Research Article, J Nucl Ene Sci Power Generat Technol Vol: 11 Issue: 6
Nanotechnology to Create Biofuels from Butchery Residue for Recyclable Catalysts of Environment
Siva Kumar Ponnusamy1*, Bazani Shaik2, Ravindra Kumar Agarwal3, Neeraj Saini4, Tasneem K. H. Khan5, S. Mohan6, Nasim Hasan7
1Department of Computer Science and Engineering, SRM Institute of Science and Technology, Uttar Pradesh, India
2Department of Mechanical Engineering, Ramachandra College of Engineering, Andhra Pradesh, India
3Department of Health and Applied Sciences, Ganpat University, Gujarat, India
4Department of Chemistry, Shree Guru Gobind Singh Tricentenary University, Haryana, India
5Department of Science and Humanities (Chemistry), Anjuman College of Engineering and Technology, Nagpur, India
6Department of English, Kalasalingam Academy of Research and Education, Tamil Nadu, India
7Department of Mechanical Engineering, Mettu University, Oromia, Ethiopia
*Corresponding Author: Siva Kumar Ponnusamy Department of Computer Science and Engineering, SRM Institute of Science and Technology, Uttar Pradesh, India; E-mail: ntltechnology2020@gmail.com
Received: 06 September, 2021, Manuscript No. JNPGT-21-41244; Editor assigned: 10 September, 2021, PreQC No. JNPGT-21-41244 (PQ); Reviewed: 24 September, 2021, QC No. JNPGT-21-41244; Revised: 28 June, 2022, QI No. JNPGT-21-41244; Manuscript No. JNPGT-21-41244 (R); Published: 26 July, 2022
Citation: Ponnusamy SK, Shaik B, Agarwal RK, Saini N, Khan TKH, et al. (2022) Nanotechnology to Create Biofuels from Butchery Residue for Recyclable Catalysts of Environment. J Nucl Ene Sci Power Generat Technol 11:6.
Abstract
Biodiesel may be used to replace fossil fuels, and effective experiments were conducted in a wide range of applications. Butchery waste is utilized to produce hydrocarbons gas and biofuels at this facility. In ambient temperature, researchers utilized a Nano catalytic and anatase type of TiO2 nanoparticles photocatalytic for all of this. Butchery waste was broken into relatively low-temperature oil, stationary phase, and natural gas during the first test. Broken gasoline was polymerized to biofuel using NaOH at ambient temperature and pressure inside experiment two. The end product was on a high-quality biofuel. Because of the low cost of raw materials product (butchery waste), which includes high quantities of saturated fats, the economy of this innovative method is significantly more commercially viable. Fossil fuels were given priority in photo catalysis. The research reveals that butchery material could be used not only for producing biodiesel but for hydrocarbons synthesis. The technique is unique in that it requires little energy, has an inexpensive and recyclable catalyst, produces less nitrogen and nitrogen-containing toxic gasses than hydrocarbon, and therefore is environmentally sound.
Keywords: Butchery residue; Photo-catalyst; Gasoline; Cobalt
particles; Nickel particles
Introduction
Questions about financial, ecological, and sustainable development as a consequence of an over-reliance on gasoline are pushing governments all over the world to look for alternatives in the form of biodiesel and bioethanol polymerization [1,2]. In the latest decade, the creation of alternative renewable energies becomes a particularly active research topic. In so many regions across the globe, the usage of biofuels generated from trans esterification, saturated fats, and waste cooking oils has risen substantially. Polymerization is among the most popular ways of reducing oil fluidity [2,3]. It is used to produce ethanol, a fuel made up of mono-alkyl ester of lengthy saturated fats. Given the technical, ecological, and strategic advantages, biofuel is perhaps the most commonly recognized alternative energy source for engines having fossil fuels [1,4]. Furthermore, biofuel is use technically comparable to petroleum-based diesel and needs little adjustments to the fuel injection system. Other benefits of biodiesel over oil and natural gas include lower emission levels, degradability, greater heating value, intrinsic moisture absorption as well as the idea that this is made in the United States [3-8].
Only with increased competition for biofuel, it is critical to decrease the load on edible oil and synthesize biofuel using discarded non-edible fatty acids to avoid contamination and lower bioethanol production costs. Due to global petroleum consumption and worries about CO2 producer's environmental impact, bioenergy has indeed been attracting a lot of support as a sustainable source of energy [9]. Biomass provides a variety of advantages over natural gas, including low SOx and Nitrogen oxide, CO2 neutrality, energy production, and local production. Combustion of fossil fuels, decomposition, combustion, and liquefied are examples of biofuel heat conversion [10]. The pyrolysis process of biofuel is thought to be a potential thermo-chemical method for biomass conversion to pyrolytic, charcoal, gases, fossil fuel and oxides [11-25]. These enhancement and innovations are not always possible with catalysts made in other methods. Cobalt-based catalysis is an example of extending the enzymatic abilities of conventional catalysts by nanoscale modification. The structure of cobalt particle catalysts, as well as other additives like Si, Ni, and Mg, might have had been an impact catalyst [9]. In comparison to the conventional catalyst and cobalt nanomaterial nanomaterial require mild conditions changes for better yield of compounds in short reaction periods [7,12].
Photo catalytic activity polymerized and transformed catalytically pyrolyzed oil into biodiesel. Whenever exposed to sunlight or a fluorescent bulb, photo catalysts create considerable oxidation, which aids in the elimination of organic molecules and microorganisms [13]. Nano composite may be utilized for different phases in cleaning a harmful effect on the environment by applying this concept to water purification, reducing NOx throughout the environmental remediation. Photo catalysts can also be used to produce energy from biomass. Many compounds oxidize gradually in the presence of catalytic electrode materials, according to Stamate and Lazar, which may be justified by kinetic considerations [21]. The magnetic moment of a process is reduced by using a photocatalyst. Set of pictures procedures frequently produce nanoparticles having high oxidation/reduction abilities [14,15]. The catalytic performance of nanocomposite coatings crystalline compound is the greatest [8]. A photoanode built on oxides TiO2 nanoparticles and hydrocarbons was utilized to remove phenolic. Catalyst decomposition and photocatalytic transesterification photocatalytic transformation of lipids to biofuel was utilized for all of this [16-22].
Materials and Methods
Production of cobalt and nickel nanoparticles
Sigma Aldrich USA provided the cobalt salt and nickel chloride, while Fluka provided the 1,10-phenanthroline. All of the solvents were reagents were of analytical quality but were not purified anymore. In 1-propanol, 0.5 M (molar) solutions of 1,10- phenanthroline and 0.5 M (molar) solutions of cobalt chloride were produced independently for the production of cobalt complex [17]. At a temp of 40°C to 50°C, the 1,10-phenanthroline sample was prepared in a drop tube and gently dripped into the cobalt chloride under steady mixing. After only a third of 1,10-phenanthroline mixture was taken to cobalt chloride solution, the bright yellow chromium oxide with cobalt/1,10-phenanthroline complicated popped up, and then going to add of the protoporphyrin solution to a sodium chloride was proceeded until full precipitate in the reaction [18].
To eliminate the un-reacted cobalt chloride, the mixture was collected and rinsed with propyl. The product was dried with infrared radiation before being vacuum-sealed. The compound was placed in a two-neck flask and decomposed in a batch reactor. The gas cylinders were attached to one bottom of the beaker, while the other served as a nitrogen and breakdown air exit. The temperature of the mixture was increased to 500°C at a ratio of 0.5°C min-1, and the materials were maintained at 500°C for 24 hours before cooling to ambient temperature in an inert argon gas environment to generate Co nanoparticles. The production of Ni nanoparticles followed the same technique [19]. The particle morphology and size were studied by scanning electron microscope, transmission electron microscope, and X-Ray Diffraction (XRD). PANalytical, Holland, spectrometer fitted with a Cu K radiation source was used to acquire the XRD of the particles powder.
Samples for biofuel production
Samples of waste animal fat were taken at a slaughterhouse in Kabul, Pakistani. 728 g solid fat, cooked at around 110°C to remove water from the fat there was a little amount of material (546 g) left behind, which included lipids. In the presence of Co and Ni nanoparticles, this material was burned in the oven in the round conical flask [20]. In the round conical flask, 100 g of fat was placed, and 0.1 g of Ni and Co nanoparticles were taken in a conical flask, which was then burned inside a kiln. The temperature was steadily raised until vapors began to emerge from the flasks at 250°C. The burner was preheated to 400°C for 10 min to 15 min before for being turned off. When a match stick was placed outlet during warming, bursts of light appeared, suggests the existence of gaseous fuel. Chemical analysis was used to analyze these gas samples (GC-MS) [21]. The findings showed hydrocarbons such as (CH4 and C2H2). After drying the mixture, which includes 57.5% viscous liquid, 18.75% solid fat, and 23.75% combination of gasses, it was left throughout the beaker.
Results
By increasing the quality of life, fuel plays an important role in socio-economic development. Humans have been using biomass as a source of energy for hundreds of years. Substantial fat butchery residue from animals was transformed into biofuels like biodiesel and hydrocarbon in this experiment [22]. Nickel is a commonly used metal in the industry in a variety of applications. Several novel properties of Ni nanoparticles have been discovered, including a high degree of surface energy, high magnetic, low thermal conductivity, high specific surface area, and lower burn spot. As a result, it may be commonly applied in industrial sectors for thermal degradation [23]. Gasification is the elevated, high-pressure thermal breakdown of lignocellulosic fuels [9]. At 1000°C at 24 hours increased with no air, heat breakdown creates hydrocarbon. The heat of the process can be reduced by up to 650°C using catalyst decomposition. The fixed bed different catalyst breakdown is the most basic form of breakdown. The ease of this type of breakdown, as well as the high carbon burning, are its main benefits in co-nano particles [24].
SEM, TEM, and XRD methods were employed to characterize cobalt, nickel, Si, Ni, Zno, Mg and K particles, as previously described [20]. Figures 1 and 2 show SEM and TEM pictures, respectively.
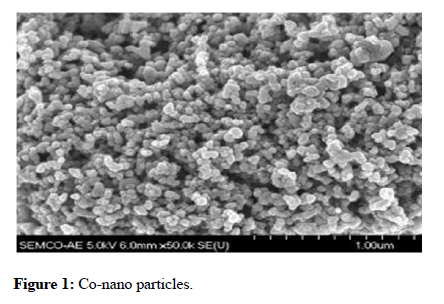
Figure 1: Co-nano particles.
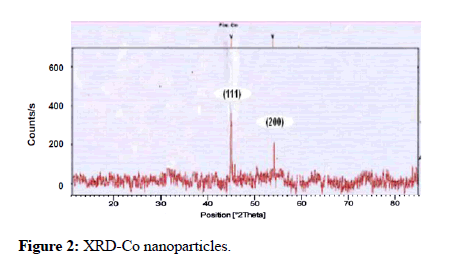
Figure 2: XRD-Co nanoparticles.
The mean particle sizes was determined to use the methods outlined in Figures 1 and 2. As they show in the morphology and on the crystalline size of copper ZnO nanoparticles produced using the co-precipitation technique. In both situations, these pictures show that the particles are homogeneous, uniform, and round putty in form. Nickel and cobalt have a range of sizes of 2 nm to 10 nm. This is equivalent to the crystalline structure estimated using the Sherrer equation using in XRD.
The metal nanoparticles of nickel and cobalt have significant peaks on the XRD. The information was used to study the XRD pattern of new cobalt particles; cubic architecture for cobalt nanoparticles was generated using normal ASTM, XRD files. The peaks on the XRD diagram (Figure 3) correlate to the indices (101) and (101) (200). The indices (101), (111), and (101) of pure faces cantered cubic at 45.4, 52.78, and 77.44, correspondingly, are similar to the peaks in reported for nickel micro particles (Figure 4). Based on the indices given above, it can be inferred that the Ni nanoparticles produced and use in this approach were pure and had a regulated phase of FCC structure.
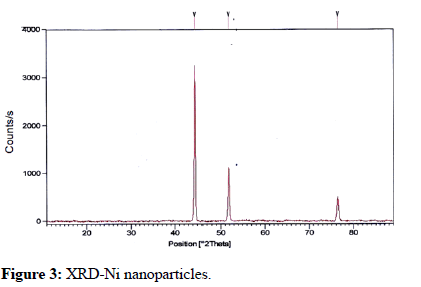
Figure 3: XRD-Ni nanoparticles.
Figure 4: Transmission.
The Ni particles operate as green catalysts for the particular factor of the aldehydic groups in the absence of many other molecules, and their yield is good (Kidwai) [6]. Aldehydes are formed when these types of alcohol interact with fatty. Excessive amounts were split into smaller one because of the elevated temperatures and action of Co and Ni. FTIR was used to examine specimens of normal petro-diesel, normal biofuel, and samples following esterification reaction [25]. The three samples were compared and found to be comparable. The FTIR spectrum of conventional biofuel, oil and natural gas, and bioethanol derived from animal fats is shown in Figures 5-7. Table 1 compares the statistics of these three diesel engines. The electronic properties of the FTIR spectrum peaks are assigned in Table 2. (in cm-1). In Table 2, the peak assignments are listed. The O-H stretching of the carboxylic acid is certainly responsible for the wide feature around 3346 cm-1 and 2816 cm-1. At such a large wavelength range, almost no component possessed such a broad and strong connection. The asymmetrical CH2 stretch and the symmetrical CH2 stretch, which were overlaid on the O-H measure, are linked to various strong links at 2915.86 cm-1 and 2816.55 cm-1, accordingly (Table 1).
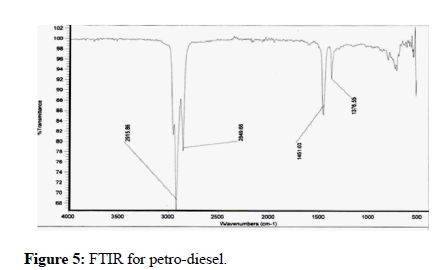
Figure 5: FTIR for petro-diesel.
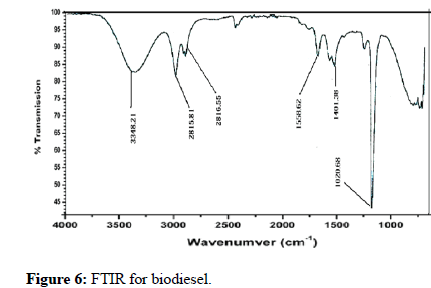
Figure 6: FTIR for biodiesel.
Table 1: FTIR comparison.
Table 2: Vibrational measurement on FTIR.
The FTIR spectra of conventional oil and natural gas, conventional biofuel, and biodiesel made from animal fat are compared. Our biodiesel samples are extremely comparable to those used in the industry. It might be because the molecules have amino acids that are almost identical. However, certain distinctions can be discerned. The location of the aldehyde line in FTIR is affected by chemical influences as well as the particle's architecture (Yang et al.) [24]. As contrasted to the convertible connection in these samples, the ethoxy carbonyl in biofuel has a different absorption location of the C=O resonance. This bond's breakpoint has shifted from 1411 cm-1 in diesel fuel to 1744 cm-1 in normal biofuel to 1588 cm-1 in the experimental sample. New bonds also were discovered in our data, which might be the result of Ni and Co particles' strong reactivity, which could change the biochemistry of animal protein. TiO2 transformed CO2 and water to methanol or ethanol utilizing energy from the sun. Such alcohols can be decreased to create alkanes or oxidized to generate lactic acid. TiO2 strong photocatalytic activity and extreme temperatures broke large chains into local molecules.
Discussion
TiO2 is a semi-conducting substance that may be triggered chemically by sunlight. In UV rays, catalysts have the maximum photo activity. Improved activity, lifespan, toxin tolerance, and other novel abilities are among the advantages of using Nano in catalytic. These enhancements and innovations are not always possible with catalysts made in those other ways. TiO2. Often called titania, is also naturally occurring titanium oxide. Paints, paper products, polymers, paper, synthetic fibers, and crayons, ceramics, electrical components, foods, and cosmetics all include TiO2. The use of TiO2 as a photo catalytic for the breakdown of organic molecules has been studied. In Uv rays, TiO2 becomes active. An object's capacity to generate a photogenerated electrons pair as a consequence of UV light is known as PCA. The radicals that arise are excellent oxidizing agents of natural produce.
For its usefulness in sterilization, sanitation, and remediation applications, TiO2 electro catalytic degradation has been widely investigated. Many additional TiO2 uses, such as printing inks and pharmaceuticals that need low PCA, rely on the ability to regulate PCA. The photo catalyst's reactive activity converts hydrocarbon to alcoholic drinks. Anatase produces the -OH radicals, which may degrade a wide range of organic molecules. For its effectiveness, cheap price, low toxicity, durability, wideband gaps, and accessibility, TiO2 has been considered an effective photo catalyst. When titanium dioxide is exposed to UV light, strong agents are formed that can oxidize and degrade a wide range of microorganisms, organic and inorganic materials. The concepts and possible uses of TiO2 catalysts were described in the subsequent sections (Stamate and Lazar) [21]. To transform a combination of co2 and water vapors into gas, titanium dioxide is used (Svoboda et al.) [23]. Researchers observed a 20-fold increase in methane production when using outside, infrared radiation, compared to prior experiments. Methane has been proposed as a byproduct of bigger alkanes, esters, and alcoholic chemicals. For this reason, nanoparticles will also provide an effective way of capturing solar energy. The photo catalyst's greater activity, smaller size, and bigger surface area were due to the conversion of carbon-based feedstock to greenhouse gases. The presence of water aided the methane production, formaldehyde, and alcohol. Jones et al. backed up this photo catalytic activity theory. The existence of free fatty acids, as well as residues of n-heptane and n-octane, is shown by photo catalysis [5].
Liquids are convenient to carry and store. In the oil test sample, genuine satisfaction is being used to remove the oily mark on colored paper, revealing the prior oxidation of various components. For the alcohol rating system, it is performed by tossing the colored papers in a heptane liquid. It is unsuitable for use in gasoline as it burns rapidly, creating motor banging, in contrast to spaced octane monomers, that fireless gradually and provide better performance. However, by incorporating n-heptane into biodiesel, it may become an important component of biodiesel with poor burning and knock ability. We can improve biodiesel's effectiveness. Nevertheless, n-octane is a frequent constituent of petrol as well as other petroleum and is utilized in biological applications, corrections, and liquid and vapor vacuum distillation. In the octane rating scale, the motor fueling and knock qualities of a monomer of n-octane are utilized as just a comparison benchmark.
Conclusion
The following findings are made from this research: Solid butchery residual fatty deposits are an excellent instrument for low-cost producing biodiesel in the industrial environment. Granular fat waste following the pyrolysis process and trans esterification, which is high in N, P, and protein, can be utilized as a bio-fertilizer. Substantial butchery wastes are a valuable source of eco-friendly hydrocarbons gaseous fuel. The addition of green power innovation to the present climate will result from the utilization of these organic wastes of biofuels. For nations in which it is produced and sold, the method would've been perfect.
References
- Enweremadu CC, Rutto HL, Oladeji JT (2011) Investigation of the relationship between some basic flow properties of shea butter biodiesel and their blends with diesel fuel. Int J Phys Sci 6: 758-767.
- Farrell AE, Plevin RJ, Turner BT, Jones AD, O'hare M, et al. (2006) Ethanol can contribute to energy and environmental goals. Science 311: 506-508.
- Haas MJ, Scott KM, Alleman TL, McCormick RL (2001) Engine performance of biodiesel fuel prepared from soybean soapstock: A high quality renewable fuel produced from a waste feedstock. Energy Fuels 15: 1207-1212.
- Hussain ST, Naheed R, Badshah A, Mehmood T (2009) Design and synthesis of nano heterogeneous supported catalysts for olefin polymerization. Afr J Pure Appl Chem 3: 247-261.
- Jones BJ, Vergne MJ, Bunk DM, Locascio LE, Hayes MA (2007) Cleavage of peptides and proteins using light-generated radicals from titanium dioxide. Analyt Chem 79: 1327-1332.
- Kidwai M, Bansal V, Saxena A, Shankar R, Mozumdar S (2006) Ni-nanoparticles: An efficient green catalyst for chemoselective reduction of aldehydes. Tetrahedr Lett 47: 4161-4165.
- Mahmood T, Hussain ST (2010) Nanobiotechnology for the production of biofuels from spent tea. Afr J Biotechnol 9: 858-868.
- Mahmood T, Hussain ST, Malik SA (2010) New nanomaterial and process for the production of biofuel from metal hyper accumulator water hyacinth. Afr J Biotechnol 9: 2381-2391.
- Navarro RM, Sanchez-Sanchez MC, Alvarez-Galvan MC, Del Valle F, Fierro JLG (2009) Hydrogen production from renewable sources: Biomass and photocatalytic opportunities. Energy Environ Sci 2: 35-54.
- Deepthi T, Balamurugan K, Uthayakumar M (2021) Simulation and experimental analysis on cast metal runs behaviour rate at different gating models. Int J Eng Syst Model Simulat 12: 156-164.
- Devaraj S, Malkapuram R, Singaravel B (2021) Performance analysis of micro textured cutting insert design parameters on machining of Al-MMC in turning process. Int J Lightweight Mater Manufact 4: 210-217.
- Garigipati RKS, Malkapuram R (2020) Characterization of novel composites from polybenzoxazine and granite powder. SN Appl Sci 2: 1-9.
- Yarlagaddaa J, Malkapuram R (2020) Influence of carbon nanotubes/graphene nanoparticles on the mechanical and morphological properties of glass woven fabric epoxy composites. Incas Bullet 12: 209-218.
- Rama Krishna M, Tej Kumar KR, Durga Sukumar G (2018) Antireflection nanocomposite coating on PV panel to improve power at maximum power point. Energy Sources Part A Recov Utiliz Environ Effects 40: 2407-2414.
- Yarlagaddaa J, Malkapuram R, Balamurugan K (2021) Machining studies on various ply orientations of glass fiber composite. Advan Indus Automat Smart Manufact.
- Ezhilarasi TP, Sudheer Kumar N, Latchoumi TP, Balayesu N (2021) A secure data sharing using IDSS CP-ABE in cloud storage. Adv Indu Automat Smart Manufact.
- Mishra P, Jimmy L, Ogunmola GA, Phu TV, Jayanthiladevi A, et al. (2020) Hydroponics cultivation using real time iot measurement system. J Phys Confer Series 1712: 012040.
- Sridharan K, Sivakumar P (2018) A systematic review on techniques of feature selection and classification for text mining. Int J Bus Inform Syst 28: 504-518.
- Vemuri RK, Reddy PCS, Kumar P, Ravi J, Sharma S, et al. (2021) Deep learning based remote sensing technique for environmental parameter retrieval and data fusion from physical models. Arab J Geosci 14: 1-10.
- Salavati-Niasari M, Davar F, Mazaheri M, Shaterian M (2008) Preparation of cobalt nanoparticles from [bis (salicylidene) cobalt (II)]-oleylamine complex by thermal decomposition. J Magnetis Magnet Mater 320: 575-578.
- Stamate M, Lazar G (2007) Application of titanium dioxide photo catalysis to create self-cleaning materials. Model Optimizat Machines Build Field 13: 280-285.
- Suryaman D, Hasegawa K, Kagaya S, Yoshimura T (2009) Continuous flow photocatalytic treatment integrated with separation of titanium dioxide on the removal of phenol in tap water. J Hazard Mater 171: 318-322.
- Svoboda K, Siewiorek A, Baxter D, Rogut J, Pohorely M (2008) Thermodynamic possibilities and constraints for pure hydrogen production by a nickel and cobalt-based chemical looping process at lower temperatures. Ener Convers Manag 49: 221-231.
- Yang WS, Gao HY, Xiang HW, Yin DH, Yang Y, et al. (2001) Cobalt supported mesoporous silica catalyst for Fischer-Tropsch synthesis. Acta Chimica Sinica 59: 1870-1877.
- Zhang C, Zhang R, Li X, Li Y, Shi W, et al. (2011) Bench-scale fluidized-bed fast pyrolysis of peanut shell for bioâ?oil production. Environ Progr Sustain Ener 30: 11-18.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 

