Research Article, J Neurosci Clin Res Vol: 3 Issue: 1
Narrow Band EEG Descriptive Parameters during Visual Habituation and Visual-Motor Association in Young Adults
Brust-Carmona H1*, Sanchez-Quezada A1, Flores-Avalos B2, Alducin-Castillo J3 and Yanez-Suarez O4
1Laboratorio EEG, Dirección de Investigacion; Instituto Nacional de Rehabilitacion (INR). Calzada Mexico
2Servicio de Neurofisiologia, Subdireccion de Electrodiagnostico. Instituto Nacional de Rehabilitación, Mexico
3Departamento de Electronica, Area de Instrumentacion UAM Azcapotzalco. Av. San Pablo Xalpa 180, Mexico
4Laboratorio de Neuroimagenologia, Universidad Autónoma Metropolitana Iztapalapa. San Rafael, Mexico
*Corresponding Author : Brust Carmona H
Laboratorio EEG, Direccion de Investigacion, Instituto Nacional de Rehabilitacion (INR), Calzada, Mexico
Tel: 55 56 733378
E-mail: hbrust@inr.gob.mx; Brusthector@gmail.com
Received: July 05, 2017 Accepted: January 09, 2018 Published: January 15, 2018
Citation: Brust-Carmona H, Sanchez-Quezada A, Flores-Avalos B, Alducin-Castillo J, Yanez-Suarez O (2018) Narrow Band EEG Descriptive Parameters during Visual Habituation and Visual-Motor Association in Young Adults. J Neurosci Clin Res 3:1.
Abstract
Oscillations, action and postsynaptic potentials in glial-neuronal ensembles integrate the spectral power of EEG and are proposed to be the building blocks of cognitive processes in attuned networks. Hence, we aimed at describing the EEG default mode and its modifications related to habituation and visual-motor association to identify possible biomarkers. The EEG was recorded at rest with closed eyes before and during repeated photo stimulations (RPh) and before (Pre) and during association of RPh with switch pressing (VM-Asso) in 64 healthy adults. The EEG was analyzed using UAM/ INR software, which removes artifacts, identifies the corresponding signals, selects 20 samples from each condition (2-s), applies Welch’s periodogram to calculate the absolute power (AP) of ᵹ, θ, α and β, before and during procedures, computes the AP averages (AAPs), and emits the data to a spreadsheet. Differences in each condition were evaluated using nonparametric tests. Cortical distribution of the AAPs per frequency during either Pre and RPh or VM-Asso was studied using a linear regression model. Delta AAP increased (synchronization or inactivation, I) during habituation and decreased (desynchronization or activation, A) during association. θ decreased (A) with the first stimulation and increased (I) with the subsequent stimulations (slope A/I) with greater synchronization (inactivation) during habituation. α presented A/I in both procedures with prolonged activation during association. β’s AAP increased with RPh and VM-Asso. The spectral power (SP) and its topography are descriptive parameters of habituation and visual-motor association, indicating the predominance of inhibitory neurons in habituation and facilitatory ones in visual-motor response.
Keywords: Narrowband EEG in habituation and association; Descriptive EEG; parameters; Habituation alpha inhibition; Visual-motor association; Beta increases; Evoked/event-related oscillations
Introduction
One core objective in neuroscience is to understand better the networks organization of the healthy brain and how it becomes disorganized by neurological disease. The hypothetical framework underlying this objective is that human behavior results from the electrical activity of glial-neuronal ensembles, which become linked together into networks by prolonged synaptic activation. This theoretical hypothesis was first proposed in 1949 (Organization of Behavior: A Neuropsychological Theory) and new data in support of the theory continues to emerge [1]. Neuronal function depends on the membrane potential, which is related to intrinsic and evoked electrical oscillations that generate field potentials of different magnitudes. These oscillations have been proposed as the building blocks of sensory-motor-cognitive processes and can be analyzed using EEG’s narrow band descriptive parameters during the learning processes of habituation and visual-motor association [2,3].
The graphical representation of absolute power (AP) of diverse frequencies bands of the spectral power density (SPD) results in topographical profiles. A set of profiles has been proposed as a basis for regulating the mechanisms of behavior [4].
The cognitive processes that result from the activity of multiple ensembles constituted in networks are tuned by the electrotonic diffusion of the field potentials and, in turn, synchronize neuronal excitability. Varela et al. proposed that synchronized, rhythmic changes in excitability are relevant for sustaining long-range neuronal communication and this is support by neuronal of short and long connections [5]. Furthermore, the neighboring neurons are also tuned by the electrotonic diffusion of membrane oscillations with the possible participation of astrocytes; both processes constitute a communications system among circuits and an integrating process that regulates the function of neuronal-glial ensembles [6-8].
In this way, multiple glial-neuronal ensembles become specialized as cortical columns and layers in subcortical nuclei with a given topographical organization [9]. Further, these organizational networks can be related to specific behaviors, such as hand movements [9]. Through this process, a greater number of attuned neurons results in a field potential with greater intensity [11,12].
Repetition of these processes generates oscillations at a specific frequency and power that will dynamically integrate the glial-neuronal network in multiple brain areas. We propose that normal function occurs within a given range of absolute power of a glial-neuronal circuit; that is, behavior will be normal within a specified range of absolute power and tuned to one or several possible frequencies [13]. In past scenarios the subject-evoked oscillations, bursts of action, and synaptic potentials in multiple glial-neuronal ensembles, which are within the networks that are integrated in the SP, generating the “basal” EEG condition, firstly described by fMRI analysis [14].
From the analysis of the EEG’s absolute power and the attuned profiles of those frequencies while the subject is at rest with closed eyes, we can infer the morphofunctional integrity of neurons and their connections, as proposed by many authors, including Catani and Ffytche [15]. The profiles can be obtained by quantifying the SP of the EEG in four frequency bands (áµ¹, θ, α, and β), integrating the basal, default condition, and when these are modified by photo stimulation or by the association of photo stimulation with a specific hand movement.
In this work, we analyzed the absolute power in four EEG frequency ranges: áµ¹, θ, α and β in a sample of healthy participants. This work allows for the establishment of descriptive parameters for different cortical areas and their modification. Modification was induced by repeated photo stimulation initiated without the knowledge of the subject. Visual-motor association was produced when the subject was asked to press a switch (normally open) when photo stimulated.
Methods
All participants were briefed on study goals, procedures, and risks. Those interested in participation then signed an informed consent letter issued by the National Institute of Rehabilitation of Mexico. The Institutional Research and Ethics Committee, following the guidelines of the Declaration of Helsinki, approved the research with protocol number 10/13.
Sixty-four healthy college students (52 female, average age 21.5 ± 2.5 years) with similar economic and social status participated in the study. All recordings were performed in a Faraday chamber that was dimly illuminated and sound attenuated. Each participant was placed in dorsal decubitus resting position and asked to remain as motionless as possible without falling asleep (we continuously observed both behavior and recordings to verify the “awake” condition). Recordings were made with an electroencephalograph (Nicolet 1) with the electrodes placed at the 10/20 distribution, and special attention was paid to the inter-electrodes distance. The impedance was maintained between 5-10 kΩ for the entire study. Recording electrodes were placed on the outer side of the eyes to register eye and eyelid movements. For analysis, bipolar leads were used in the parasagittal left (Fp1F3, F3C3, C3P3 and P3O1) and right hemispheres (Fp2F4, F4C4, C4P4 and P4O2). The EEG recording paradigm was as follows: a no-task and eyes-closed condition followed by a 20 times repeated photo stimulation, each at 5 Hz, during 2 s at variable intervals of 20-25 s, applied with a Nicolet lamp placed 70 cm from the subject’s face (photo stimulations were applied without the knowledge of the subjects). Then, a second eyes-closed condition was recorded followed by a similar photo stimulation procedure, but with the addition of an indication to press a switch (normally open) when perceiving the light and to hold the switch until the end of the series of flashes.
Data Analysis
A computer program (UAM/INR) was utilized to remove eyelid, ocular and EMG artifacts using the blind separation sources, and the algorithm FastICA. Using windows of one second for each frequency and Shapiro-Wilk statistics, we eliminated electromorphogram with intensity higher than two standard deviation [16].
Then, the program identified the signal of the flashes and took 20 pre-stimulation samples (Pre-Habi) of 2-s duration and samples from each series of 20-repeated photo stimulations (RPh). The flashing signal was again identified and 20 pre-stimulation samples were taken (Pre-VM-Asso). The software identified the signal of voluntary motor response. Samples of each of the 20 associations (VM-Asso) were obtained and their response latency was measured. By estimating power spectral density using Welch’s method (with 256 Hz sample rate), the program integrates the absolute power (AP) data per frequency (áµ¹ [1.6-4 Hz], θ [4.5-8 Hz], α [8.5-13 Hz] and β [13.5-30 Hz]), calculates the averages of the AP (AAP) for each of the samples per frequency and lead, and transfers the data into Excel® spreadsheets. Statistically significant differences were obtained using an ANOVA and a Kruskal Wallis corrected by Dunnett’s test (p ≤ 0.05). However, by averaging the AP values, the evolution of the AP in each condition was lost. This evolution was studied through the AAP distribution by applying a Spearman model of linear regression to each condition. The level of significance was set at p ≤ 0.05. The software used for these analyses was Prisma® (GraphPad Prism version 6.00 for Windows, GraphPad Software, La Jolla, CA, USA).
Results
Delta’s and theta’s AAP modifications before and during both RPh alone and RPh with switch-pressing (normally open) (VM-Asso) in left and right parasagittal (PS) leads
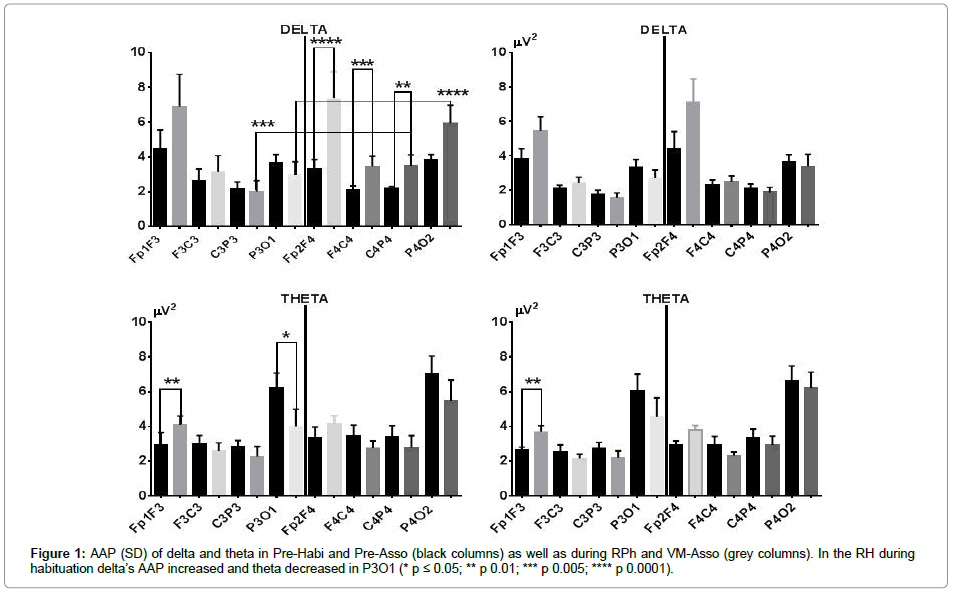
During Pre-Habi, the AAP was higher in the right hemisphere (RH) and yielded a significant asymmetry difference as compared to the corresponding left leads (Figure 1, right side). RPh produced an increase in the AAP of delta in the four PS leads of the RH. The difference was only statistically significant in the first three (Figure 1). In the left hemisphere (LH), the increase occurred in the first two leads, although with a lesser intensity as compared to the RH. In the two posterior leads, the AAP diminished (Figure 1).
During visual-motor associations (VM-Asso), the increase in the AAP of delta was of low intensity and higher in the frontal-frontal (FF) leads of both hemispheres. In the other leads, delta’s AAP did not change practically, or only diminished slightly (Figure 1, left side).
RPh induced an increase in the AAP of theta in the FF leads of both hemispheres, but was significant only in Fp1F3. Further, it diminished in all other leads and reached statistical significance in P3O1 (Figure 1, lower right side). During VM-Asso, the increase of theta’s AAP was significant in lead Fp1F3; in all other leads, it diminished and this diminution was of greater intensity in the LH (Figure 1, lower part). However, averages mask the AP changes in each condition; therefore, in the following, the distribution of delta’s and theta’s AP in each of the conditions before and during either RPh or VM-Asso is presented below.
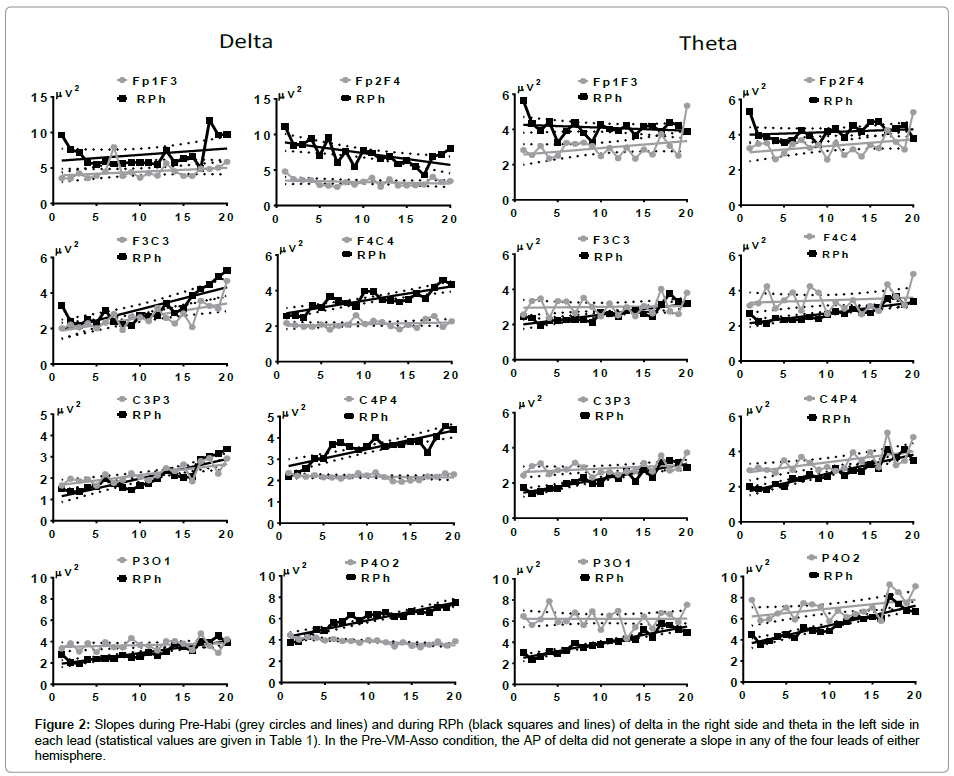
Spearman’s linear regression of delta’s AP generated different slopes in the right and left hemispheres. Thus, during Pre-Habi, the slope was ascending in fronto-central (FC), centro-parietal (CP), and parieto-occipital (PO) leads, but not in FF of the left LH. No slope was observed in the RH during the Pre-Habi condition. RPh produced an ascending slope in leads FC, CP, and PO with steeper inclination in the RH; however, in F2F4 the slope was descending (Figure 2, right side).
Figure 2: Slopes during Pre-Habi (grey circles and lines) and during RPh (black squares and lines) of delta in the right side and theta in the left side in each lead (statistical values are given in Table 1). In the Pre-VM-Asso condition, the AP of delta did not generate a slope in any of the four leads of either hemisphere.
In contrast, the AP of theta during Pre-Habi in the LH did not generate a slope except in FF, where it was ascending. In the RH, no slope was recorded in FC, but the slope was ascending in the other three leads (statistical data are provided in Table 1). RPh did not produce a slope in FF of either hemisphere; in the other three leads the slope was ascending, starting with an AP desynchronization (activation A), follow by synchronization (inactivation I). Slopes were steeper in PO leads and the steepest in the LH (Figure 2, left side).
| Habituation | F1F3 | RPh | F3C3 | RPh | C3P3 | RPh | P3O1 | RPh | F2F4 | RPh | F4C4 | RPh | C4P4 | RPh | P4O2 | RPh | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta R2 | 0.1 | 0.08 | 0.51 | 0.61 | 0.57 | 0.77 | 0.051 | 0.78 | 0.059 | 0.37 | 0.061 | 0.69 | 0.036 | 0.7 | 0.57 | 0.86 | |
| F | 2 | 1.6 | 18 | 29 | 24 | 59 | 0.98 | 62 | 1.1 | 11 | 1.2 | 40 | 0.67 | 42 | 24 | 110 | |
| P value | 0.175 | 0.227 | 4E-04 | <0.0001 | 1E-04 | <0.0001 | 0.336 | <0.0001 | 0.302 | 0.004 | 0.296 | <0.0001 | 0.423 | <0.0001 | 1E-04 | <0.0001 | |
| Theta R2 | 0.13 | 0.044 | 0.004 | 0.63 | 0.12 | 0.81 | 8E-06 | 0.87 | 0.14 | 0.043 | 0.02 | 0.72 | 0.34 | 0.86 | 0.22 | 0.83 | |
| F | 2.6 | 0.82 | 0.069 | 30 | 2.5 | 77 | 1E-04 | 118 | 3 | 0.81 | 0.37 | 47 | 9.3 | 108 | 5.1 | 89 | |
| P value | 0.125 | 0.377 | 0.796 | <0.0001 | 0.132 | <0.0001 | 0.991 | <0.0001 | 0.099 | 0.38 | 0.548 | <0.0001 | 0.007 | <0.0001 | 0.036 | <0.0001 | |
| Alpha R2 | 0.21 | 0.36 | 0.85 | 0.51 | 0.66 | 0.87 | 0.75 | 0.84 | 0.077 | 0.48 | 0.18 | 0.85 | 0.64 | 0.89 | 0.61 | 0.83 | |
| F | 4.9 | 10 | 104 | 19 | 34 | 116 | 53 | 96 | 1.5 | 17 | 4 | 105 | 32 | 149 | 28 | 88 | |
| P value | 0.04 | 0.005 | <0.0001 | 4E-04 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.237 | 7E-04 | 0.061 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Beta R2 | 0.059 | 0.37 | 0.061 | 0.69 | 0.036 | 0.7 | 0.57 | 0.86 | 0.003 | 0.018 | 0.22 | 0.81 | 0.13 | 0.82 | 0.63 | 0.7 | |
| F | 1.1 | 11 | 1.2 | 40 | 0.67 | 42 | 24 | 110 | 0.055 | 0.33 | 5 | 75 | 2.6 | 85 | 30 | 43 | |
| P value | 0.302 | 0.004 | 0.296 | <0.0001 | 0.423 | <0.0001 | 1E-04 | <0.0001 | 0.818 | 0.571 | 0.039 | <0.0001 | 0.122 | <0.0001 | <0.0001 | <0.0001 | |
| ASSO VM | F1F3 | VM | F3C3 | VM | C3P3 | VM | P3O1 | VM | F2F4 | VM | F4C4 | VM | C4P4 | VM | P4O2 | VM | |
| Delta R2 | 0.32 | 0.022 | 0.035 | 0.18 | 0.15 | 0.58 | 0.42 | 0.59 | 0.027 | 0.039 | 0.054 | 0.19 | 0.033 | 0.55 | 0.11 | 0.26 | |
| F | 8.3 | 0.4 | 0.65 | 3.9 | 3.1 | 25 | 13 | 26 | 0.49 | 0.74 | 1 | 4.4 | 0.61 | 22 | 2.2 | 6.2 | |
| P value | 0.01 | 0.533 | 0.431 | 0.065 | 0.095 | <0.0001 | 0.002 | <0.0001 | 0.492 | 0.402 | 0.323 | 0.051 | 0.446 | 2E-04 | 0.153 | 0.023 | |
| Theta R2 | 0.024 | 0.3 | 0.55 | 0.71 | 0.2 | 0.61 | 0.13 | 0.6 | 0.002 | 0.24 | 0.38 | 0.7 | 0.31 | 0.62 | 0.006 | 0.61 | |
| F | 0.44 | 7.8 | 22 | 45 | 4.5 | 28 | 2.7 | 27 | 0.039 | 5.7 | 11 | 43 | 7.9 | 29 | 0.1 | 29 | |
| P value | 0.517 | 0.012 | 2E-04 | <0.0001 | 0.048 | <0.0001 | 0.115 | <0.0001 | 0.846 | 0.028 | 0.004 | <0.0001 | 0.011 | <0.0001 | 0.75 | <0.0001 | |
| Alpha R2 | 0.16 | 0.01 | 0.056 | 0.65 | 0.36 | 0.71 | 0.31 | 0.67 | 0.35 | 0.32 | 0.14 | 0.67 | 0.56 | 0.74 | 0.69 | 0.49 | |
| F | 3.3 | 0.18 | 1.1 | 33 | 10 | 44 | 8.1 | 36 | 9.6 | 8.3 | 2.9 | 36 | 22 | 52 | 40 | 18 | |
| P value | 0.085 | 0.676 | 0.314 | <0.0001 | 0.005 | <0.0001 | 0.011 | <0.0001 | 0.006 | 0.01 | 0.104 | <0.0001 | 2E-04 | <0.0001 | <0.0001 | 6E-04 | |
| Beta R2 | 0.026 | 0.13 | 0.002 | 0.82 | 0.044 | 0.86 | 0.51 | 0.83 | 4E-04 | 0.61 | 0.018 | 0.89 | 0.19 | 0.91 | 0.8 | 0.69 | |
| F | 0.48 | 2.8 | 0.032 | 84 | 0.83 | 111 | 19 | 86 | 0.007 | 28 | 0.32 | 140 | 4.3 | 185 | 72 | 39 | |
| P value | 0.495 | 0.114 | 0.861 | <0.0001 | 0.375 | <0.0001 | 4E-04 | <0.0001 | 0.932 | <0.0001 | 0.578 | <0.0001 | 0.053 | <0.0001 | <0.0001 | <0.0001 |
Table 1: Statistical data of the slope of each frequency by left and right parasagittal leads before (Pre) and during RPh (habituation), as well as before and during visual-motor associations (VM-Asso).
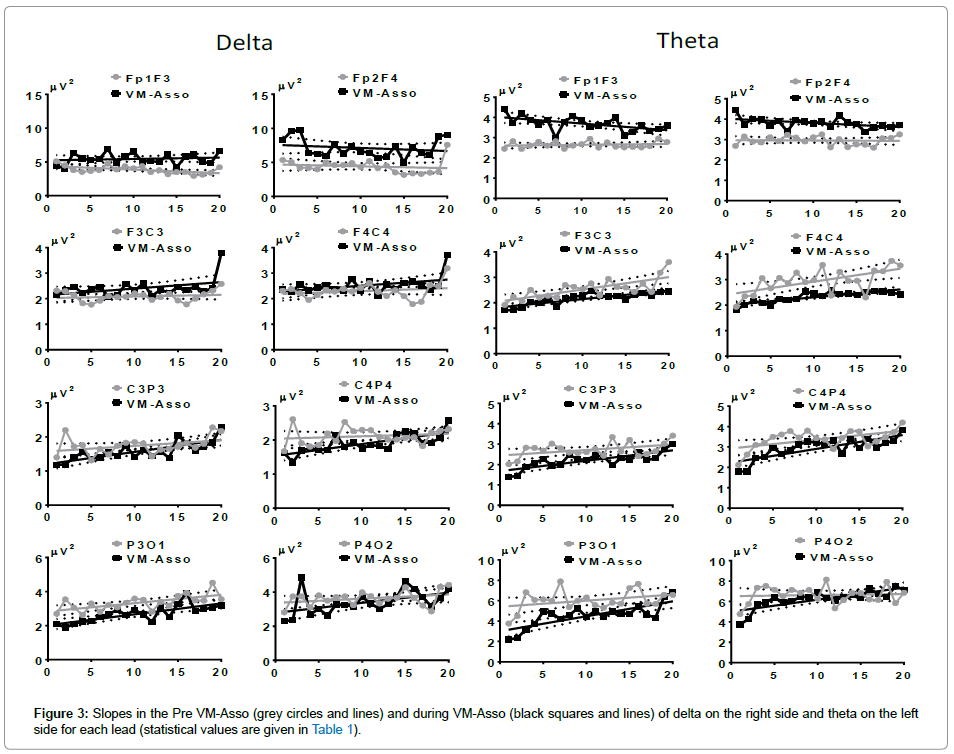
During VM-Asso, an ascending slope was generated in the three posterior leads and was steeper in parieto-occipital (PO), where the ascent was started after a slight diminution (A) (Figure 3, right side). During Pre-VM-Asso, the AP of theta generated ascending slopes except in FF of both hemispheres. The steepest slope was recorded in leads FC, particularly in the RH (Figure 3, right side). During VM-Asso, an ascending slope was generated for all leads with the exception of FF in both hemispheres. The ascent started with a desynchronization (activation A), followed by synchronization (inactivation I) in leads CP and PO, and with a steeper slope in leads PO of both hemispheres (Figure 3, left side).
Modifications of alpha’s and beta’s AAP before and during both RPh alone and RPh with switch-pressing (Normally open) (VM-Asso) in left and right parasagittal (PS) leads
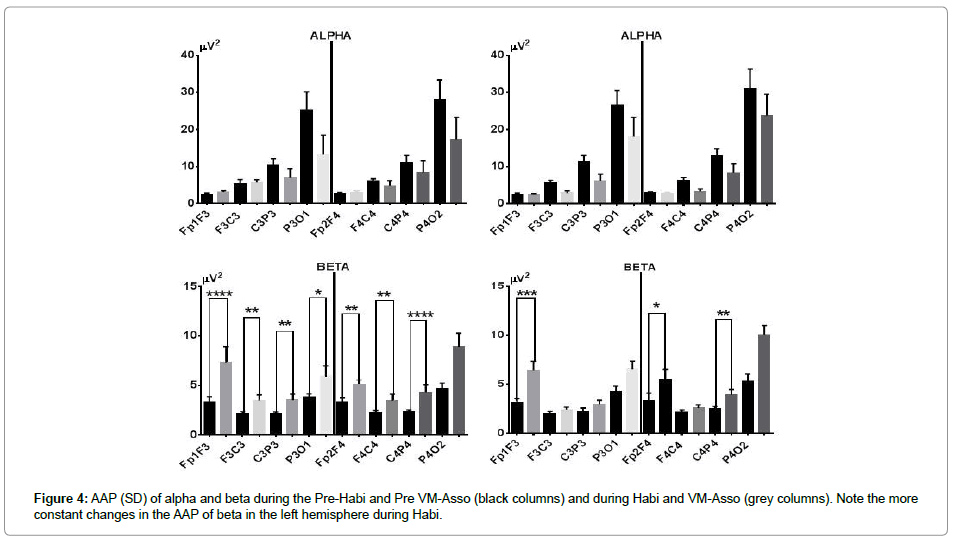
Alpha’s AAP increased in FF and FC, but diminished in the other two leads of the LH during RPh; in the right hemisphere, it diminished in three leads and showed a slight increase in FF. During VM-Asso, the AAP of alpha did not change in FF, but diminished in the other three leads of the LH; in the RH, AAP of alpha did not change and diminished in the other three leads (Figure 4, upper part).
RPh increased beta’s AAP in all leads and the increment was significant with the exception of P4O2. VM-Asso induced a significant increase in FF in both hemispheres, with more intensity in the LH and in C4P4; the increase was recorded in all leads, but did not reach statistical significance (Figure 4, lower part).
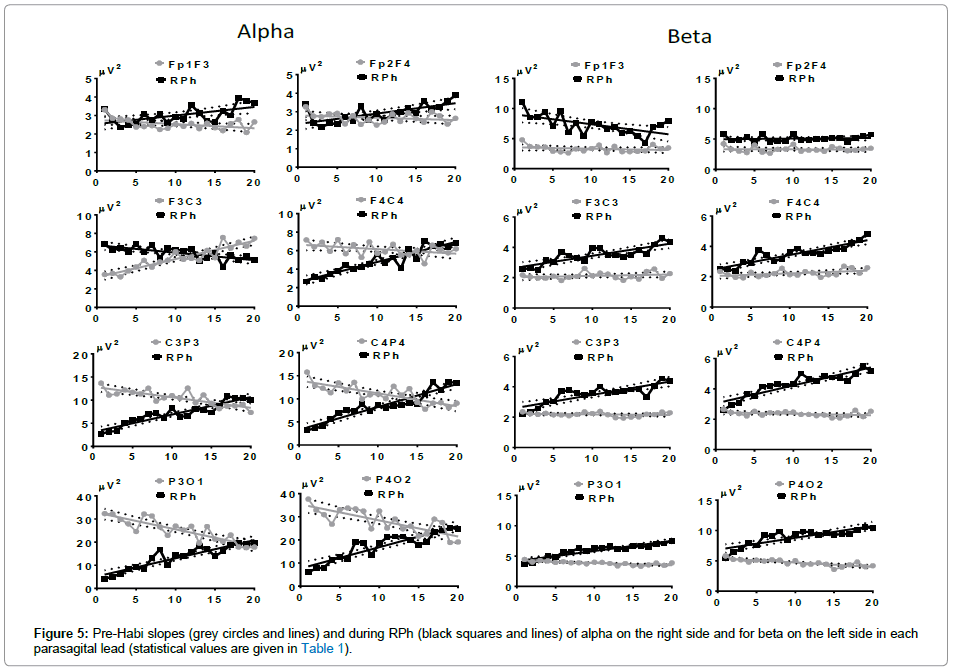
The analysis of alpha AP (Spearman’s linear regression) in both conditions before both stimulation conditions (RPh or VM-Asso) reveals an ascending slope in F3C3, whereas the slope was negative in F4C4; in the other two posterior leads, the slope was negative and statistically significant (activation A). RPh produced an ascending slope in FF of both hemispheres; in FC, the slope was descendent in the LH and ascendant in the RH, with a marked decrease at the beginning of RPh. In the other leads, the slope was positive and statistically significant, with a marked decrease (activation A) at the beginning of RPh; the final values exceed those shown during the Pre- Habi condition, particularly in CP of both hemispheres (inactivation I) (Figure 5, right side).
The AP of beta in the Pre-Habi condition did not change, except in leads PO, where a slightly descending slope was found. RPh induced an ascending slope in all leads with the exception of FF. In Fp1F3, the slope was significantly descending and in the corresponding right lead, no slope was observed. In the other leads, the AP of beta gave rise to significant ascending slopes, which started at the same value of the AP recorded during the Pre-Habi condition. That is, beta did not depict initial desynchronization as was observed with alpha and theta (Figure 5, left side).
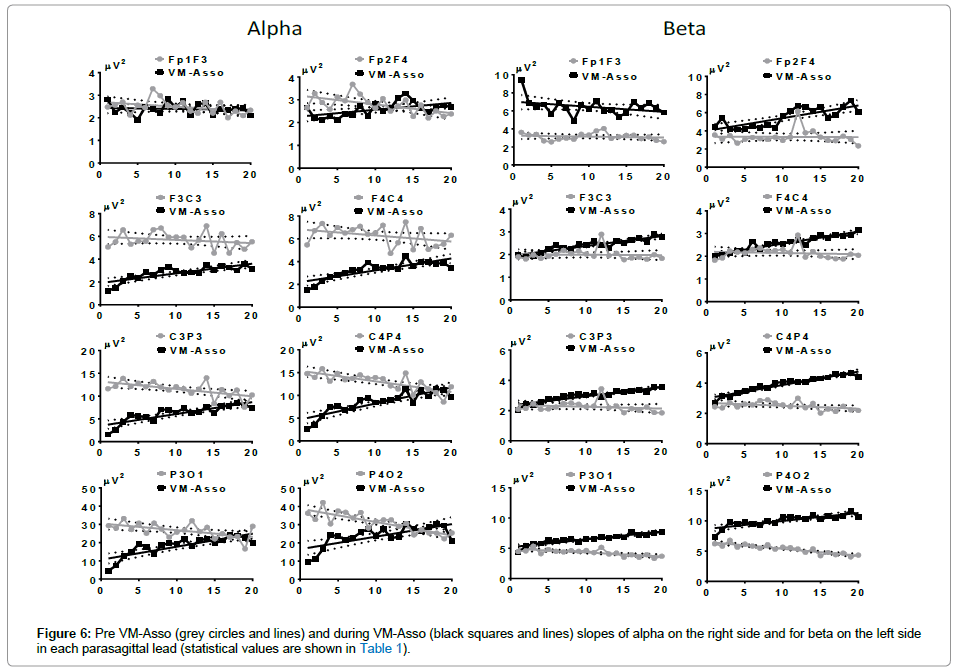
The AP of alpha in the Pre VM-Asso condition generated descending slopes in all leads, which were steeper in CP and PO. During VM-Asso, no slope was generated in Fp1F3, but the slopes in all other leads were ascendant. All slopes started with a marked initial diminution and final values only reached the Pre VM-Asso values in C4P4 and P4O2 (Figure 6, right side).
The AP of beta during Pre VM-Asso did not generate a slope in FF or FC in either hemisphere. However, a descending slope was observed in CP and PO of both hemispheres, with a steeper slope in the RH. During VM-Asso, the slope was statistically ascendant in all leads except in FF, where it was descendent.
During VM-Asso, all slopes of alpha and beta were less steep than those observed during habituation as revealed by R2 data in Table 1, which presents all the corresponding statistical values.
Discussion
The spectral power density of EEG seems to be the integrated functional block of the cerebral networks that participates in sensation, perception, memory, somatic, and vegetative responses [17-20]. Thus, the average absolute power (AAP) of the four frequencies analyzed behaved differently in each cortical region before and during habituation (RPh) or before and during visualmotor association (VM-Asso).
During habituation, the AAP of δ (synchronization) increased in almost all cortical regions except in the left parieto-occipital, in which AAP decreased. However, the δ increment reached statistical significance only in the RH. During VM-Asso, synchronization occurred in fronto-frontal and fronto-central regions of both hemispheres and desynchronization was recorded in the other two regions. These spectral δ power modifications likely participate in controlling the neuro-vegetative activity that occurs as part of the decreased response to a non-significant stimulation during habituation. The greater synchronization of δ could represent an inhibitory process, as proposed for α suggesting that the δ rhythm contributes to the inhibition of those circuits intervening in vegetative responses [21,22]. However, we are not able to discard the participation of δ in attention, sensory, and/or motor integration processes [23-25]. Furthermore, other studies on human δ (0.5-3.5 Hz) have shown its involvement in perception, attention, learning, decision, and working memory [26,27].
These interpretations pose a dilemma, as δ is the EEG activity described most frequently in the presence of cerebral lesions [28]. However, a different function could be explained in relation to the distribution of delta’s power. For instance, a gradual decrease of δ occurs in healthy subjects as compared to subjects with mild cognitive impairment and Alzheimer patients; furthermore, the decrease in the δ response is also significant in bipolar disorder and schizophrenia [8].
In contrast to habituation, during visual-motor association, δ synchronization was recorded in frontal regions and desynchronization in parieto-occipital regions with a trend towards significance. We were expecting larger changes induced by VM-Asso and thus, we interpret that less inhibitory processes are necessary during VM association.
The θ AAP decreased (desynchronization, meaning increased neuronal activity) during habituation, as well as during VM-Asso in all regions except in fronto-frontal regions. In this sense, Steriade et al. proposed that the term desynchronization be replaced by activation and that synchronization be replaced by inactivation when discussing neuronal circuits’ functions [29]. The θ slopes during Pre- Habi and RPh were ascendant in LH and during RPh in RH, but not during Pre-Habi. Thus, the right hemisphere seems to have higher regulatory memory processes during repeated stimulation, while the left hemisphere identifies and responds to specific stimulation during the preparation processes. Thus, we interpret that the increment in theta activity is related to memory processes necessary in both types of learning, as mentioned in the introduction.
The AAP of α did not change in fronto-frontal and fronto-central regions during Pre-Habi, but diminished (desynchronization) in the two other regions, whereas in Pre of VM-Asso, alpha’s AAP revealed desynchronization in the four leads. We related the frontal alpha increment with the motor activity exerted during VM-Asso. The desynchronization in the other cortical regions, considered as an increase in neuronal activity, could be the neural process used to identify and analyze the meaning of the RPh and the expected conditioning during association. This would result in feedback activation of the descending circuits, the reticular-thalamic-limbicbasal ganglia, as well as to facilitate motivation, vegetative and motor networks responsible for the orientation reflex and sympathetic activation. All of these actions are manifestations of the descending regulatory function of the α rhythm [30].
In both types of learning, habituation and VM association, the first stimulations produce alpha desynchronization, which represents activation of different circuits of the visual pathway and of the mesencephalic-reticular-thalamic-cortical system. This desynchronization increases the state of vigilance and activates the frontal cortical and limbic circuits of association, which participate in the integration of the meaning of the sensory stimulation [31-34]. These processes of preparation explain the desynchronization found in the Pre-conditions, which has been described as stimulus expectation.
Repeated stimulation in the same scenario without important changes in an organism’s homeostasis increases the activity of inhibitory interneurons, which leads to decreased responses, such as the orienting reflex and has been called “habituation” by [35]. If stimulation continues, information is rated as “non-significant”, and the organism learns not to respond. Thus, the α desynchronization decreased and yielded an ascending slope of desynchronization/ synchronization (activation/inactivation), which we interpreted as EEG manifestation of habituation.
However, assigning a meaning to photo stimulation modifies the processes producing desynchronization and leading to an increase in the activity of facilitatory interneurons that activate cortical and subcortical regions (mainly extrapyramidal circuits) involved in the motor response. At the same time, a diminution of the inhibitory activity of interneurons is produced, which explains the higher desynchronization in fronto-central region. However, in the parieto-occipital regions, the desynchronization was lower, leading to synchronization during VMAsso repetition and producing a D/S slope, which suggests a diminution of cortical participation in the simple motor response.
The AAP of β increased in habituation and could indicate incremental increases in the attention filtering processes attempting to concentrate the activity in specific networks to diminish the nonsignificant responses. The increase in VM-Asso retained the gating control of different circuits. However, while we were expecting higher beta modifications during VM-Asso, only a significant increment was observed in the right centro-parietal region, which is probably related to the motor response. The possible explanation of less change of beta activity is that, during the visual-motor association, beta’s gating activity has to be more localized to cortical regions.
The descriptions presented herein provide support to the hypothesis that, when beta syntonizes in the different cortical regions, it coordinates the attention and sensory-perception processes and motor functions [36-38]. Other authors have described this condition as the manifestation of the bottom-down regulation of organisms [39].
Tuning the different frequencies of the circuits involved in behavior could result from activation of thalamic nuclei and corticalthalamic feedback circuits; this could also hold for the condition of suppressing nonspecific, irrelevant responses. All of these changes are complemented with the “functional connection” to the pyramidal and extrapyramidal motor modules, which are concatenated in the regulatory processes of the still unknown reticular-thalamic-corticocortical- thalamic-basal nuclei; these are mechanisms that regulate the sensory-motor information in learning processes and are reflected in the modifications of the EEG power spectrum density.
Limitations
A limitation of the present paper is that we did not identify the origin and sequence of the spectral power modifications. Therefore, we recognize the low capacity of the EEG to identify the source of electrical changes. A major weakness of EEG is that recordings represent changes in the local field potentials of thousands of neurons of diverse circuits, which may have different functions and times of participation that are inherent to the changes in the EEG rhythms. However, qEEG was used, as it is more applicable than neuroimaging studies; it is safe, does not have medical contraindications, is easier to perform in different subjects (even children) and patient conditions, and is less expensive.
Conclusion
For the neuro-rehabilitation programs, the descriptive parameters of a narrow band could be a possible biomarker of the EEG that could be used to compare patients that have acquired chronic neurological diseases. Brain oscillations in multiple frequency windows can be used as physiological markers for the recognition and progression of diseases or to improve rehabilitation effects, as shown in simple learning processes like habituation and association of a sensorial signal and a voluntary motor response. Spatial coherences in diseases may be helpful in analyzing integrative brain functions and, thus, are important for future research [40].
Acknowledgement
We would like to thank Ingrid Mascher for editorial assistance and Dr. Teodoro Flores for administrative support. We would also like to thank Amber Rochette and the Research and Editing Consulting Program of the International Neuropsychological Society for editorial assistance.
This study was performed with support from CONACyT-Mexico, Protocol Salud- 2011 No. 1 161587.
Declaration of Conflicting Interests
The authors declare no conflicts of interest with respect to research, authorship, and/or publication of this article.
References
- Basar E (2012) A review of alpha activity in integrative brain function: Fundamental physiology, sensory coding, cognition and pathology. Int J Psychophysiol 86: 1-24.
- Buzsaki G (2006) Rhythms of the Brain. Oxford University Press. New York, USA.
- Fingelkurts AA, Fingelkurts AA (2010) Morphology and dynamic repertoire of EEG short-term spectral patterns in rest: Explorative study. Neurosci Res 66: 299-312.
- Basar E, Eroglu EC, Karakas S, Schurmann M (2000) Gamma, alpha, delta and theta oscillations govern cognitive processes. Int J Psychophysiol 39: 241-248.
- Varela F, Lachaux JP, Rodriguez E, Martinerie J (2001) The brainweb: Phase synchronization and large-scale integration. Nat Rev Neurosci 2: 229-239.
- Fries P (2015) Rhythms for cognition: Communication through coherence. Neuron.
- Wyart V, Sergent C (2009) The phase of on-going eeg oscillations uncovers the fine temporal structure of conscious perception. JNEUROSCI29: 12839-12841.
- Basar E, Eroglu BC, Guntekin B, Yener GG (2013) Brain’s alpha, beta, gamma, delta and theta oscillations in neuropsychiatric diseases: Proposal for biomarker strategies. Supplements to Clinical Neurophysiology 62: 19-54.
- Grossberg S (1999) How does the cerebral cortex work? Learning, attention and grouping by laminar circuits of the visual cortex. Spat Vis 12: 163-185.
- de Klerk CCJM, Johnson MH, Southgate V (2015) An EEG study on the somatotopic organisation of sensorimotor cortex activation during action execution and observation in infancy. Dev Cogn Neurosci 15: 1-10.
- Michels L, Muthuraman M, Luchinger R, Martin E, Anwar AR, et al. (2013). Developmental changes of functional and directed resting-state connectivities associated with neuronal oscillations in EEG. NeuroImage 81: 231-242.
- Pfurtscheller G (2006) The cortical activation model (CAM), pp: 19-27.
- Wang XJ (2010) Neurophysiological and computational principles of cortical rhythms in cognition. Physiol Rev90: 119-1268
- Fransson P (2005) Spontaneous low-frequency BOLD signal fluctuations: An fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp 26: 15-29.
- Catani M, Fytche DH (2005) The rises and falls of disconnection syndromes. Brain 128: 2224-2239.
- Shapiro SS, Wilk MB (1965) An analysis of variance test for normality (complete samples). Biometrika 52: 591.
- da Silva LF (2013) Principles of neural coding from EEG signals. Boca Raton. FL.: Taylor & Francis Group.
- Simon BE, Podlipsky I, Arieli A, Zhdanov A, Hendler T (2008) Never resting brain: Simultaneous representation of two alpha related processes in humans. PLoS ONE 3: e3984.
- Kopell N, Kramer MA Malerba P, Whittington MA (2010) Are different rhythms good for different functions? Front Hum Neurosci 4: 187.
- Carmona BH, Valadez G, Avalos FB, Martinez JA, Sanchez A, et al. (2013). Absolute power of cortical oscillations and their topographical distribution in a sample of young adults during resting wakefulness and unspecific attention. Revista de Investigación Cl’inica; Organo Del Hospital de Enfermedades de La Nutrición 65: 52-64.
- Klimesch W, Sauseng P, Hanslmayr S (2007) EEG alpha oscillations the inhibition-timing hypothesis. Brain Res Rev.
- Knyazev GG (2012) EEG delta oscillations as a correlate of basic homeostatic and motivational processes. Neurosci Biobehav Rev.
- Bruns A, Eckhorn R (2004) Task-related coupling from high- to low-frequency signals among visual cortical areas in human subdural recordings. Int J Psychophysiol 51: 96-116.
- Harmony T, Fernández T, Silva J, Bernal J, Comas DL et al. (1996). EEG delta activity: An indicator of attention to internal processing during performance of mental tasks. Int J Psychophysiol 24: 161-171.
- Womelsdorf T, Fries P (2006) Neuronal coherence during selective attentional processing and sensory-motor integration. J Physiol Paris 100: 182-193.
- Savas ED, Guntekin B, Yener GG, Basar E (2016) Decrease of delta oscillatory responses is associated with increased age in healthy elderly. Int J Psychophysiol 103: 103-109.
- Karamacoska D, Barry RJ, Steiner GZ (2017) Resting state intrinsic EEG impacts on go stimulus-response processes. Psychophysiol 54: 894-903.
- Thatcher RW, North DM, Curtin RT, Walker RA, Biver CJ et al. (2001). An EEG Severity Index of Traumatic Brain Injury. J Neuropsychiatr Clin Neurosci 13: 77–87.
- Steriade M, Amzica F, Contreras D (1996) Synchronization of fast (30-40 Hz) spontaneous cortical rhythms during brain activation. J Neurosci 16: 392-417.
- Engel AK, Fries P, Singer W (2001) Dynamic predictions: Oscillations and synchrony in top–down processing. Nat Rev Neurosci 2: 704-716.
- Basar E, Guntekin B (2012) A short review of alpha activity in cognitive processes and in cognitive impairment. Int J Psychophysiol 86: 25-38
- Gvilia I (2010) Underlying brain mechanisms that regulate sleep-wakefulness cycles. Int Rev Neurobiol 93: 1-21.
- Hummel F, Gerloff C (2005) Larger interregional synchrony is associated with greater behavioral success in a complex sensory integration task in humans. Cerebral Cortex 15: 670-678.
- Moruzzi G, Magoun HW (1949) Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol 1: 455-73.
- Sokolov EN (1963) Higher Nervous Functions: The Orienting Reflex. Ann Rev Physiol 25: 545-580.
- Basar E, Guntekin B (2013) Review of delta, theta, alpha, beta, and gamma response oscillations in neuropsychiatric disorders. Suppl Clin Neurophysiol.
- Steriade M, Llinas RR (1988) the functional states of the thalamus and the associated neuronal interplay. Physiol Rev 68: 649–742.
- Wrobel A (2000) Beta activity: A carrier for visual attention. Acta Neurobiol Exp 60: 247-60
- Taylor AG, Goehler LE, Galper DI, Innes KE, Bourguignon C (2010) Top-down and bottom-up mechanisms in mind-body medicine development of an integrative framework for psychophysiological research. EXPLORE J Sci Heal 6: 29-41.
- Basar E, Duzgun A (2016) How is the brain working Research on brain oscillations and connectivities in a new take-off. Int J Psychophysiol 103: 3-11.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi