Research Article, J Forensic Toxicol Pharmacol Vol: 7 Issue: 2
Identification of Synthetic Cannabinoid 5-fluoro-ADB in Human Performance and Postmortem Samples: A Case Series
Reidy L1*, Seither J1 and Boland D2
1Department of Pathology and Laboratory 6 Medicine, Toxicology Laboratory, Miller School of Medicine, University of Miami, Miami, Florida, USA
2Miami Dade Medical Examiners, Number one Bob Hope Road, Miami, Florida, USA
*Corresponding Author : Lisa Reidy
Department of Pathology and Laboratory 6 Medicine, Toxicology Laboratory, Miller School of Medicine, University of Miami, Miami, Florida, USA
Tel: +3052431337
Fax: 3052431339
E-mail: lreidy@med.miami.edu
Received: July 18, 2018 Accepted: July 27, 2018 Published: August 03, 2018
Citation: Reidy L, Seither J, Boland D (2018) Identification of Synthetic Cannabinoid 5-fluoro-ADB in Human Performance and Postmortem Samples: A Case Series. J Forensic Toxicol Pharmacol 7:2. doi: 10.4172/2325-9841.1000162
Abstract
In recent years, various types of synthetic cannabinoids have become widely distributed and are causing social and health problems throughout many parts of the world. These drugs are considered novel psychoactive substances (NPS) and were created to imitate the cannabimimetic effects similar to Δ9-tetrahydrocannabinol (THC) through interaction with the CB1 and CB2 receptors of the human endocannabinoid system. This paper reports the detection of the recently identified synthetic cannabinoids and metabolites in both blood and urine matrices and presents both postmortem and human performance cases In Miami, Florida. The paper also correlates the identification of these drugs with observed behavior in human performance cases and post-mortem cases. There is limited information available regarding the confirmation of 5-fluoro-ADB involving fatal poisonings.
Results highlight the need for expanded toxicology testing for synthetic cannabinoids in clinical and forensic specimens especially when characteristic symptoms such as memory impairment in a traffic accident and unresponsive cardio-pulmonary arrest are observed.
Keywords: 5-Fluoro-ADB; Postmortem; Human performance; LC-MS/MS
Introduction
In recent years, various types of synthetic cannabinoids have become widely distributed and are causing social and health problems throughout many parts of the world. Synthetic cannabinoids were first developed in a research setting as a potential therapeutic agent and also to assist in research [1]. They are considered novel psychoactive substances (NPS) and were created to imitate the cannabimimetic effects similar to Δ9-tetrahydrocannabinol (THC) through interaction with the CB1 and CB2 receptors of the human endocannabinoid system. CB1 receptors are primarily found in the central and peripheral nervous systems, bone, heart and reproductive system [2]. CB2 receptors are found predominantly in the immune system and are thought to mediate immunosuppression through control of apoptosis, cell proliferation and cytokine modulation [3]. CB2 receptors are located in the central nervous system (CNS) but at a significantly lower frequency when compared to the CB1 receptors [4]. Some synthetic cannabinoid users reported effects similar to those produced by cannabis which includes feelings of relaxation, elevated mood, and altered perception. Although synthetic cannabinoids can produce effects similar to cannabis, there have been numerous reports of individuals who have used these drugs and experienced more severe side effects; including nausea and vomiting, agitation, hallucinations, paranoia, violent behavior, acute psychosis, seizures, cardiac complications, brain damage, and death [5]. The main pharmacologically active compounds in these herbal mixtures are frequently evolving. The components can be structurally diverse and have varying affinities for the CB1 and CB2 receptors when compared to THC. Limited human pharmacological data make their effects dangerously unpredictable as demonstrated by the adverse health effects associated with some of these compounds [6,7].
Synthetic cannabinoids are synthesized in clandestine laboratories and typically marketed as “herbal incense.” Dried plant material is sprayed with a solution containing one or more synthetic cannabinoids and this material is sold in colorful foil packaging with labels typically stating “not for human consumption.” Users typically smoke the dried plant material by itself or in combination with marijuana. Similarly to traditional cannabis, vaping synthetic cannabinoids material is also becoming increasingly popular with the use of electronic cigarette devices. The desirability of these drugs may be attributed to the ease of availability of the substances in drug paraphernalia shops (headshops), gas stations, and the Internet alongside their perceived lack of detectability in standard workplace drug testing and evading drug legislation. Clandestinely manufactured drugs have limited, or no quality control procedures, resulting in a heterogeneous material that contains chemical compounds that are not intended to be present in the material such as precursors or synthesis by-products.
In the late 2000s, synthetic cannabinoids were identified by laboratories in “Spice” or “K2” and related herbal incense products. The compounds found in the “first generation” of synthetic cannabinoid products were primarily the C8 homologs of the nonclassical cannabinoid CP-47,497 and the aminoalkylindole, JWH-018, both of which are agonists of the CB1 and CB2receptors. The binding affinity of the synthetic cannabinoids to the CB receptors is dependent on the compound. For example, JWH-018 has a binding affinity for the CB1 receptor that is four times greater than that of THC and approximately ten times higher than THC for the CB2 receptor [8,9].
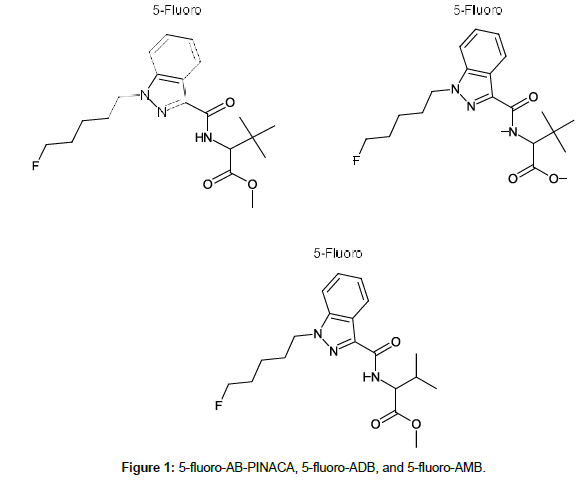
Subsequent synthetic cannabinoid generations containing slight chemical modifications resulted in varying affinities for the CB1 and CB2 receptors. In the last several years, a series of novel and extremely potent synthetic cannabinoids of the indazole-3-carbinoxamide family became available; they include 5-fluoro-AB-PINACA, 5-fluoro-ADB, and 5-fluoro-AMB (Figure 1). Of particular concern is 5-fluoro- ADB (5F-MDMB-PINACA or methyl (R)-2-[1-(5-fluoropentyl)- 1H-indazole-3-carboxamido]-3, 3-dimethylbutanoate), a full agonist of the CB1 receptor that is 300 times more potent than THC [8,9]. Although publications regarding 5-fluoro-ADB are limited, there are reports on the toxic effects of this drug, predominantly referencing emergency department admissions and human performance effects, specifically in drivers [5,6,10-12].
Barcelo et al. reported the acute intoxication of five patients following the smoking of herbal mixtures containing 5-fluoro-ADB and MMB-2201 [6]. Toxic effects included psychomotor agitation, confusion, anxiety, psychosis, and tachycardia. Significantly, these substances were identified in the herbal blends smoked by the patients; however only their phase I metabolites (5-fluoro-ADB 5-OH-pentyl metabolite, 5-fluoro-ADB ester hydrolysis product, and MMB-2201 amide/ester hydrolysis product) were identified in the urine; no blood was drawn from the patients for testing in these cases.
Kaneko described a series of motor vehicle collisions in which 5-fluoro-ADB was identified in twenty-four impaired drivers [11]. All motor vehicle collisions involving 5-fluoro-ADB were minor offenses which the authors attribute to the driver’s inability to drive very far before losing consciousness. Approximately 50% of the drivers under the influence of 5-fluoro-ADB reported vomiting, and the most common finding amongst drivers is that they were not likely to remember what they were doing before or after the collision, suggesting that the use of the synthetic cannabinoids caused retrograde amnesia and disabled learning. A limitation of this study is that 5-fluoro-ADB was only identified in the urine of two of the drivers and was not identified in any of the blood specimens.
Publications regarding the occurrence of 5-Fluoro-ADB in postmortem samples are limited to one report from Japan which describes ten fatal cases involving its use. Hasegawa et al. published data regarding the tissue distribution of 5-fluoro-ADB in blood, urine, stomach contents, and nine solid organs including brain, liver, lung, heart and adipose tissue [7]. 5-fluoro ADB concentrations were highest in the adipose tissue with none detected in the blood or urine at a limit of detection of 0.1 ng/mL.
The detection of the synthetic cannabinoids, however, is based on the laboratory’s ability to adapt and validate methods that can identify new drugs in an ever-changing environment. The laboratory should have the ability to identify the parent drug and any possible metabolites. Testing for these drugs, as they are continually evolving, can be challenging for laboratories and quickly drains resources from both forensic and public health laboratories. Screening techniques such as immunoassays, including enzyme-linked immunosorbent assay (ELISA), are becoming a less valuable tool due to the everchanging chemical structure of these compounds and adapting antibodies in a timely fashion. Laboratories are having more success identifying these compounds when employing a methodology that couples gas or liquid chromatography to a mass spectrometer. This methodology provides the necessary selectivity and sensitivity needed to elucidate these compounds in toxicological investigations. This paper reports the detection of the recently identified synthetic cannabinoids and metabolites in both blood and urine matrices and presents both postmortem and human performance cases. The paper also correlates the identification of these drugs with observed behavior in human performance cases and post-mortem cases. There is limited information available regarding the confirmation of 5-fluoro-ADB involving fatal poisonings.
Case Histories
Table 1 summarizes the observational data for the eleven cases where synthetic cannabinoids, were implicated in the case history. The two human performance cases involve motor vehicle collisions (Cases 1 and 2) with drivers (20-40-year-old) in possession of drug paraphernalia. In both cases the accidents were severe; one resulting in serious bodily injuries and the other in a fatality. The driver in Case 1 had no memory of the incident at the time and when interviewed by police several days later. The driver appeared dazed, confused, and disorientated immediately after the incident. The driver in Case 2 was observed to be impaired and had drug paraphernalia in his mouth at the time of the collision. The nine postmortem cases (Cases 3-11) involved male decedents (5 black, 4 white) with an average age of 47 years (range 29-62 years, median age 46 years). Only one decedent, Case 3, was observed before death and was pacing on a roadway before collapsing. All other postmortem cases involved terminal events that were not witnessed, and the decedents were found unresponsive. The majority of the postmortem cases has histories of alcohol abuse, illicit substance abuse, and was known to smoke synthetic cannabinoids. In five of the cases, drug paraphernalia were submitted to the laboratory for testing.
| Case ID | Age | Gender | Race | Case history | Symptoms | Paraphernalia found |
|---|---|---|---|---|---|---|
| Case 1 | 20-40 | Male | White | The driver was observed driving at high speed in the wrong lane of travel and had a head-on collision with another vehicle resulting in serious bodily injuries to the passenger in the vehicle. The suspect was observed discarding two baggies of what appeared to be synthetic cannabinoids labeled “Death Grip.” The subject was sent to the hospital with critical injuries and survived the crash. The subject’s family had reported the person as missing for the past 48 h. | The driver had no memory of the crash at the time or several days later. He was dazed, confused and disorientated. | A black plastic bag labeled “Death Grip” incense |
| Case 2 | 20-40 | Male | White | The driver caused an accident whereby he pinned another Individual against his vehicle which caused a fatality. The driver was found and observed to have material in his mouth at the time of the collision. Blood samples were taken approx. 5 h. post-collision. In addition the individual had previously, on that day, admitted themselves into hospital and additional blood was taken at that time. | The driver had been in the ER that day complaining of psychological disturbance and had discharged himself from the hospital. Sometime later he was involved in the crash |
“Lotus” Half smoked “Blunt” |
| Case 3 | 51 | Male | Black | The decedent was found unconscious along the side of a road. Witnesses advised that the decedent had consumed several beers and smoked “tobacco that was synthetic marijuana”. The decedent was known to heavily consume alcoholic beverages and smoke tobacco. | The descendant was observed pacing the roadway, and collapsed. | A black, plastic bag labeled “Death Grip” Incense |
| Case 4 | 46 | Male | White | The decedent was found unresponsive in an alley behind a business. There were no witnesses to the terminal event. Family members indicated that the decedent was homeless and known to abuse alcohol and other illicit substances. | Unresponsive | Bag of green, leafy material |
| Case 5 | 59 | Male | Black | The decedent was found unresponsive in a dormitory. According to family members, he was not known to smoke cigarettes, drink alcohol, or use illicit drugs. | Unresponsive | None |
| Case 6 | 44 | Male | Black | The decedent was found unresponsive on the floor. According to the investigation, the deceased had previously overdosed on “K2”. His roommate, when questioned, appeared to be under the influence of drugs. | Unresponsive | Hand-rolled cigarette |
| Case 7 | 29 | Male | White | The decedent was found slumped over in bed by his roommates. According to the family, the decedent was known to smoke cigarettes, drink alcohol, and had a history of illicit substance abuse. A bag of “White Tiger” herbal incense was found in close proximity to the decedent. | Unresponsive | “White Tiger” herbal incense |
| Case 8 | 39 | Male | Black | The decedent was found unresponsive in a park, slumped over a picnic table bench. A witness on the scene indicated that the decedent was observed smoking “some type of synthetic marijuana”, as well as “popping pills”. Family members indicated that the decedent also had a history of cocaine use. | Unresponsive | None |
| Case 9 | 46 | Male | White | The decedent was found lying on the ground unresponsive and not breathing in an alleyway behind an auto-repair shop. The decedent was disabled and known to smoke cigarettes, drink alcohol, and smoke synthetic marijuana. | Unresponsive | None |
| Case 10 | 62 | Male | Black | The decedent was observed by witnesses to become unresponsive after apparently smoking “K2”. Family was not aware of any drug or alcohol abuse by the decedent. | Unresponsive | None |
| Case 11 | 50 | Male | White | The decedent was found on the floor unresponsive by his son. Decedent had a history of cocaine and marijuana use. The son reported that he and his father had been habitual users of synthetic marijuana and were using it prior to his death. | Unresponsive | Small packet containing plant residue |
Table 1: Demographics and case history information.
Routine toxicological analysis
The human performance cases were analyzed by the University of Miami Toxicology Laboratory (UMTL). Routine toxicological analysis performed at the UMTL included a volatile screen using headspace-gas chromatography/flame ionization detector ( H S - GC/FID), ELISA screen (amphetamines, methamphetamine, cocaine, cannabis, benzodiazepines, opiates, fentanyl and buprenorphine) and a basic drug screen utilizing gas chromatography-mass spectrometry coupled with a nitrogen-phosphorus detector (GC-NPD/MS). Specimens are analyzed further using targeted confirmation methods based on the screening results and the case history.
The postmortem cases were analyzed by Miami-Dade Medical Examiner Department (MDME) Toxicology Laboratory. Routine toxicology testing at the MDME Toxicology Laboratory included a volatile analysis (ethanol, acetone, isopropanol, and methanol) using HS-GC/FID, as well as an enzyme multiplied immunoassay technique (EMIT) for amphetamines, benzodiazepines, benzoylecgonine, cannabinoids, opiates, and oxycodone. If urine is not available for testing, blood is submitted to ELISA testing for benzodiazepines, benzoylecgonine, cannabinoids, opiates, and oxycodone. A comprehensive drug screen using solid-phase extraction followed by GC- NPD/MS analysis was also performed for each case. Further testing was assigned based on the results of the screen and based on case history and investigation.
All of the human performance and postmortem cases were analyzed in duplicate using the validated liquid chromatographytandem mass spectrometry (LC-MS/MS) methods that targeted synthetic cannabinoids.
Materials and Methods
Confirmatory and quantitative analysis for synthetic cannabinoids
Drug standards for the synthetic cannabinoid panel were purchased from Cayman Chemical Co. (Ann Arbor, MI, USA) and included the following targets: 5-fluoro AB-PINACA, ADBICA N-(4- hydroxypentyl) metabolite, 5-fluoro-ADBICA, ADBICAN- pentanoic acid metabolite, 5-fluoro-ADB, ADB-PINACA N-(5-hydroxypentyl) metabolite , 5-Fluoro-ADB metabolite #7, ADB-PINACA pentanoic acid metabolite, 5-fluoro-AMB, AKB48 N-(5-hydroxypentyl) metabolite, 5-fluoro SDB-006, AM-2201, 5-fluoro AB-PINACA N-(4-hydroxypentyl) metabolite, AM-2201 N-(4 Hydroxy pentyl) metabolite, 5-fluoro NNEI, FUB-PB-22, 5-fluoro- PB-22 3-carboxyindole metabolite, MAB-CHMINACA, 5-fluoro- AKB48 N-(4-hydroxypentyl) metabolite, MAB-CHMINACA metabolite M1, AB-CHMINACA, MAB-CHMINACA metabolite M2, AB-CHMINACA metabolite M2, MAM-2201, AB-CHMINACA metabolite M1A, MAM-2201 N-(4-hydroxypentyl) metabolite, ABCHMINACA metabolite M6, MAM2201 N-pentanoic acid metabolite, AB-FUBINACA, MMB-2201, AB- FUBINACA metabolite 2, MMB-FUBINACA, AB-PINACA N-(4-hydroxypentyl) metabolite, UR-144 N-pentanoic acid metabolite, AB-PINACA pentanoic acid metabolite XLR-11 N-(4-hydroxypentyl) metabolite. Four internal standards were utilized, AB-CHMINACA metabolite M4-D4, ABFUBINACA- D4, and UR-144 N-pentanoic acid-D5 were purchased from Cayman Chemical Company (Ann Arbor, MI, USA); JWH-018 4-hydroxypentyl metabolite-D5 from Cerilliant Corp (Round Rock, TX, USA). LC- MS grade water, acetonitrile, dichloromethane, nheptane, methanol, ammonium acetate, ammonium formate, ammonium hydroxide, and formic acid were purchased from Fisher Scientific (Pittsburgh, PA). All solvents and reagents were of analytical grades or higher. The enzyme, β-glucuronidase BG100, from the Red abalone, was purchased from Kura Biotec (Inglewood, CA, USA)
Sample preparation and solid phase extraction (SPE)
Bloods: The target synthetic cannabinoids were extracted from blood using Bond Elute Plexa PCX solid phase-extraction (SPE) cartridges (Agilent Technologies, Santa Clara, CA, USA). Samples were first prepared by fortifying 1mL of blood with the working internal standard solution (25 ng/mL). Protein precipitation was performed using 1mL of cold acetonitrile while vortexing. Specimens were then centrifuged at 3500 rpm for 10 min at -10°C, and the supernatant was transferred to a clean silanized tube. Ammonium acetate buffer (0.1 M, pH 4.8, 2 mL) was added and vortexed.
Before introducing samples to the SPE cartridges, the columns were preconditioned with methanol (1 mL) and ammonium acetate buffer (1 mL). Samples were added to the cartridge and subsequently washed with the ammonium acetate buffer (3 mL), deionized water/ methanol (50:50v/v, 3 mL). Columns were dried for 1 min at maximum pressure (40 psi) and eluted with acetonitrile/ammonium hydroxide (98:2v/v, (1 mL). The eluent was then evaporated at 40°C with N2, and the residue reconstituted with 100 μL of the initial mobile phase composition.
Urines: An enzymatic hydrolysis step was performed by the addition of 100 units/mL of beta-glucuronidase BG100, with an incubation at 68°C for 10 min to cleave any potential conjugated metabolites. After cooling to room temperature, the target synthetic cannabinoids were extracted from urine by adding 9 mL of a dichloromethane/nheptane (7:3v/v) solution and then rotating the samples for 15 min. Specimens were then centrifuged at 3500 rpm for 10 min at -10°C; the aqueous layer was discarded and the organic layer was transferred to a clean silanized tube. The organic layer was then evaporated at 35°C with N2 gas at 8 psi, and the residue was reconstituted with 100 μL of the initial mobile phase composition.
Paraphernalia: For analysis of the paraphernalia found at the scene, 1 mg of each of the items were dissolved in methanol and sonicated for 10 mins. The top layer was then removed and decanted to a clean test tube. The samples were then diluted at various concentrations with the initial mobile phase and analyzed using the same methodology as the biological matrices. Four blank injections followed every case sample to ensure that contamination across the injections did not occur.
Instrumental analysis: The analysis was conducted using a 1290 high-performance liquid chromatograph (HPLC) coupled to a 6460 triple quadrupole mass spectrometer (Agilent Technologies). Chromatographic separation was achieved using a Poroshell 120 PFP column (3.0 × 50 mm i.d particle size 4 μm) analytical column with an aqueous mobile phase of 5 mM ammonium formate with 0.1% formic acid in LC-MS grade water (A), and an organic mobile phase of acetonitrile with 0.1% formic acid (B). Isocratic run at 25% B held until 15 min, then 100% B for 1 min with a flow rate of 0.8 mL/min to wash. Injection volume was 5 μL; column temperature was held at 40°C.
The mass spectrometer was operated in positive mode with an electrospray ionization source (ESI) with jet stream technology. Ion source parameters included gas temperature of 320°C and a flow of 7 L/min. The nebulizer was set at 45 psi, and the capillary voltage was set to 3500 V. The mass spectrometer was operated in dynamic multiple reaction monitoring (dMRM) mode with all compounds having two transitions at each optimal fragment or voltage and collision energy for each target synthetic cannabinoid (Table 2).
| Compound | Precursor ion (m/z) | Product ion (m/z) | Fragment or voltage (V) | Collision energy (V) | LOD (ng/mL) blood | LOD (ng/mL) urine |
|---|---|---|---|---|---|---|
| 5-fluoro AB-PINACA | 349.2 | 233.1 | 80 | 22 | 0.1 | 0.05 |
| 349.2 | 304.2 | 80 | 10 | |||
| 5-fluoro-ADBICA | 362.2 | 345.2 | 80 | 4 | 0.5 | 0.1 |
| 362.2 | 232.1 | 80 | 18 | |||
| 5-fluoro-AB-PINACA N-(4- hydroxypentyl) metabolite | 365.2 | 348.2 | 80 | 4 | N/A | 0.5 |
| 365.2 | 249.1 | 80 | 22 | |||
| 5-fluoro AKB48 N-(4-hydroxypentyl) metabolite | 400.2 | 135.1 | 80 | 18 | 0.5 | |
| 400.2 | 107.1 | 80 | 54 | |||
| 5-fluoro-AMB | 364.2 | 304.2 | 104 | 10 | 0.1 | 0.5 |
| 364.2 | 233.1 | 104 | 18 | |||
| 5-fluoro-ADB | 378.2 | 318.1 | 128 | 10 | 5 | 0.5 |
| 378.2 | 233 | 128 | 22 | |||
| 5-fluoro-ADB metabolite # 7 | 364.2 | 318.2 | 104 | 10 | N/A | 0.5 |
| 364.2 | 233.1 | 104 | 18 | |||
| 5-Fluoro-SDB-006 | 339.2 | 232.1 | 176 | 18 | 1 | 0.5 |
| 339.2 | 206.1 | 176 | 18 | |||
| 5-fluoro-NNEI | 375.2 | 232.1 | 152 | 18 | 0.5 | 0.5 |
| 375.2 | 206.1 | 152 | 14 | |||
| 5-fluoro PB-22 3-carboxyindole metabolite | 250.1 | 206.2 | 104 | 10 | N/A | 0.5 |
| 250.1 | 118 | 118 | 22 | |||
| AB-CHMINACA | 357.2 | 340.3 | 80 | 6 | 0.1 | 0.1 |
| 357.2 | 241.1 | 80 | 26 | |||
| AB-CHMINACA metabolite M2 | 358.2 | 241.1 | 80 | 18 | N/A | 0.1 |
| 358.2 | 145 | 80 | 38 | |||
| AB-CHMINACA metabolite M1A | 373.2 | 356.2 | 80 | 4 | 0.5 | |
| 373.2 | 257.1 | 80 | 26 | |||
| AB-CHMINACA metabolite M6 | 358.2 | 241.1 | 80 | 18 | N/A | 5 |
| 358.2 | 145 | 80 | 38 | |||
| AB-FUBINACA | 369.2 | 324.2 | 80 | 10 | 0.1 | 0.1 |
| 369.2 | 109 | 80 | 46 | |||
| AB-FUBINACA metabolite 2 | 399.2 | 382.1 | 104 | 6 | N/A | 1 |
| 399.2 | 253.1 | 104 | 22 | |||
| AB-PINACA | 331.2 | 286.2 | 80 | 10 | 0.5 | 0.1 |
| 331.2 | 215.1 | 80 | 22 | |||
| AB-PINACA N-(4-hydroxypentyl) metabolite | 347.2 | 302.2 | 80 | 10 | N/A | 0.5 |
| 347.2 | 213.1 | 80 | 26 | |||
| AB-PINACA pentanoic acid metabolite | 361.2 | 227.1 | 80 | 30 | 0.1 | |
| 361.2 | 217.1 | 80 | 30 | |||
| ADB-CHMICA | 370.2 | 353.3 | 104 | 6 | 0.5 | 0.5 |
| 370.2 | 240.2 | 104 | 18 | |||
| ADBICA | 344.2 | 327.2 | 80 | 4 | 0.5 | N/A |
| 344.2 | 214.1 | 80 | 18 | |||
| ADBICA N-(4-hydroxypentyl) metabolite | 360.2 | 343.2 | 104 | 6 | N/A | 0.5 |
| 360.2 | 230.1 | 104 | 18 | |||
| ADBICA N-pentanoic acid metabolite | 374.2 | 357.2 | 80 | 4 | 5 | |
| 374.2 | 244.1 | 80 | 18 | |||
| ADB-PINACA | 345.2 | 328.2 | 80 | 6 | 1 | N/A |
| 345.2 | 215.1 | 80 | 26 | |||
| ADB-PINACA N-(5-hydroxypentyl) metabolite | 361.2 | 344.2 | 80 | 4 | N/A | 5 |
| 361.2 | 213.1 | 80 | 30 | |||
| ADB-PINACA pentanoic acid metabolite | 375.2 | 358.2 | 80 | 4 | N/A | 0.5 |
| 375.2 | 330.2 | 80 | 14 | |||
| AKB48 N-(5-hydroxypentyl) metabolite | 382.2 | 246.2 | 128 | 10 | N/A | 5 |
| 382.2 | 213.1 | 128 | 30 | |||
| 360.2 | 232.1 | 152 | 22 | 0.1 | 5 | |
| AM-2201 | 360.2 | 155.1 | 152 | 26 | ||
| AM-2201 N-(4-hydroxypentyl) metabolite | 376.2 | 248.1 | 176 | 22 | N/A | 0.1 |
| 376.2 | 155 | 176 | 26 | |||
| FUB-PB-22 | 397.1 | 252.1 | 80 | 10 | 1 | 0.5 |
| 397.1 | 224.1 | 80 | 26 | |||
| MAB-CHMINACA | 371.2 | 326.2 | 80 | 14 | 0.5 | 0.1 |
| 371.2 | 241.1 | 80 | 26 | |||
| MAB-CHMINACA M1 | 387.2 | 342.3 | 80 | 14 | N/A | 0.1 |
| 387.2 | 257.2 | 80 | 26 | |||
| MAB-CHMINACA M2 | 372.2 | 326.3 | 80 | 10 | 0.5 | |
| 372.2 | 241.2 | 80 | 22 | |||
| MAM-2201 | 374.2 | 232.1 | 152 | 22 | 0.5 | 0.1 |
| 374.2 | 169.1 | 152 | 26 | |||
| MAM-2201 N-(4-hydroxypentyl) metabolite | 390.2 | 248.1 | 152 | 22 | N/A | 0.1 |
| 390.2 | 169 | 152 | 26 | |||
| MAM-2201 N-pentanoic acid metabolite | 386.2 | 244.1 | 152 | 22 | 0.1 | |
| 386.2 | 169.1 | 152 | 22 | |||
| 262.1 | 144 | 80 | 22 | |||
| MMB-FUBINACA | 384.2 | 253 | 80 | 10 | 0.5 | 0.1 |
| 384.2 | 324 | 80 | 18 | |||
| PX-1 | 396.2 | 379.2 | 80 | 6 | 0.5 | N/A |
| 396.2 | 232.1 | 80 | 18 | |||
| 397.2 | 233.1 | 80 | 22 | |||
| RCS-4 | 322.2 | 214.1 | 152 | 22 | 5 | N/A |
| 322.2 | 158.1 | 152 | 30 | |||
| 312.2 | 214.1 | 152 | 22 | |||
| UR-144 N-pentanoic acid metabolite | 342.2 | 244.1 | 152 | 22 | N/A | N/A |
| 342.2 | 125.1 | 152 | 22 | |||
| UR-144 N-pentanoic acid met-D5 | 347.2 | 125.1 | 152 | 22 | N/A | |
| 347.2 | 97.1 | 152 | 30 | |||
| 330.2 | 232.1 | 152 | 22 | |||
| XLR-11 N-(4-hydroxypentyl) metabolite | 346.2 | 248.1 | 128 | 22 | N/A | 0.5 |
| 346.2 | 144.1 | 128 | 38 |
Table 2: Transition and source parameters for the target synthetic cannabinoid analysis by LC-MS/MS together with the limits of detection in blood and urine.
Results
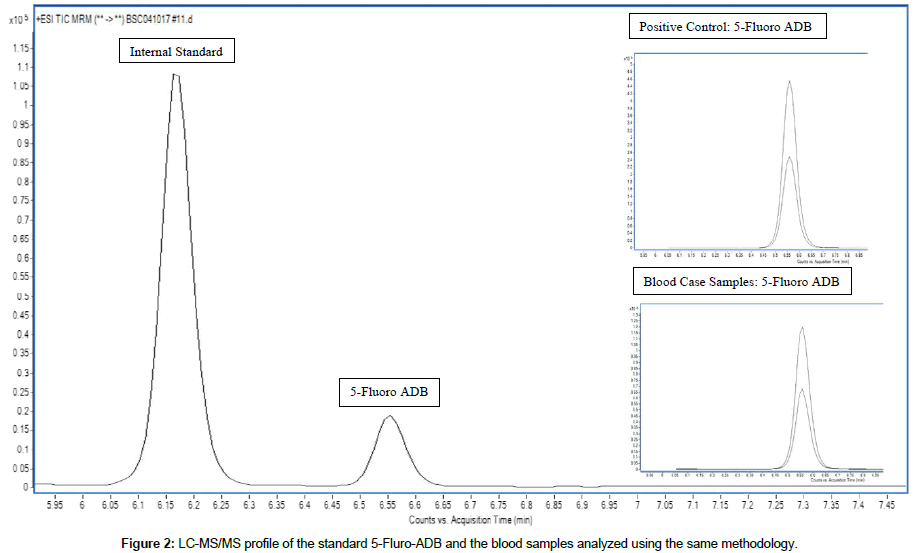
The method described utilized a LC-MS-MS targeted screen for synthetic cannabinoids that have been reported in the literature and local drug seizure reports (Figure 2). The method validation, including LOD studies (Table 2), carryover, and interference and ionization suppression/enhancement was carried out in accordance with the scientific Working Group for Forensic Toxicology (SWGTOX) guidelines. The target analytes and their respective LOD’s are listed in Table 1. Ionization enchantment and suppression observed were within acceptable ranges. No appreciable interference was observed in endogenous and exogenous interference studies.
Case results
All eleven of the human performance and postmortem cases, where synthetic cannabinoid use was suspected, confirmed positive for 5-fluoro-ADB in at least one matrix. In eight of the nine postmortem cases where a urine sample was collected, the 5-fluoro- ADB ester hydrolysis metabolite was confirmed in the urine matrix. The full toxicological results for each case are presented in Table 3. This table also includes details of the samples collected and other toxicological findings for each case.
| Case # | Initial toxicology results | Confirmatory synthetic cannabinoid results | ||
|---|---|---|---|---|
| Source | Results | Source | Results | |
| 1 | Blood | No drugs found | Blood Paraphernalia ‘Death Grip’ |
5-fluoro-ADB 5-fluoro-ADB MMB-FUBINACA |
| 2 | Blood | No drugs found | Blood Paraphernalia- ‘Lotus’ |
5-fluoro-ADB 5-fluoro-ADB MMB-FUBINACA |
| 3 | Blood Urine Paraphernalia –‘Death Grip’ | Ethanol: 0.30 g/100mL 11-nor-9-carboxy-Δ9-THC 5-fluoro-ADB | Blood Urine Paraphernalia–‘Death Grip’ | 5-fluoro-ADB AM-2201 AM-2201 metabolite (N-Hydroxy pentyl) JWH-018 metabolite (N-pentanoic acid) 5-fluoro-ADB 5-fluoro-ADB metabolite #7 5-fluoro-ADB |
| 4 | Blood Urine Green leafy material | Cocaine metabolites Quinine Cocaine and metabolites 11-nor-9-carboxy-Δ9-THC 9-hydroxyrisperidone 5-fluoro-ADB |
Blood Urine Green leafy material | 5-fluoro-ADB 5-fluoro-ADB metabolite #7 5-fluoro-ADB AB -CHMINACA MAB-CHMINACA MMB-FUBINACA |
| 5 | Blood | 5-fluoro-ADB N-ethylpentylone Diphenhydramine Meperidine |
Blood Urine | 5-fluoro-ADB 5-fluoro-ADB metabolite #7 |
| 6 | Blood Hand-rolled cigarette | N-ethylpentylone N-Ethylpentylone N,N-dimethylpentylone AB-FUBINACA 5-fluoro-ADB |
Blood Urine Hand-rolled cigarette |
5-fluoro-ADB 5-fluoro-ADB 5-fluoro-ADB metabolite #7 5-fluoro-ADB AB-FUBINACA, MAB-CHMINACA, MMB-FUBINACA |
| 7 | Blood Urine Paraphernalia – ‘White Tiger’ | Nortriptyline Olanzapine + metabolite Ibuprofen 5-Fluoro-ADB |
Blood Urine Paraphernalia – ‘White Tiger’ | None Identified 5-fluoro-ADB metabolite #7 5-fluoro-ADB 5-fluoro-ADB MMB- FUBINACA AB-FUBINACA AB-CHMINACA 5-fluoro-AMB |
| 8 | Blood Urine | Ethanol: 0.23 g/100mL Cocaine: < 0.01 mg/L Benzoylecgonine: 0.09 mg/L Cocaethylene: < 0.01 mg/L Levamisole Cocaine + metabolites |
Blood Urine | 5-fluoro-ADB 5-fluoro-ADB 5-fluoro-ADB metabolite #7 |
| 9 | Blood Urine | Paroxetine Trazodone + metabolite Atorvastatin Levetiracetam Aripiprazole Paroxetine Trazodone + metabolite Atorvastatin Aripiprazole |
Blood Urine | 5-fluoro-ADB 5-fluoro-ADB metabolite #7 |
| 10 | Blood | Azithromycin Quetiapine |
Blood Urine | 5-fluoro-ADB None Identified |
| 11 | Blood Packet containing residue | Ethanol 0.047 g/100mL Gabapentin Ibuprofen Quetiapine + metabolite 5-fluoro-ADB |
Blood Urine Packet containing residue |
5-fluoro-ADB 5-fluoro-ADB metabolite #7 5-fluoro-ADB |
Table 3: Toxicology specimen’s results, cause and manner of death determination.
Case #1 and Case #2 are both human performance cases where driving under the influence investigations were performed. Case #1 involved a crash where the subject had no recollection of the events that took place either leading up to or post-crash. The driver was observed speeding in the opposite lane of travel before being involved in a head-on-collision with another vehicle. The subject was seen to discard bags that contained herbal material labeled “Death Grip.” Two blood samples and seized material were obtained and submitted to the laboratory for analysis. Alcohol and drugs were not detected in the blood samples after routine blood testing. The seized material was analyzed, and 5-fluoro-ADB and MMB-FUBINACA were identified in the “Death Grip” material. Following this discovery, a targeted blood synthetic cannabinoid LC MS/MS method was developed and validated to include these two compounds. Subsequent analysis of the two blood samples (blood sample containing ethylene-di-aminetetra- acetic-acid EDTA and a second containing sodium citrate as the anticoagulant) confirmed 5-fluoro-ADB as the only drug present in both collection tubes.
Cases #2 involved an individual colliding with a stationary vehicle which resulted in the death of a bystander. The subject was apprehended with a “joint” that contained plant material in his mouth. Also, a packet of “Lotus” potpourri was also recovered from the vehicle and submitted to the laboratory for analysis. Routine blood testing did not identify ethanol or drugs in the blood sample. Similar to Case #1, the seized material was analyzed and confirmed 5-fluoro- ADB and MMB-FUBINACA in the material. Due to this finding, the blood sample was then analyzed by the blood synthetic cannabinoid analysis. Analysis of the blood sample drawn from the subject using both an EDTA vacutainer and a sodium fluoride/potassium oxalate tube confirmed the identification of 5-fluoro-ADB in both collection tubes.
The nine other cases (Case #3-Case #11) are postmortem cases. They were submitted for synthetic cannabinoids analysis based on preliminary testing, case histories, or drug paraphernalia found or observed at the scene. Six of the postmortem cases analyzed had a documented history of synthetic cannabinoid abuse. All of the postmortem cases reported 5-fluoro-ADB and/or metabolite in the blood and/or urine specimens as well as one or more additional substances. One case (Case 3) reported another synthetic cannabinoid, AM-2201, in addition to 5-fluoro-ADB. Three cases (#3, #8, and #11) contained ethanol; two of which (#3 and #8) had concentrations well above the legal limit to drive a motor vehicle. Two cases (#4 and #8) revealed the presence of cocaine and metabolites, with one (Case 8) reporting a measurable concentration of benzoylecgonine with cocaine and cocaethylene concentrations less than the lower limit of quantitation for the analytical method. Two cases (#5 and #6) reported the presence of a synthetic cathinone, N-Ethylpentylone. The remaining additional drugs identified in the postmortem cases include THC its metabolites, antidepressants (trazodone and paroxetine), antipsychotics (olanzapine, quetiapine, 9-hydroxyrisperidone, and aripiprazole), cholesterol medication (atorvastatin), and pain medication (gabapentin and ibuprofen).
Discussion
To date this is the largest synthetic cannabinoids case series describing 5-fluoro-ADB. As recreational use of synthetic compounds increases, atypical pharmacological effects have been observed with synthetic cannabinoid use when compared to traditional cannabis use. It has been suggested that the CB1 and CB2 receptor affinities affect the potency of an individual synthetic cannabinoid compound. The exact mechanism of 5-Fluroro-ADB toxicity, if any, is unknown. The affinities of the SC’s are their metabolites are higher at the CB1 and CB2 receptors than that of THC and are hypothesized to results in the deaths and symptoms observed. The difference in receptor affinities could also produce synthetic cannabinoids interacting with only one of the CB receptors [8-10]. This is not observed with traditional cannabis where THC acts as a partial agonist of both the CB1 and CB2 receptors [1]. The atypical effects could attribute to increased potency, and the specific receptor binding of the synthetic cannabinoid interacts. Other hypothesized mechanism of action includes activation of non-cannabinoid receptors and drug-drug synergistic effects. Effects contributed to acute synthetic cannabinoid intoxication include hospitalization, severe injury and, as highlighted in this paper, death [7,11]. A review of the current literature [12] identified that while adverse side effects may require hospitalization, most recovered in >24 h. post-exposure. While with the older generation of synthetic cannabinoids the adverse effects were observed, such as psychosis and cardiotoxicity, there is limited data to correlate with the cause of death. It is hypothesized that, as the group of drugs evolves and increases in potency, more deaths are associated with the use of drugs in the synthetic cannabinoid family. Due to the pharmacological effects mentioned in previous work and documented in this paper, the identification of synthetic cannabinoids is of interest for forensic toxicological investigations as questions of impairment or cause of death typically arise. Atypical pharmacological effects were observed in both human performance and postmortem cases presented in this paper. In the human performance cases, the observations in the behavior of subjects analyzed for human performance when impaired by 5-fluoro-ADB are consistent with those previously reported in respects to memory loss and lack of knowledge of the event [11]. No other psychoactive active compounds were identified in the blood samples submitted to the laboratory, leading to the conclusion that the psychoactive agent attributed to the observed impairment was the synthetic cannabinoid, 5-Fluoro-ADB. The exact mechanism of 5-Fluoro-ADB toxicity is known.
This study illustrates the need for forensic and clinical laboratories to be able to identify synthetic cannabinoids in both human performance and postmortem toxicological investigations. The identification of 5-fluoro-ADB was significant for both human performance and post-mortem cases where no other psychoactive substance was identified. Investigators might have arrived at an alternative/incorrect conclusion if 5-fluoro-ADB was not reported in these case samples, resulting in both criminal and civil implications. For example, the decision to proceed with a driving-under-theinfluence criminal investigation might not have been carried out as no psychoactive substances were identified.
It is also worthwhile to note the importance of obtaining and analyzing the drug paraphernalia that was discovered at the accident/ scene of death. The identification of synthetic cannabinoids, 5-fluoro ADB and MMB-FUBINACA, in the material assisted the toxicological investigation. Once identified, these compounds were added to the targeted LC-MS/MS method which enabled for the laboratories to detect 5-Fluoro ADB and metabolites in the blood and urine samples submitted for analysis.
The MMB-FUBINACA compound was not identified in any of the blood or urine samples. It was hypothesized that MMBFUBINACA was a minor component of the two herbal materials that were submitted with the human performance cases as the peak area response of MMB-FUBINACA was over 100 times smaller when compared to the of the peak area of 5-fluoro-ADB. It is unknown whether this is an impurity in the production of 5-fluoro-ADB or if this compound was added intentionally to the material.
One limitation of this study is that concentration of 5-Fluoro- ADB in each matrix was not determined at the time of this manuscript due to the lack of availability of a certified reference standard which is needed for quantitative methodologies.
Conclusion
This paper highlights the need for expanded toxicology testing for synthetic cannabinoids in clinical and forensic specimens especially when characteristic symptoms such as memory impairment in a traffic accident and unresponsive cardio-pulmonary arrest are observed. This is emphasized in case results #4, 9 and 11 which list 5-fluoro-ADB in the cause of death with no other drugs found. Without such testing, the possible significance of identifying these compounds, may underrepresent the toxic effects of this newer generation of synthetic cannabinoids. In addition, in the human performance cases (#1 and #2), no other psychoactive compounds were detected which might have led investigators to the conclusion that the subjects were not impaired by a controlled substance at the time of the accidents. From the pharmacological effects noted, the need for extended testing for synthetic cannabinoids of this finding cannot be underestimated.
It is also important to note the significance of recovering the paraphernalia in the cases and identifying the synthetic cannabinoid should not be underestimated. This material allowed the identification of these synthetic cannabinoids and guided the testing of the biological specimens. It is recommended that any drug paraphernalia seized during a toxicological investigation be analyzed before or concurrently with the biological specimen analysis to help direct the toxicological analysis.
References
- Pertwee RG (2004) Receptors and pharmacodynamics: natural and synthetic cannabinoids and endocannabinoids. Pharmaceutical Press, London, UK.
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, et al. (2002) International union of pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev 54: 161-202.
- Reider SA, Chauhan A, Singh U, Nagarkatti M, Nagarkatti P (2010) Cannabinoid-induced apoptosis in immune cells as a pathway to immunosuppression. Immunobiology 215: 598-605.
- Ashton JC, Friberg D, Darlington CL, Smith PF (2006) Expression of the cannabinoid CB2 391 receptor in the rat cerebellum: an immunohistochemical study. Neurosci Lett 396: 113-116.
- Musshoff F, Madea B, Kernbach-Wighton G, Kneisel S, Hutter M, et al. (2014) Driving under the influence of synthetic cannabinoids (“Spice”): a case series. Int J Leg Med 128: 59-64.
- Barcelo B, Pichini S, Lopez-Corominas V, Gomila I, Yates C, et al. (2017) Acute intoxication caused by synthetic cannabinoids 5F-ADB and MMB-2201: A case series. For Sci Int 273: 10-14.
- Hasegawa K, Wurita A, Minakata K, Gonmori K, Yamasishi I, et al. (2015) Identification and quantification of 5F-ADB, one of the most dangerous synthetic cannabinoids, in the stomach contents and solid tissue of a human cadaver and in some herbal products. Forensic Toxicol 33: 112-121.
- Huffman JW, Zengin G, Wu MJ, Lu J, Hynd G, et al. (2005) Structure-activity relationships for 1-alkyl-3-(1-naphthoyl)indoles at the cannabinoid CB1 and CB2 receptors: steric and electronic effects of naphthoyl substituents. New highly selective CB2 receptor agonists. Bioorg Med Chem 13: 89-112.
- Aung MM, Griffin G, Huffman JW, Wu M, Keel C, et al. (2000) Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB1 and CB2 receptor binding. Drug Alcohol Depend 60: 133-140.
- Banister SD, Longworth M, Kevin R, Sachdev S, Santiago M, et al. (2016) Pharmacology of valinate and tert-leucine synthetic cannabinoids 5F-AMBICA, 5F-AMB, 5F-ADB, AMB-FUBINCACA, MDMB-FUBINACA, MDMB-CHMICA, and their analogs. ACS Chem Neurosci 7: 1241-1254.
- Kaneko S (2017) Motor vehicle collisions caused by ‘super strength’ synthetic cannabinoids, MAM-2201, 5F-PB-22, AF-AB-PINACA, 5F-AMB and 5F-ADB in Japan experienced from 2012 to 2014. Forensic Toxicol 35: 244-251.
- Castaneto MS, Gorelick DA, Desrosiers NA, Hartman RL, Pirad S, et al. (2014) Synthetic cannabinoids: epidemiology, pharmacodynamics and clinical implications. Drug Alcohol Depend 144: 12-41.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi