Case Report, Clin Oncol Case Rep Vol: 3 Issue: 3
Disease Progression after Discontinuation of Nivolumab for Intolerable ImmunotherapyAssociated Polymyalgia Rheumatica in a Patient with Metastasized Adrenocortical Carcinoma: A Case Report and Review of the Literature
Dennis Christoph Harrer1*, Martin Fassnacht2, Matthias Kroiss2, Laura Landwehr2, Dirk Hellwig3, Christina Wendl4, Wolfgang Herr1, Tobias Pukrop1 and Martin Vogelhuber1
1Department of Hematology and Internal Oncology, University Hospital Regensburg, Franz-Josef-Strauss-Allee 11, D-93053 Regensburg, Germany
2Department of Internal Medicine, Division of Endocrinology and Diabetes, University Hospital, University of Wurzburg, Oberdurrbacher Strauss 6, D-97008 Wurzburg, Germany
3Department of Nuclear Medicine, University Hospital Regensburg, Franz-Josef- Strauss-Allee 11, D-93053 Regensburg, Germany
4Departmentof Radiology, University Hospital Regensburg, Franz-Josef-Strauss- Allee 11, D-93053 Regensburg, Germany
*Corresponding Author : Dennis Harrer, Department of Hematology and Internal
Oncology
University Hospital Regensburg, D-91052 Erlangen, Germany
Franz-
Josef-Strauss-Allee 11, D-93053 Regensburg, Germany
Tel: 09419445510
E-mail: dennis.harrer@ukr.de
Received: March 27, 2020 Accepted: April 15, 2020 Published: April 27, 2020
Citation: Harrer DC, Fassnacht M, Kroiss M, Landwehr L, Hellwig D, et al. (2020) Disease Progression after Discontinuation of Nivolumab for Intolerable Immunotherapy-Associated Polymyalgia Rheumatica in a Patient with Metastasized Adrenocortical Carcinoma: A Case Report and Review of the Literature. Clin Oncol Case Rep 3:3.
Abstract
Background: Metastasized Adrenocortical Carcinoma (ACC) ranks high among tumors with a dismal prognosis. Antibodies blocking PD-1, such as nivolumab, have proven efficacy against a variety of metastasized malignancies. In this case report, we present a long-term survivor with metastasized ACC who responded well to treatment with nivolumab but showed disease progression after discontinuation of PD-1 blockade due to intolerable immunotherapyassociated Polymyalgia Rheumatica (PMR). Case Presentation: A 38-year-old woman was subjected to adrenalectomy due to newly diagnosed adrenocortical carcinoma. Over the next 15 years, multiple metastases were treated using surgery, radiotherapy, chemotherapy, mitotane and targeted radionuclide therapy. The histological examination of brain metastases that occurred 16 years after primary diagnosis revealed high PD-L1 expression on adrenocortical carcinoma cells prompting the initiation of nivolumab therapy. The patient displayed a pronounced response to immunotherapy with significant regression of pulmonary and abdominal metastases. After 28 cycles of nivolumab, however, the patient complained about intolerable pain and stiffness of proximal muscles compatible with polymyalgia rheumatica. Thus, nivolumab was discontinued and corticosteroids, as well as cyclophosphamide, were administered. While the muscle pain could be alleviated using intensified immunosuppression, Positron Emission Tomography (PET) imaging performed after suspension of nivolumab showed progressive disease of formerly controlled metastases and the manifestation of entirely new metastatic lesions. Conclusion: This case report showcases the clinical efficacy of PD-1 blocking therapy in a heavily pre-treated patient with metastasized adrenocortical carcinoma. Moreover, we present the first case report of disease progression after discontinuation of PD-1 blocking therapy due to PMR highlighting PMR as a rare but severe immunotherapy-related adverse event. Finally, we compiled published cases about PMR arising during therapy with nivolumab or pembrolizumab.
Keywords: Nivolumab; Immunotherapy; Polymyalgia rheumatica; Metastasized adrenocortical carcinoma
Introduction
Adrenocortical Carcinoma (ACC) constitutes rare endocrine neoplasia with a poor prognosis. In advanced ACC, five-year-survival rates are clearly below 20%, and the long-term survival of patients with metastasized ACC is extremely rare [1-3]. The current therapeutic armamentarium for ACC comprises surgery, the adrenolytic agent mitotane, other localized treatment modalities and cytotoxic chemotherapy [4].
The Programmed Death 1 (PD-1)/Programmed Death 1 Ligand (PD-L1) axis assumes a crucial role in protecting tumor cells from T-cell mediated anti-cancer immune attack. Antibodies blocking PD-1 on T cells, such as nivolumab or pembrolizumab, have caused spectacular tumor regressions across a variety of different cancer entities [5]. Nevertheless, the unselected induction of T cell activation by PD-1 blocking antibodies harbors the potential for serious autoimmune toxicities. Recurrent immune-related adverse events (irAEs) include hepatitis, colitis, pancreatitis, hypophysitis, thyroiditis, dermatitis, neurotoxicity, myositis and arthritis. Depending on the severity,irAEs are treated with immunosuppression and discontinuation of immunotherapy.
Polymyalgia Rheumatica (PMR), which predominantly affects females above 50 years of age, is characterized by pain and morning stiffness of proximal muscles, such as thighs, shoulders, hips, and neck [6]. Whereas elevation of Creatine-Kinase (CK) is uncommon in patients suffering from PMR, C-Reactive Protein (CRP) and Erythrocyte Sedimentation Rate (ESR) are suitable indicators for active PMR [6]. Therapy of PMR usually requires the administration of moderate-dose corticosteroids resulting in the swift resolution of symptoms [6].
In this case report, we present a rare long-term survivor with metastasized ACC who responded well to treatment with nivolumab but showed disease progression after discontinuation of PD-1 blockade due to intolerable immunotherapy-associated polymyalgia rheumatica. This report aims to corroborate the clinical efficacy of nivolumab in the treatment of metastasized ACC and to highlight PMR as a rare but severe and treatment-limiting immunotherapyrelated adverse event.
Case Presentation
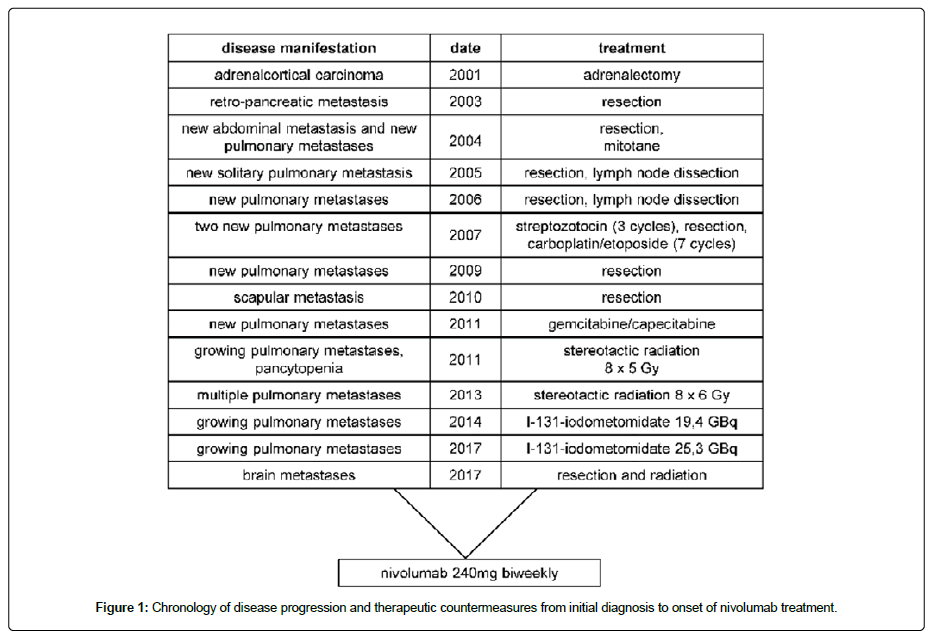
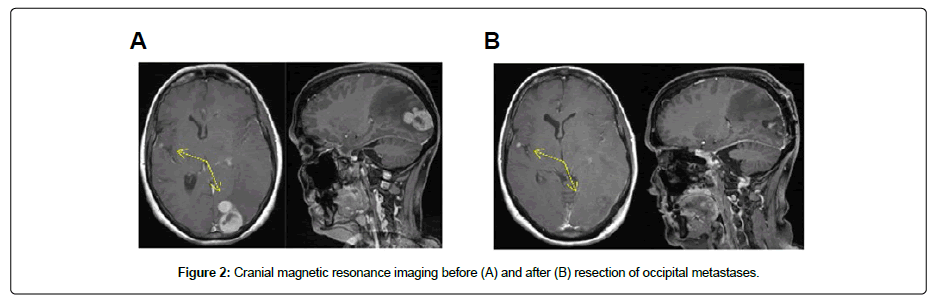
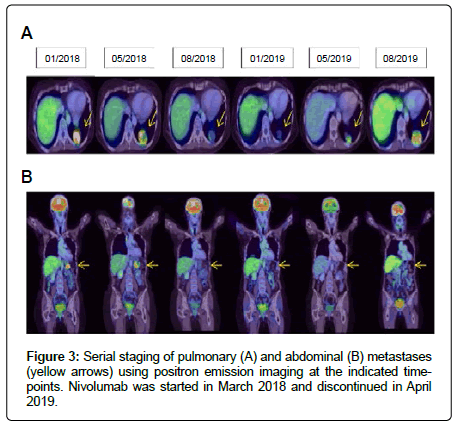
A 38-year-old woman diagnosed with glucocorticoid-secreting adrenocortical carcinoma underwent laparoscopic adrenalectomy in 2001. One year later, a retro-pancreatic metastasis was detected and surgically removed. In the following year, another abdominal metastasis was resected, and on the suspicion of pulmonary metastases, mitotane treatment was initiated. Over the ensuing six years, progressive disease was observed in both lungs prompting repetitive surgical interventions and the application of three cycles of streptozotocin as well as seven cycles of carboplatin/etoposide (Figure 1). In 2010, a scapular metastasis was excised. Within the next seven years, continuous progression of pulmonary metastases necessitated counteraction with a multimodal therapeutic approach involving gemcitabine/capecitabine chemotherapy, stereotactic radiation and targeted radionuclide therapy using I-131-iodometomidate (Figure 1). In December 2017, the patient was hospitalized with sudden cognitive impairments and headache. Magnetic resonance imaging revealed metastases in the temporal and occipital lobes (Figure 2). Histopathological examination of the fully resected occipital lesions revealed less differentiated epithelial carcinoma cells showing high proliferation and an immunohistochemistry profile concordant with the known adrenal carcinoma. Also, carcinoma cells displayed high grade microsatellite instability (MSI-H). Moreover, five percent of the tumor cells stained positive for PD-L1 (2/6 Cologne proportion score), and PD-L1 could be detected on up to 25% percent of the tumor surrounding cells. Hence, after radiotherapy of the temporal metastasis and the occipital resection field, immunotherapy with the PD-1 blocking antibody Nivolumab was started at 240 mg biweekly in March 2018. After two months, Positron Emission Tomography (PET) imaging revealed metabolicresponse in pulmonary metastases (Figure 3A). In August 2018, both pulmonary and abdominal lesions showed significant tumor regression (Figure 3A and 3B).
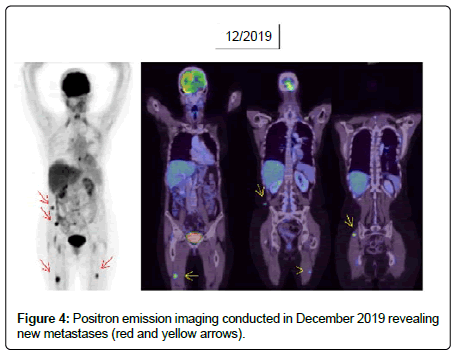
However, approximately one year after initiation of immunotherapy the patient complained about progressive diffuse muscle pain clustered around the shoulders, the pelvic girdle, and the thighs. Physical examination revealed subfebrile temperatures. Indicators of muscle damage, such as CK and Lactate Dehydrogenase (LDH) remained at baseline. Elevated levels of C-Reactive Protein (CRP: 39 mg/dl, normal: <5 mg/dl) suggested an ongoing inflammatory process. Based on those clinical findings a diagnosis of immunotherapy-associated PMR was made. Nivolumab therapy was suspended after 29 cycles. Furthermore, Prednisolone was started at 50mg daily resulting in mitigation of muscle pain and normalization of CRP. Subsequent efforts to taper the Prednisolone dose led to a re-emergence of PMR with a concomitant rise in CRP up to 100 mg/dl (normal <5 mg/dl). Finally, sufficient control of PMR could be achieved with 100 mg Prednisolone daily in combination with oral cyclophosphamide at 50 mg twice daily. Gradually, the Prednisolone dose could be successfully lowered to 10mg without additional cyclophosphamide. Currently, no clinical manifestations of PMR are present. Unfortunately, PET imaging performed after discontinuation of Nivolumab treatment revealed the progressive disease of previously controlled pulmonary metastases (Figure 3A). Besides, a new metastasis was found in the cerebellum. Given the initially difficult control of PMR requiring ongoing intensified immunosuppression, a re-exposure to Nivolumab was dismissed. Pulmonary metastases were surgically removed, and the patient underwent radiotherapy for the cerebellar lesion. Most recently, PET-imaging performed seven months after discontinuation of Nivolumab treatment showed new soft-tissue metastases located around the pelvic girdle (Figure 4). The patient is currently being prepared for re-exposure to Nivolumab therapy.
Discussion
Metastasized ACC is a rare entity with a sinister prognosis. While localized ACC (stage I and II according to ENSAT classification) exhibit survival rates well above 50%, 5-year survival for stage III (lymph node involvement)is around 50% and for patients with metastasized ACC (stage IV) overall survival rates approximate 13% [3]. Thus, clinical courses with stage IVACC extending over more than a decade are rather uncommon. Given the paucity of effective treatment options in case of ineligibility for surgery, novel treatment strategies beyond chemotherapy and mitotane are urgently needed. Unfortunately, the molecular architecture of ACC is rather heterogenous lacking signature oncogenic drivers that are druggable [7-9].
Antibodies blocking the immune checkpoint molecule programmed death 1 (PD-1), up-regulation of which is frequently observed on tumor-infiltrating T cells impeding tumor-destruction by tumor-specific T cells, have mediated spectacular tumor regressions in metastasized solid tumors, such as melanoma, renal cell carcinoma and non-small cell lung cancer [5]. Concerning ACC, a phase-II clinical trial evaluating the efficacy of the anti-PD1 antibody nivolumab administered at 240 mg biweekly to ten patients with metastasized ACC pretreated with mitotane and platinumbased chemotherapy was published recently [10]. Stable disease was found in two patients lasting several months and one patient showed an unconfirmed partial response [10]. Two trials exploring the PD-1 blocking antibody pembrolizumab yielded similar results with objective response rates of 14% and 25%, respectively [11,12]. No complete remissions could be induced in either trial [11,12]. To date, the only durable complete remission of metastasized ACC using PD-1 blocking agents was achieved in a patient concomitantly harboring a high mutational burden and a mismatch-repair deficiency [13]. The patient in the present case-report did also evince mismatchrepair deficiency and the decision to initiate nivolumab therapy was corroborated by the abundant presence of PD-L1 on carcinoma cells and stromal cells. Interestingly, PD-L1 expression on ACC cells is not essential for the anti-tumor activity of PD-1 blocking agents as evidenced by impressive partial responses of patients with PDL1 negative ACC to pembrolizumab therapy [12,14]. In aggregate, PD-1 blocking antibodies demonstrate variable anti-tumor activity against ACC necessitating the quest for suitable biomarkers to a priori identify ACC patients benefiting from immunotherapy.
During treatment with PD-1/PD-L1 blocking agents, immunerelated adverse events (irAEs) are observed (or develop/occur) in more than half of treated patients within the first six months [5]. Mechanistically, irAEs are attributed to a general lowering of selftolerance resulting inactivation of auto-reactive T cells with ensuing autoimmune reactions. Here, we report a case of polymyalgia rheumatica occurring approximately one year after the start of nivolumab therapy. PMR is an autoimmune disease caused by synovitis of the proximal joints resulting in morning stiffness and pain of the proximal muscle groups [6]. Four other case reports on nivolumab-induced PMR [15-18] and four other patients with pembrolizumab-induced PMR [15,19,20] have been published. Therapeutically, PMR is managed by discontinuation of immunotherapy and initiation of moderate-dose steroids followed by tapering of the steroid dose and resumption of immunotherapy with variable corticosteroid maintenance after a restart of immunotherapy [21]. Strikingly, in the present case report standard immunosuppression with corticosteroids did not suffice to entirely relieve from PMR symptoms after discontinuation of nivolumab. Hence, intensified immunosuppression using higher doses of prednisone combined with oral cyclophosphamide was necessary preventing swift tapering of immunosuppression required for a rapid restart of nivolumab. Unfortunately, the patient showed disease progression after discontinuation of nivolumab. Currently, the immunosuppression could be lowered to 10 mg prednisone daily and the re-introduction of immunotherapy is planned. While data obtained from melanoma patients treated with corticosteroids for irAEs induced by nivolumab did not indicate a disadvantage concerning overall survival [22], a similar study evaluating lung cancer patients found reduced overall survival as well as reduced progression-free survival if the prednisone doses were greater than 10 mg per day [23]. Nevertheless, comprehensive studies evaluating cancer control after initiation of immunosuppression to treat irAEs are still rare. Especially, reports on cancer control after the start of combinational immunosuppression as utilized in this case are missing.
Conclusion
In sum, we report a profound regression of metastases in a patient with ACC during nivolumab treatment. Furthermore, we present the fifth case of PMR-associated with nivolumab therapy and the first case showing disease progression after discontinuation of PD-1 blocking therapy due to intolerable PMR. Thus, we want to raise awareness for PMR as a severe irAE requiring appropriate immunosuppressive therapy and enhanced vigilance for potential disease progression during immunosuppression.
References
- Creemers SG, Hofland LJ, Korpershoek E, Franssen GH, Kemenade FJ, et al. (2016) Future directions in the diagnosis and medical treatment of adrenocortical carcinoma. Endocr Relat Cancer 23: 43-69.
- Else T, Kim AC, Sabolch A, Raymond VM, Kandathil A, et al. (2014) Adrenocortical carcinoma. Endocr Rev 35: 282-326 .
- Fassnacht M, Kroiss M, Allolio B (2013) Update in adrenocortical carcinoma. J Clin Endocrinol Metab 98: 4551-4564.
- Fassnacht M, Dekkers O, Else T, Baudin E, Berruti A, et al. (2018) European society of endocrinology clinical practice guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European network for the study of adrenal tumors. Eur J Endocrinol 179: 1-46.
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, et al. (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366: 2443-2454.
- Salvarani C, Cantini F, Boiardi L, Hunder GG (2002) Polymyalgia rheumatica and giant-cell arteritis. N Engl J Med 347: 261-271.
- Assie G, Letouze E, Fassnacht M, Jouinot A, Luscap W, et al. (2014) Integrated genomic characterization of adrenocortical carcinoma. Nat Genet 46: 607-612.
- Zheng S, Cherniack AD, Dewal N, Moffitt RA, Danilova L, et al. (2016) Comprehensive pan-genomic characterization of adrenocortical carcinoma. Cancer Cell 29: 723-736.
- Lippert J, Appenzeller S, Liang R, Sbiera S, Kircher S, et al. (2018) Targeted molecular analysis in adrenocortical carcinomas: A strategy toward improved personalized prognostication. J Clin Endocrinol Metab 103: 4511-4523.
- Carneiro BA, Konda B, Costa RB, Costa RB, Sagar V, et al. (2019) Nivolumab in metastatic adrenocortical carcinoma: Results of a phase 2 trial. J Clin Endocrinol Metab 104: 6193-6200.
- Habra MA, Stephen B, Campbell M, Hess K, Tapia C, et al. (2019) Phase II clinical trial of pembrolizumab efficacy and safety in advanced adrenocortical carcinoma. J Immunother Cancer 7: 253.
- Raj N, Zheng Y, Kelly V, Katz SS, Chou J, et al. (2020) PD-1 Blockade in advanced adrenocortical carcinoma. J Clin Oncol 38: 71-80.
- Mota JM, Sousa LG, Braghiroli MI, Siqueira LT, Neto JB, et al. (2018) Pembrolizumab for metastatic adrenocortical carcinoma with high mutational burden: Two case reports. Medicine (Baltimore) 97: e13517.
- Lindsey A, Hagman T, Hawk NC, Pablo B (2019) Impressive response to immunotherapy in a patient with metastatic adrenocortical carcinoma. Clin Oncol: Case Rep 2019: 2.
- Belkhir R, Le Burel S, Dunogeant L, Marabelle A, Hollebecque A, et al. (2017) Rheumatoid arthritis and polymyalgia rheumatica occurring after immune checkpoint inhibitor treatment. Ann Rheum Dis 76: 1747-1750.
- Bernier M, Guillaume C, Leon N, Alexandre J, Hamel SL, et al. (2017) Nivolumab causing a polymyalgia rheumatica in a patient with a squamous non-small cell lung cancer. J Immunother 40: 129-131.
- Nakamagoe K, Moriyama T, Maruyama H, Yokosawa M, Hara T, et al. (2017) Polymyalgia rheumatica in a melanoma patient due to nivolumab treatment. J Cancer Res Clin Oncol 143: 1357-1358.
- Imai Y, Tanaka M, Fujii R, Uchitani K, Okazaki K (2019) Effectiveness of a low-dose corticosteroid in a patient with polymyalgia rheumatica associated with nivolumab treatment. Yakugaku Zasshi 139: 491-495.
- Garel B, Kramkimel N, Trouvin AP, Frantz C, Dupin N (2017) Pembrolizumab-induced polymyalgia rheumatica in two patients with metastatic melanoma. Joint Bone Spine 84: 233-234.
- Robilliard B, Arnaud E, Gastaud L, Broner J (2018) A case of pembrolizumab-induced autoimmune haemolytic anaemia with polymyalgia rheumatica. Eur J Cancer 103: 281-283.
- Steven NM, Fisher BA (2019) Management of rheumatic complications of immune checkpoint inhibitor therapy: An oncological perspective. Rheumatology (Oxford) 58: 29-39.
- Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, et al. (2017) Safety profile of nivolumab monotherapy: A pooled analysis of patients with advanced melanoma. J Clin Oncol 35: 785-792.
- Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, et al. (2018) Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol 36: 2872-2878.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi