in animal [12-19] and botanical [7,8,28,20-27] field, but there is a paucity of research regarding the ASE technique applied to human biological samples [29-34]. The validation procedure for this new method is still in progress, the validation technique was already applied to meconium samples [29,30] searching for cotinine and nicotine cocaine and metabolites or to whole blood samples as in this study, measuring dioxins or fatty acids. Therefore, in this research the validation method was applied on whole blood samples, an essential matrix in forensic toxicological analyses, validating 36 molecules of toxicological interest. Future studies could be focus on other biological matrices, as urine or gastric content human samples. The SPE is a traditional extraction technique tested and validated through the years in several scientific fields, as botanical, animal, and forensic one [38].
Moreover, the new method reported some advantages in respect with the SPE extraction. Indeed, this extractive method reduces solvent consumption, improves extractive processes, cuts down the time required for multiple extractions, greatly decreases the bias caused by the operator and increases sample throughputs. ASE extraction has the advantage of a solid robustness as variation due operator-dependent steps mostly eliminated due to the almost complete automatization of the procedure.
Conclusion
During this study, the reliability of the Accelerated-Solvent Extraction technique was validated under a qualitative and quantitative point-of-view, in respect with the Solid-Phase Extraction analyzing many molecules of toxicological-forensic interest on whole blood matrix.
After the comparison of the recoveries of substances obtained from both the extractive methods (ASE and SPE), it can be asserted that it is possible to consider the ASE as an efficient alternative method of extraction and purification of this type of biological matrix. The versatility of this extractive procedure allows method customization for peculiar molecules or samples of different nature. Especially, pressure, temperature, and time of extraction and solvent mixtures can be modified to target specific molecules and increase the process efficiency. Considering all these advantages, the accelerated-solvent extraction should be considered fundamental in forensic toxicology and in research laboratories. More methods should be developed to further increase the sensitivity of this extractor on different molecules and method validation procedures should be developed for other matrices of cadaveric origin.
References
- Bratinčević MV, Visković T, Sutlović D (2017) Comparison of the solid phase and liquid-liquid extraction methods for methadone determination in human serum and whole blood samples using gas chromatography/mass spectrometry. Arh Hig Rada Toksikol 68: 308-314. [Crossref] , [Google Scholar], [Indexed]
- Ötles S, Kartal C (2016) Solid-Phase Extraction (SPE): Principles and applications in food samples. Acta Sci Pol Technol Aliment 15: 5-15. [Crossref], [Google Scholar], [Indexed]
- Richter BE, Jones BA, Ezzell JL, Porter NL, Avdalovic N, et al. (1996) Accelerated solvent extraction: A technique for sample preparation. Anal Chem 68: 1033-1039. [Crossref] , [Google Scholar],
- Tsakelidou E, Virgiliou C, Valianou L, Gika HG, Raikos N, et al. (2017) Sample preparation strategies for the effective quantitation of hydrophilic metabolites in serum by multi-targeted HILIC-MS/MS. Metabolites 7: 13. [Crossref], [Google Scholar], [Indexed]
- Humbert L (2010) Extraction en phase solide (SPE): Théorie et applications. Ann Toxicol Anal 22: 61-68. [Crossref], [Google Scholar]
- Labella GF, Bousova K, Hollosi L, Arioli F (2016) Comparison between Accelerated Solvent Extraction (ASE) with clean up in-line and Quick, Easy, Cheap, Effective, Rugged, and Safe (QuEChERS) extraction in honey. Int J Heal Anim Sci Food Saf 3: 3927. [Crossref], [Google Scholar]
- Popp P, Keil P, Möder M, Paschke A, Thuss U (1997) Application of accelerated solvent extraction followed by gas chromatography, high-performance liquid chromatography and gas chromatography-mass spectrometry for the determination of polycyclic aromatic hydrocarbons, chlorinated pesticides and polychlorin. J Chromatogr A 774: 203-211. [Crossref], [Google Scholar]
- Conte E, Milani R, Morali G, Abballe F (1997) Comparison between accelerated solvent extraction and traditional extraction methods for the analysis of the herbicide diflufenican in soil. J Chromatogr A 765: 121-125. [Crossref], [Google Scholar]
- Kellogg J, Wallace E, Graf T, Oberlies NH, Cech NB (2017) Conventional and accelerated-solvent extractions of green tea (Camellia sinensis) for metabolomics-based chemometrics. J Pharm Biomed Anal 145: 604-610. [Crossref], [Google Scholar], [Indexed]
- Wang G, Lee AS, Lewis M, Kamath B, Archer RK (1999) Accelerated solvent extraction and gas chromatography/mass spectrometry for determination of polycyclic aromatic hydrocarbons in smoked food samples. J Agric Food Chem 47: 1062-1066. [Crossref], [Google Scholar], [Indexed]
- Mottaleb MA, Sarker SD (2012) Accelerated solvetn extraction for natural products isolation. Nat Prod Isol 864: 1-25. [Crossref], [Google Scholar], [Indexed]
- Abend AM, Chung L, McCollum DG, Wuelfing WP (2003) Development and validation of an automated extraction method (accelerated solvent extraction®) and a reverse-phase HPLC analysis method for assay of ivermectin in a meat-based chewable formulation. J Pharm Biomed Anal 31: 1177-1183. [Crossref], [Google Scholar], [Indexed]
- Ottonello G, Ferrari A, Magi E (2014) Determination of polychlorinated biphenyls in fish: Optimisation and validation of a method based on accelerated solvent extraction and gas chromatography-mass spectrometry. Food Chem 142: 327-333. [Crossref], [Google Scholar], [Indexed]
- Sun H, Zuo Y, Qi H, Lv Y (2012) Accelerated solvent extraction combined with capillary electrophoresis as an improved methodology for simultaneous determination of residual fluoroquinolones and sulfonamides in aquatic products. Anal Methods 4: 670-675. [Crossref], [Google Scholar]
- Tao Y, Chen D, Wei H, Yuanhu P, Liu Z, et al (2012) Development of an accelerated solvent extraction, ultrasonic derivatisation LC-MS/MS method for the determination of the marker residues of nitrofurans in freshwater fish. Food Addit Contam-Part A Chem Anal Control Expo Risk Assess 29: 736-745. [Crossref], [Google Scholar], [Indexed]
- Wang B, Xie X, Zhao X, Xie K, Diao Z, et al (2019) Development of an accelerated solvent extraction-ultra-performance liquid chromatography-fluorescence detection method for quantitative analysis of thiamphenicol, florfenicol and florfenicol amine in poultry eggs. Molecules 24: 1-16. [Crossref], [Google Scholar], [Indexed]
- Wang B, Pang M, Zhao X, Xie K, Zhang P, et al (2019) Development and comparison of liquid-liquid extraction and accelerated solvent extraction methods for quantitative analysis of chloramphenicol, thiamphenicol, florfenicol, and florfenicol amine in poultry eggs. J Mass Spectrom 54: 488-494. [Crossref], [Google Scholar], [Indexed]
- Wang B, Pang M, Zhao X, Xie K, Zhang P, et al (2019) Development of an accelerated solvent extraction approach for quantitative analysis of chloramphenicol, thiamphenicol, florfenicol, and florfenicol amine in poultry eggs. Food Anal Methods 54: 488-494. [Crossref], [Google Scholar], [Indexed]
- Wu JJ, Mak YL, Murphy MB, Lam JCW, Chan WH, et al (2011) Validation of an accelerated solvent extraction liquid chromatography- tandem mass spectrometry method for Pacific ciguatoxin-1 in fish flesh and comparison with the mouse neuroblastoma assay. Anal Bioanal Chem 400: 3165-3175. [Google Scholar], [Crossref],[Indexed]
- Kellogg JJ, Wallace ED, Graf TN, Oberlies NH, Cech NB (2017) Conventional and accelerated-solvent extractions of green tea (camellia sinensis) for metabolomics-based chemometrics. J Pharm Biomed Anal 145: 604-610. [Crossref], [Google Scholar], [Indexed]
- Chuang YH, Zhang Y, Zhang W, Boyd SA, Li H (2015) Comparison of accelerated solvent extraction and quick, easy, cheap, effective, rugged and safe method for extraction and determination of pharmaceuticals in vegetables. J Chromatogr A 1404: 1-9. [Crossref], [Google Scholar], [Indexed]
- Marchese S, Perret D, Gentili A, Curini R, Marino A (2001) Development of a method based on accelerated solvent extraction and liquid chromatography/mass spectrometry for determination of arylphenoxypropionic herbicides in soil. Rapid Commun Mass Spectrom 15: 393-400. [Crossref], [Google Scholar], [Indexed]
- Pallaroni L, Björklund E, Von Holst C (2002) Alternative extraction methods for zearalenone: Microwave assisted extraction and accelerated solvent extraction. Mycotoxin Res 18: 74-77. [Crossref], [Google Scholar], [Indexed]
- Ge X, Wu X, Liang S, Sun H (2014) A sensitive and validated HPLC method for the determination of cyromazine and melamine in herbal and edible plants using accelerated solvent extraction and cleanup with SPE. J Chromatogr Sci 52: 751-757. [Crossref], [Google Scholar], [Indexed]
- Yang G, Sun Q, Hu Z, Liu H, Zhou T, et al (2015) Optimization of an accelerated solvent extraction dispersive liquid-liquid microextraction method for the separation and determination of essential oil from ligusticum chuanxiong hort by gas chromatography with mass spectrometry. J Sep Sci 38: 3588-3598. [Crossref], [Google Scholar], [Indexed]
- Zhang Y, Yang J, Shi R, Su Q, Gao Y, et al (2011) Development of an analytical method based on accelerated solvent extraction, solid-phase extraction clean-up, then GC-ECD for analysis of fourteen organochlorine pesticides in cereal crops. Chromatographia 73: 385-391. [Crossref], [Google Scholar]
- Zhang YN, Yang XL, Bian YR, Gu CG, Wang DZ, et al (2016) An accelerated solvent extraction-solid phase extraction-high performance liquid chromatographic method for determination of polycyclic aromatic hydrocarbons in soil and earthworm samples. Chinese J Anal Chem 44: 1514-1520. [Crossref], [Google Scholar]
- Zhu QZ, Sun Q, Su ZG, Xie MM, Song JY, et al (2014) A soil water extraction method with accelerated solvent extraction technique for stable isotope analysis. Chinese J Anal Chem 42: 1270-1275. [Crossref], [Google Scholar]
- Sant'Anna SG, Oliveira CDR, Diniz EMA, Yonamine M (2012) Accelerated solvent extraction for gas chromatographic analysis of nicotine and cotinine in meconium samples. J Anal Toxicol 36: 19-24. [Crossref], [Google Scholar], [Indexed]
- Mantovani CC, Lima MB, Oliveira CDR, Menck RA, Diniz EMA, et al (2014) Development and practical application of accelerated solvent extraction for the isolation of cocaine/crack biomarkers in meconium samples. J Chromatogr B Anal Technol Biomed Life Sci 957: 14-23. [Crossref],[Google Scholar], [Indexed]
- Giordano G, Gomez LB, Seneci P, Cattaneo C, Candia DD (2021) Detecting drugs in dry bone : A pilot study of skeletal remains with a post-mortem interval over 23 years. Int J Legal Med 135: 19-23. [Crossref], [Google Scholar], [Indexed]
- Franceschetti L, Candia DD, Giordano G, Carabelli I, Vignali G, et al (2020) Drugs in bone: Detectability of substances of toxicological interest in different states of preservation. J Forensic Sci 66: 677-686. [Crossref], [Google Scholar], [Indexed]
- Iida T, Todaka T (2003) Measurement of dioxins in human blood: Improvement of analytical method. Ind Health 41: 197-204. [Crossref], [Google Scholar], [Indexed]
- Kuriki K, Tajima K, Tokudome S (2006) Accelerated solvent extraction for quantitative measurement of fatty acids in plasma and erythrocytes. Lipids 41: 605-614. [Crossref], [Google Scholar], [Indexed]
- Di Candia D, Boracchi M, Muccino E, Gentile G, Zoja R (2021) The lethal cutting: An unexpected cause of death-A methomyl acute intoxication. J Anal Toxico l00: 1-8. [Crossref], [Google Scholar], [Indexed]
- https://www.aafs.org/asb-standard/standard-practices-method-validation-forensic-toxicology. [Crossref],[Google Scholar]
- Kollipara S, Bende G, Agarwal N, Varshney P, Paliwal J (2011) International guidelines for bioanalytical method validation: A comparison and discussion on current scenario. Chromatographia 73: 201-217. [Crossref], [Google Scholar]
- Ferenc AZ, Biziuk M (2006) Solid phase extraction technique-trends, opportunities and applications. Pol J Environ Stud 15: 677-690. [Crossref], [Google Scholar]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi