Case Report, J Sleep Disor Vol: 9 Issue: 1
Excessive Daytime Sleepiness without Sleep Apnea in a Weight Gain Boy with Prader-Willi Syndrome: A Case Report
Hitomi Ida1,2, Taro Adachi1,3,*, Mariko Morita1,2, Teruyoshi Kawamoto1, Yoshio Watanabe1,2, Toshiro Shinke3 and Hironori Sagara1,2
1Sleep Medicine Center, Showa University East Hospital, Tokyo, Japan
2Department of Medicine, Division of Respiratory Medicine and Allergology, Showa University, School of Medicine, Tokyo, Japan
3Department of Medicine, Division of Cardiology, Showa University, School of Medicine, Tokyo, Japan
*Corresponding Author: Taro Adachi
1-5-8, Hatanodai, Shinagawa-Ku, Tokyo, 142-8666, Japan
Tel: +81-3-3784-8539
Fax number: +81-3-3784-8622
E-mail: adachitaro@med.showa-u.ac.jp
Received: January 16, 2020 Accepted: February 10, 2020 Published: February 17, 2020
Citation: Ida H, Adachi T, Morita M, Kawamoto T, Watanabe Y, et al. (2020) Excessive Daytime Sleepiness without Sleep Apnea in a Weight Gain Boy with Prader-Willi Syndrome: A Case Report. J Sleep Disor: Treat Care 9:1. doi: 10.37532/jsdtc.2020.9(1).228
Abstract
Prader-Willi Syndrome (PWS) is a genetic disorder with several clinical features, such as excessive daytime sleepiness (EDS). However, it is difficult to delineate which sleep disorder causes EDS in patients with PWS because they have several risks of EDS, including obstructive sleep apnea (OSA), narcolepsy, and manifestation of PWS. We present the case of an obese 11-year-old boy with PWS who experienced EDS and excessive weight gain concurrently. He was diagnosed with PWS at the age of 5 months. At the age of 10 years, he gradually gained weight and developed symptoms of EDS. His attending family doctor referred him to our clinic to assess OSA using nocturnal polysomnography; however, there were no signs of sleep apnea. Results of the multiple sleep latency test did not fulfil the diagnostic criteria for narcolepsy. We concluded that the EDS in this patient was due to a direct symptom of PWS.
Keywords: Prader-Willi Syndrome, Hypersomnia, Obstructive Sleep apnea, Narcolepsy
Abbreviations
AHI: Apnea Hypopnea Index; BMI: Body Mass Index; EDS: Excessive Daytime Sleepiness; MSLT: Multiple Sleep Latency Test; Na-2: Narcolepsy Type 2; OSA: Obstructive Sleep Apnea; PSG: Polysomnography; PWS: Prader-Willi Syndrome; REM: Rapid Eye Movement
Introduction
Prader-Willi Syndrome (PWS) is a genetic disorder caused by deletion or inexpression of the paternal chromosome 15q11-q13. It is characterized by an unusual physical appearance, mental or neurological disabilities, and endocrinological abnormalities, which can differ, based on age [1]. During maturation, patients with PWS often experience weight gain due to hyperphagia and behavioural difficulties, such as stealthy eating as a toddler or young child [2]. In addition to obesity, they have elevated risk of obstructive sleep apnea (OSA) due to hypotonia and/or facial dysmorphia [3]. Recently, it has been reported that excessive daytime sleepiness (EDS), which can be caused by sleep apnea, narcolepsy, or as a direct symptom of PWS, is present in patients with PWS [4]. We present the case of an 11-yearold boy with PWS who experienced symptoms of EDS and weight gain concurrently.
Case Description
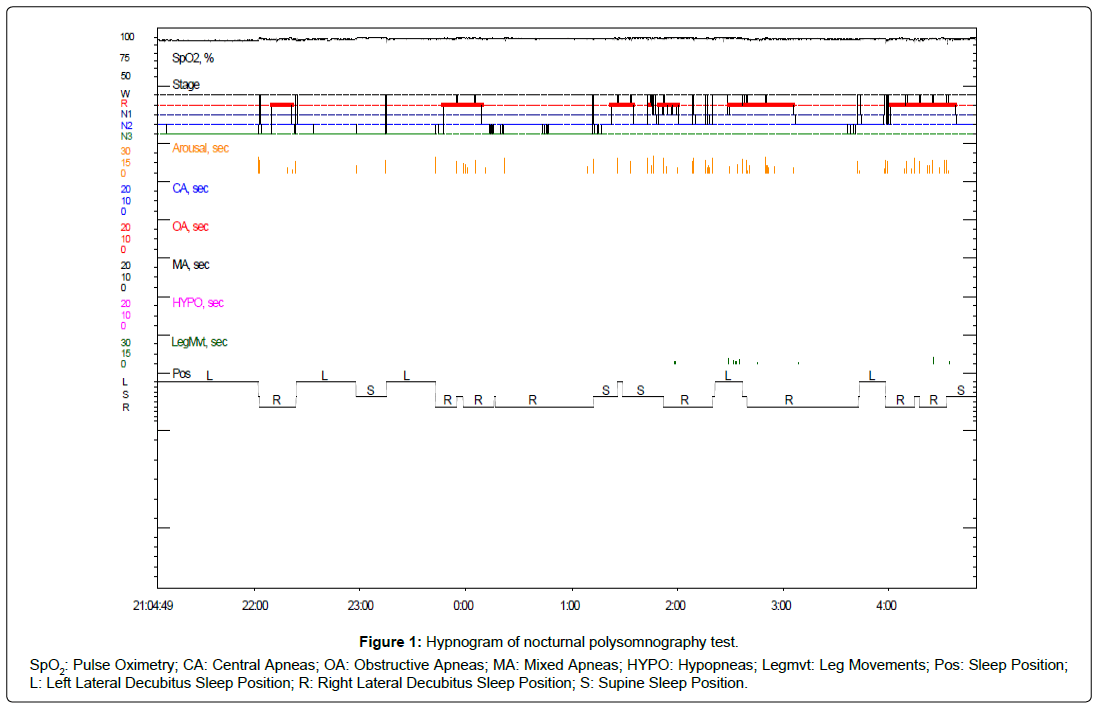
Our patient was born at 38 weeks of gestation, weighing 2.72 kg, by caesarean section because of a prior myomectomy. At birth, hypotonia, feeding difficulties and micrognathia were observed. He was diagnosed with PWS by genetic testing that revealed uniparental disomy at the age of 5 months. The delayed development was seen only until he reached 1 year of age, and he was able to hold his head up at the age of 7 months. When he was 11 months old, he underwent orchiopexy for cryptorchidism in his left testicles. He began to snore at the age of 4 years and underwent maxillary protraction using the right-angled maxillary protraction appliance system at the age of 6 years. He was never treated with growth hormones. Between the ages of 9 and 10 years, his weight increased from 32.5 kg (91.5th percentile for body mass index [BMI]-for-age) to 37.7 kg (93.0th percentile for BMI-for-age). At the same time, he began to develop symptoms of EDS. At the age of 10 years and 4 months, he began to fall asleep in the morning during school and he could not stay awake. His mother suspected OSA and took him to an otorhinolaryngology clinic, where he was prescribed a leukotriene receptor antagonist to improve his symptoms, but there was no improvement. A screening test for OSA was conducted using a portable sleep-monitoring device that revealed an apnea hypopnea index (AHI) score of 12 events per hour. Subsequent laboratory testing at the age of 10 years and 11 months confirmed that the endocrine functions affecting sleepiness, such as hypothalamic and thyroid functions, were normal. At the age of 11 years and 2 months, he was referred to our clinic for the diagnosis of OSA. While visiting our clinic, he woke up at 06:00 and went to bed at 20:30 daily, and practiced proper sleep hygiene. At the time of his examination, he was 134 cm in height, and 38 kg in weight (BMI: 21.2 kg/m2), his neck had a circumference of 31 cm, and his abdominal circumference was 78 cm. The oral examination revealed a narrow oropharynx, but normal palate tonsils and adenoids. We observed hyperextension of his neck while sleeping, but did not observe any snoring. His Epworth sleepiness scale score was 10. The nocturnal polysomnography test (PSG) showed excellent sleep efficiency (94.3%) and sleep architecture (3.0% stage N1, 31.9% N2, 38.1% N3, and 27.0% rapid eye movement [REM]) (Figure 1 and Table 1). Moreover, sleep apnea was not observed (AHI score = 0 event per hour). Since he did not have any episodes of cataplexy, hypnagogic hallucinations, or sleep paralysis during infancy, we suspected that the symptoms of EDS were due to direct symptoms of PWS. At the age of 11 years and 4 months, a multiple sleep latency test (MSLT) was performed after sleeping for 9.5 hours. The results of the MSLT (with four nap opportunities) demonstrated the following sleep latencies: 10 min and 30 sec, 13 min and 30 sec, 7 min and 30 sec, and 7 min and 40 sec. The overall mean sleep latency was 9 min and 45 sec without any sleep-onset REM periods (SOREMPs) (Table 1). These findings revealed that the patient did not meet the diagnostic criteria for narcolepsy including a mean sleep latency of ≤ 8 minutes and ≥ 2 SOREMPs on the MSLT [5]. Based on the PSG and MSLT results, we concluded that his symptoms of EDS were due to a direct symptom of PWS. The patient has been advised to undergo a second PSG in his adolescence to verify the existence of OSA. This case was approved by the ethics committee of Showa University School of Medicine. The patient and his caregiver provided informed consent for the data concerning this case to be submitted for publication.
Figure 1: Hypnogram of nocturnal polysomnography test.
SpO2: Pulse Oximetry; CA: Central Apneas; OA: Obstructive Apneas; MA: Mixed Apneas; HYPO: Hypopneas; Legmvt: Leg Movements; Pos: Sleep Position; L: Left Lateral Decubitus Sleep Position; R: Right Lateral Decubitus Sleep Position; S: Supine Sleep Position.
| Nocturnal polysomnography test | |||||
| EEG data | EMG data and video | ||||
| Time at lights off | 21:04:49 | Periodic limb movements (PLM) index | N/A | ||
| Time at lights on | 4:49:43 | ||||
| Total sleep time (TST) (min) | 438.4 | Respiratory data | |||
| Wake time after sleep onset (WASO) (min) | 26.5 | 3% oxygenation desaturation index (ODI) (/hr) | 0.8 | ||
| Sleep efficiency (SE) (%) | 94.3 | Apnea hypopnea index (AHI) | 0 | ||
| Sleep onset latency (SL) (min) | 0 | Mean O2 saturation (%) | 98 | ||
| REM latency (min) | 65 | Lowest O2 saturation (%) | 91 | ||
| Sleep stages | |||||
| % Stage N1 (%) | 3 | ||||
| % Stage N2 (%) | 31.9 | ||||
| % Stage N3 (%) | 38.1 | ||||
| % REM (%) | 27 | ||||
| Arousal index (AI) (/hr) | 8.5 | ||||
| Multiple sleep latency test | |||||
| Nap1 | Nap2 | Nap3 | Nap4 | ||
| Time at lights off | 9:03:00 | 11:02:00 | 13:07:00 | 15:03:00 | |
| Sleep onset | 9:13:00 | 11:15:30 | 13:14:30 | 15:10:30 | |
| Time at lights on | 9:33:00 | 11:32:00 | 13:32:00 | 15:26:00 | |
| Total recording time (TRT) (min) | 30 | 30 | 25 | 23 | |
| Total sleep time (TST) (min) | 9.5 | 9.5 | 17.5 | 14.5 | |
| Sleep latency (min) | 10.5 | 13.5 | 7.5 | 7.5 | |
| REM latency (min) | - | - | - | - | |
| Mean sleep latency (min) | 9.8 | ||||
| Mean REM latency (min) | 0 | ||||
| Number of SOREMPs (time) | 0 | ||||
Table1: Results of nocturnal polysomnography test and multiple sleep latency test.
Discussion
We presented the case of an 11-year-old boy with PWS who began to develop symptoms of EDS and gain weight concurrently. The nocturnal PSG test showed excellent sleep efficiency and no signs of sleep apnea (AHI score = 0 event per hour). Because his daily sleep duration was around 9 hours and he practiced proper sleep hygiene, we suspected hypersomnia. The MSLT findings did not fulfil the diagnostic criteria for narcolepsy. Based on these results, we concluded that his symptoms of EDS were due to direct symptoms of PWS.
In patients with PWS, EDS could adversely affect daily functions and learning is the most common type of sleep disturbance. Maas et al reported that 33% of the patients with PWS suffered from severe EDS [6]. Furthermore, EDS could adversely impact the sleep of caregivers of the patients with PWS [7]. The risks for OSA that cause EDS in patients with PWS include obesity, facial dysmorphia, and hypotonia [4]. It is necessary to diagnose OSA early to prevent the occurrence of life-threatening complications during adolescence and adulthood [1]. In children with PWS, a delay in treatment for OSA has been associated with various health problems, including lower cognitive function, cardiovascular complications, attention problems, hyperactivity, and other psychiatric problems [8]. However, there are not enough findings that demonstrate a relationship between sleepdisordered breathing and EDS in patients with PWS. EDS could occur in patients with PWS as a direct symptom of PWS itself, rather than by sleep disturbance caused by sleep-disordered breathing [4].
Hypothalamic dysfunction is thought to play an important role in EDS in patients with PWS [1]. Moreover, Sedky et al. reported that 35% of the children with PWS were diagnosed with narcolepsy, based on the MSLT results and/or symptoms of cataplexy. However, the authors were concerned that OSA, as a comorbidity, led to narcoleptic patterns on the MSLT by interrupting sleep [8]. Nevsimalova et al. suggested that hypothalamic dysfunction could be the cause of EDS in patients with PWS by assessing the level of cerebrospinal fluid hypocretin [9]. However, the presence of abnormalities of the hypocretin gene in narcolepsy has not yet been investigated in PWS. Furthermore, the influence hypocretin on EDS symptoms in patients with PWS has not been clarified. Hence, further studies regarding the various factors causing EDS in young patients with PWS are necessary. In this case, we observed weight gain and EDS concurrently. Our results suggested that weight gain and EDS in the patient with PWS could occur from a shared etiology. In a previous study, authors hypothesized that the obesity and EDS in PWS are linked by a primary central hypothalamic dysfunction that might be responsible for the respiratory disturbances and abnormal cardiorespiratory responses to hypoxia and hypercapnia [10].
There were several limitations in this study. First, we had to consider the uncertainty of the results of MSLT because the patient was pre-pubertal. MSLTs are known to produce uncertain results, especially in children with narcolepsy type 2 (Na-2). The use of adult normative sleep latency values for diagnosis of Na-2 in pre-pubertal children remains controversial. For some children with narcolepsy, repeated MSLTs are necessary to confirm its diagnosis [11]. Second, even though the patient had several findings that were suggestive of OSA, such as the narrow oropharynx, neck hyperextension, and high AHI values assessed by a portable screening device, our diagnostic evaluation showed an no signs of sleep apnea. There is a possibility that the presence of sleep-disordered breathing was captured at home, but not in the lab or as an upper airway resistance syndrome (UARS) when the patient developed the symptoms at that time. In our case, UARS could not be strictly excluded, because end-tidal carbon dioxide (ETCO2) monitoring and esophageal pressure monitoring was not performed at his PSG testing. Therefore, the patient has been advised to undergo a second PSG with ETCO2 monitoring and MSLT in his adolescence.
Conclusion
We presented a case of EDS and excessive weight gain in an 11-year-old boy with PWS. Although OSA was suspected, PSG showed excellent sleep efficiency and no OSA. As he maintained proper sleep hygiene with daily sleep duration of approximately 9 hours, we suspected hypersomnia, but MSLT showed no findings of narcolepsy. Therefore, we concluded that his EDS was a direct symptom of PWS. This case reinforces the importance of sleep studies in young patients with PWS to identify the risks of EDS. Physicians trained in sleep medicine should be aware of the various factors causing EDS in young patients with PWS.
Declaration
All authors have no financial conflicts of interest to declare.
Acknowledgements
The authors would like to thank Ms. Ai Hanyu, Ms. Mitsuki Kato, and Mr. Seiichi Tsuchida for their assistance in PSG and MSLT. The authors also thank Kuniaki Hirai, MD, PhD and Shintaro Suzuki, MD, PhD for their assistance in the critical review of this manuscript.
References
- Butler MG, Manzardo AM, Forster JL (2016) Prader-Willi Syndrome: Clinical Genetics and Diagnostic Aspects with Treatment Approaches. Curr Pediatr Rev 12: 136-166.
- Angulo MA, Butler MG, Cataletto ME (2015) Prader‑Willi syndrome: a review of clinical, genetic, and endocrine findings. J Endocrinol Invest 38: 1249-1263.
- Zaffanello M, Antoniazzi F, Tenero L, Nosetti L, Piazza M, et al. (2018) Sleep-disordered breathing in paediatric setting: existing and upcoming of the genetic disorders. Ann Transl Med 6: 343.
- Nixon GM, Brouillette RT (2002) Sleep and Breathing in Prader-Willi Syndrome. Pediatr Pulmonol 34: 209-217.
- Aldrich MS, Chervin RD, Malow BA (1997) Value of the multiple sleep latency test (MSLT) for the diagnosis of narcolepsy. Sleep 20: 620-629.
- Maas AP, Sinnema M, Didden R, Maaskant MA, Smits MG, et al. (2010) Sleep disturbances and behavioural problems in adults with Prader-Willi syndrome. J Intellect Disabil Res 54: 906-917.
- Kayadjanian N, Schwartz L, Farrar E, Comtois KA, Strong TV (2018) High levels of caregiver burden in Prader-Willi syndrome. PLoS One 13: e0194655.
- Sedky K, Bennett DS, Pumariega A (2014) Prader Willi syndrome and obstructive sleep apnea: co-occurrence in the pediatric population. J Clin Sleep Med 10: 403-409.
- Nevsimalova S, Vankova J, Stepanova I, Seemanova E, Mignot E, et al. (2005) Hypocretin deficiency in Prader-Willi syndrome. Eur J Neurol 12: 70-72.
- Bruni O, Verrillo E, Novelli L, Ferri R (2010) Prader-Willi syndrome: sorting out the relationships between obesity, hypersomnia, and sleep apnea. Curr Opin Pulm Med 16: 568-573.
- Littner MR, Kushida C, Wise M, Davila DG, Morgenthaler T, et al. (2005) Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep 28: 113-121.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi