Research Article, J Neurosci Clin Res Vol: 9 Issue: 1
Pain and Spasticity in Multiple Sclerosis: A Short Treatment Guide
Athanasios Papathanasiou*
1Department of Neurology, Nottingham Centre for Multiple Sclerosis and Neuroinflammation, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, NG7 2UH, UK
*Corresponding Author: Athanasios Papathanasiou,
Department of Neurology,
Nottingham Centre for Multiple Sclerosis and Neuroinflammation, Queen’s Medical
Centre, Nottingham University Hospitals NHS Trust, Nottingham, NG7 2UH, UK
E-mail: tpapathanasiou@gmail.com
Received Date: 16 February, 2024, Manuscript No. JNSCR-24-127697;
Editor assigned Date: 19 February, 2024, Pre QC No. JNSCR-24-127697 (PQ);
Reviewed Date: 04 March, 2024, QC No JNSCR-24-127697;
Revised Date: 11 March, 2024, Manuscript No JNSCR-24-127697 (R);
Published Date: 18 March, 2024, DOI: DOI: 10.4172/Jnscr.1000179.
Citation: Papathanasiou A. (2024) Pain and Spasticity in Multiple Sclerosis: A Short Treatment Guide. J Neurosci Clin Res 9:1.
Abstract
Objective: Multiple Sclerosis (MS) causes many disabling symptoms that can lead to physical and psychological burden for patients. Pain and spasticity are common MS symptoms that affect patient’s quality of life. Effective management of MS symptoms reduces their impact on daily activities and helps patients to continue in employment. The aim of the study is to provide a short treatment guide for pain and spasticity in patients with MS, in order to assist clinicians in every-day clinic practice.
Materials and Methods: PubMed, Medline, and Cochrane library were searched using the keywords ‘symptom management’, ‘symptomatic treatment’, ‘spasticity’, ‘pain’ and ‘Multiple Sclerosis’. The published guidelines from the American Academy of Neurology (AAN), the European Academy of Neurology (EAN)/European Federation of Neurological Societies (EFNS) and the United Kingdom National Institute for Health and Care Excellence (NICE) were also reviewed.
Results: We present an evidence-based guidance for pharmacological and non-pharmacological treatment of pain and spasticity in patients with MS.
Conclusions: Symptomatic treatment of pain and spasticity in MS can be difficult and may lead to polypharmacy which can cause serious side effects and worsening of other MS symptoms. An individualised and holistic approach that includes non-pharmacological treatments such as physical exercise, physiotherapy, occupational therapy and cognitive behavioural therapy is suggested. There is an increased need for randomised controlled trials, to find which specific exercise interventions are the most helpful, and to look for medicines that can be effective in more than one MS symptoms.
Keywords: Multiple Sclerosis; Spasticity; Pain; Treatment; Symptom Management
Introduction
Multiple Sclerosis (MS) is a chronic inflammatory, demyelinating and neurodegenerative condition of the central nervous system [1]. It is the most common cause of disability in young adults, affecting 2.3 million people worldwide [1]. MS can cause many disabling symptoms that result in high psychological and physical burden for patients [1-3]. Pain and spasticity are common MS symptoms that can co-exist and affect patients’ quality of life [2,4,5]. Effective management of patients’ symptoms reduces their impact on daily activities and helps patients to continue in employment [2,4,5]. Currently there are many different pharmacological agents that are widely used to treat pain and spasticity, however, the evidence base for efficacy in patients with MS is modest [1-3,5].
Symptomatic treatment in MS can be difficult and requires a multimodal and individualised approach [6]. The aim of this study was to provide a short treatment guide for the management of pain and spasticity in patients with MS, to assist clinicians in every-day clinic practice.
Materials and Methods
PubMed, Medline, and Cochrane library were searched using the keywords ‘symptom management’, ‘symptomatic treatment’, ‘spasticity’, ‘pain’ AND ‘Multiple Sclerosis’. Articles were selected based on relevance and quality. Additional articles were identified through reference lists. The published guidelines from the American Academy of Neurology (AAN), the European Academy of Neurology (EAN)/European Federation of Neurological Societies (EFNS) and the United Kingdom National Institute for Health and Care Excellence (NICE) were also reviewed.
Pain
Pain is a frequent manifestation of MS, even in the early stages [7], with a pooled pain prevalence of 62.8% [8]. Patients with MS report pain as one of the most troublesome symptoms of their disease [9]; it interferes with quality of life, activities of daily living, sleep, mental health, social functioning, and employment [10]. Pain in MS has been classified as intermittent/paroxysmal neuropathic pain (i.e. trigeminal neuralgia, painful tonic spasms, Lhermitte’s phenomenon), continuous neuropathic pain (i.e. persistent burning dysesthesia), musculoskeletal pain (i.e secondary to immobility and spasticity) and mixed neuropathic and non-neuropathic pain (i.e. migraine, tension type headache) [11]. Pain in MS lasting more than 12 weeks is considered chronic pain, although other studies described chronic pain as lasting more than 1 month [10]. Based on numeric rating scales ranging from 0-10, mild pain is usually classified as 0-4, moderate pain as 5-7, and severe pain as 8-10 [12]. There are numerous scales used to characterise the impact of pain in patients with MS, but only the pain interference scale of the brief pain inventory, the graded chronic pain disability score, and the pain effect scale are validated for MS [10].
Pain in MS can be difficult to manage [13] and treatment recommendations are based on research in other disorders, as randomized controlled trials in MS-associated pain are lacking [2]. Anti-epileptic medication is the treatment of choice for all types of paroxysmal neuropathic pain [2]. First line treatment for trigeminal neuralgia is carbamazepine [14,15]. Other anticonvulsants used for paroxysmal neuropathic pain (including trigeminal neuralgia) [16] are shown in Table 1. Surgical treatment for MS patients with trigeminal neuralgia (microvascular decompression, gamma-knife radiosurgery) is less effective compared to idiopathic trigeminal neuralgia [17].
| Class | Drug | Mechanism of action | Daily dose | Side effects | Recommendation |
|---|---|---|---|---|---|
| Anticonvulsants | Gabapentin | Block of calcium channel reduction of excitatory neurotransmitters | 900-3600 mg | Dizziness, drowsiness, unsteadiness, impotence cognitive dysfunction, leukopenia, visual changes weight changes, mood changes, respiratory depression | First line for continuous neuropathic pain first line for spasticity-related pain second line for intermittent neuropathic pain |
| Pregabalin | Block of calcium channel reduction of excitatory neurotransmitters | 150-600 mg | Dizziness, drowsiness, unsteadiness, visual changes sexual dysfunction, cognitive dysfunction, weight changes mood changes, respiratory depression, dependence | First line for continuous neuropathic pain second line for intermittent neuropathic pain | |
| Lamotrigine | Block of sodium channel | 100-400 mg | Rash, gastrointestinal, dry mouth, arthralgia dizziness, drowsiness, tremor, visual changes loss of coordination, sleep changes, agitation | Second line for continuous and intermittent neuropathic pain* | |
| Levetiracetam | Modulation of neurotransmitter release through binding to synaptic vesicle protein | 500-3000 mg | Dizziness, drowsiness, headache, cognitive dysfunction insomnia, anxiety, mood and personality changes fatigue, nasopharyngitis, rash, tremor, gastrointestinal | Second line for continuous and intermittent neuropathic pain* | |
| Topiramate | Block of sodium channel increase of GABA activity | 50-300 mg | Dizziness, drowsiness, visual changes, tingling increased eye pressure, kidney stones, confusion unsteadiness, cognitive dysfunction, mood changes | Second line for continuous and intermittent neuropathic pain* | |
| Lacosamide | Sodium channel modulation, interaction with collapsing-response mediator protein 2 | 100-300 mg | Gastrointestinal, dizziness, drowsiness, headache blurred vision, lack of coordination, tremor, fatigue mood changes, cognitive dysfunction | Second line for continuous and intermittent neuropathic pain* | |
| Sodium Valproate | Block of sodium channel increase of GABA activity | 400-1500 mg | Gastrointestinal, weight gain, dizziness drowsiness, anaemia, thrombocytopenia, fatigue cognitive dysfunction, extrapyramidal disorder sleep changes, tremor, transient hair loss | Second line for continuous and intermittent neuropathic pain* | |
| (Ox) Carbamazepine | Block of sodium channel | 400-1600 mg | Gastrointestinal, dry mouth, dizziness, drowsiness visual changes, loss of coordination, allergic reaction anaemia, blood disorders, leukopenia hyponatremia thrombocytopenia, fatigue | First line for trigeminal neuralgia, including other forms of intermittent neuropathic pain | |
| Hypnotics, Sedatives, Anxiolytics | Benzodiazepines (Clonazepam/Diazepam) | Increase GABA-A receptor activity in CNS | 1-8 mg/2-30 mg | Dizziness, drowsiness, tolerance, dependence confusion, cognitive dysfunction, gastrointestinal suicidal thoughts, fatigue, respiratory depression | Second or third line for spasticity-related pain particularly for nocturnal spasms |
| Antidepressants | Duloxetine | Serotonin and noradrenaline re-uptake inhibitor | 30-120 mg | Gastrointestinal, dry mouth, sleep changes, changes in weight fatigue, sweating, sexual dysfunction, dizziness, drowsiness tremor, visual changes, paraesthesia, anxiety, suicidal behaviour | First line for continuous neuropathic pain |
| Venlafaxine | Serotonin and noradrenaline re-uptake inhibitor | 75-225 mg | Anxiety, suicidal behaviour, asthenia, abnormal dreams, confusion drowsiness, gastrointestinal, headache, hypertension, sleep changes sexual dysfunction, changes in cholesterol, tremor, visual changes | Second line for continuous neuropathic pain | |
| Tricyclic (Amitriptyline/Nortriptyline) | Serotonin and noradrenaline re-uptake inhibitor inhibit acetylocholine activity, | 10-75 mg | dry mouth, difficulty passing urine, gastrointestinal, hypertension fatigue, oedema, palpitation, restlessness, stomatitis, weight changes | First line for continuous neuropathic pain | |
| Opioids | Morphine | Mu-Opioid receptor agonist | 20-40 mg | Agitation, headache, agitation, mood changes, dry mouth drowsiness, confusion, cognitive dysfunction, fatigue, dependence gastrointestinal, difficulty passing urine, respiratory depression | Second or third line for continuous neuropathic pain* |
| Cannabinoids | Tetrahydrocannibinol/Cannabidiol oromucosal spray | Activate presynaptic CB1 and CB2 receptors reducing neuronal excitability | 1-12 puffs/day | Dry mouth, gastrointestinal, drowsiness, cognitive dysfunction weight changes, weakness, loss of balance, oral disorder vertigo, blurred vision, malaise, dysarthria | Second or third line for non-neuropathic pain related to immobility or spasticity |
| Muscle relaxants | Baclofen | Pre and postsynaptic GABA-B agonist at spinal level | 20-80 mg | Drowsiness, dizziness, weakness, confusion, gastrointestinal cognitive dysfunction, mood changes, hallucinations, headache paraesthesia, urinary disorder, skin reactions, blurred vision | First line for spasticity-related pain |
| Tizanidine | A2-adrenergic receptor agonist in CNS and spinal level | 8-36 mg | Drowsiness, dizziness, fatigue, dry mouth, arrhythmias hypotension, rebound hypertension, weakness, blurred vision urinary disorder , cognitive dysfunction, skin reaction | First line for spasticity-related pain | |
| Dantrolene | Calcium channel block at muscle level | 25-300 mg | Abnormal liver function, dizziness, drowsiness, weakness fatigue, gastrointestinal, skin disorder, respiratory depression speech disorder | Second or third line for spasticity-related pain |
Note:*NICE recommends prescription only with a specialist advice Adapted from: An update on the pharmacological management of pain in patients with multiple sclerosis16
Table 1: Pharmacological treatments for MS-associated pain.
MS-associated continuous neuropathic pain is treated similarly to general causes of neuropathic pain. UK NICE guidelines recommend a choice of amitriptyline, duloxetine, gabapentin or pregabalin as first-line treatment for neuropathic pain [14]. Second-line treatment options [2,5,14,16] are demonstrated in Table 1. Combination therapy in smaller doses can be helpful [2], however, the EFNS investigated the evidence base and suggests combined treatment only for tricyclic antidepressants-gabapentin and opioids-gabapentin [18].
Since early 2000, many studies explored the safety and efficacy of cannabinoids in MS-associated pain and demonstrated modest efficacy [2,16,19]. Although head-to head trials comparing them with other commonly used drugs are currently lacking [2,16,19], it is recommended by the AAN for the treatment of pain and spasticity (excluding neuropathic pain) [20].
Botulinum toxin treatment has demonstrated benefits in neuralgiform pain, pain associated with spasticity, painful spasms and migraine [21]. A referral to specialized pain service is recommended for patients with severe pain affecting quality of life that has not responded to the commonly used treatment options [14] (Table 1).
Various non-pharmacological treatment approaches have been proposed and showed some potential therapeutic effects in reducing pain intensity in patients with MS [22]. Moreover, cognitive behavioral therapy [23] and its newer forms [24] are traditionally considered an effective treatment for chronic pain. However, a Cochrane review of the efficacy of transcutaneous electrical nerve stimulation, psychotherapy, hydrotherapy, reflexology, transcranial direct stimulation and transcranial random noise stimulation in MS-associated pain concluded that the evidence is limited or/ and insufficient [25]. A systematic review and meta-analysis of the available randomized controlled trials demonstrated some evidence of the effectiveness of physical exercise alleviating MS-associated pain compared to passive control, however, there were limitations, particularly a high risk of bias and heterogeneity between studies [26]. Furthermore, a recent review of the literature found acupuncture, as a complementary and alternative medicine, to be helpful with some MS symptoms, including pain [27].
Spasticity
Around 60%-90% of patients with MS will develop spasticity (increased muscle tone) during their illness [5]. Spasticity can be localised or generalised and its common clinical manifestations include stiffness, spasms and pain leading to reduced mobility, contractures, and pressure sores [2,5,28]. It can cause sleep deprivation, reduced social participation and impaired quality of life [2,5,28,29].
Clinicians can assess spasticity using the Ashworth scale, the modified Ashworth scale or the Tardieu scale, based on the degree of resistance to passive movement [30]. Patient reported scales such as the numeric rating scale, visual analogue scale and the multiple sclerosis spasticity scale are widely used in every-day clinic practice [31].
The evidence base for the commonly used drugs for spasticity in MS is considered poor and comparative studies have been inconclusive [32-34]. Before starting any pharmacological treatment for spasticity, clinicians are encouraged to rule out infection, constipation, noxious stimuli, emotions, inappropriately fitted mobility aids, pressure ulcers and posture, as they can aggravate spasticity [32,33]. UK NICE guidelines suggest oral baclofen or gabapentin as a first line treatment for spasticity in MS [32]. They can also be used in combination, if one drug did not offer adequate relief, or the patient can only tolerate low dose due to side effects [32]. Second line treatment options are tizanidine or dantrolene, while benzodiazepines (clonazepam, diazepam) are considered third line option, with emphasis on their benefit in treating nocturnal spasms [32]. Other experts consider tizanidine as a first-line option and gabapentin as second-line [2,33,34]. Patients that do not respond to the usual first line treatments should be referred to a specialised spasticity clinic with a multidisciplinary approach [33,35].
Since early 2000, many studies explored the safety and efficacy of cannabinoids in MS spasticity, including a large placebo-controlled trial with a long follow up [36,37]. Cannabinoids are particularly helpful when spasticity is associated with pain and are used as an add-on treatment in patients with moderate to severe spasticity that do not adequately respond to other available treatment options [2,20]. A 4-week trial of Tetrahydrocannibinol (THC)/Cannabidiol (CBD) oromucosal spray is usually offered and if there is no benefit (more than 20% improvement on a patient reported numeric spasticity scale) after 4 weeks, the treatment should be stopped [2,32]. The efficacy and safety of nabiximols in the treatment of MS spasticity has been highlighted in review studies [38,39].
Moreover, there is recent growing evidence that symptoms such as pain, spasms, bladder dysfunction, insomnia and fatigue, that are commonly associated with spasticity in patients with MS, constitute a broad ‘spasticity-plus syndrome’ that can be treated effectively with cannabinoids [40-42]. A recent Cochrane review showed that cannabinoids improve spasticity, but there was an uncertainty regarding the effect on chronic pain and health-related quality of life [43]; the authors concluded that the evidence is limited by the shortterm duration of the included randomised controlled trials [43].
Botulinum toxin demonstrated its efficacy in MS-associated spasticity, when applied into hip adductor muscles, in a double-blind parallel study of 74 patients with MS [44]. The Inter-disciplinary task force for movement disorders published a report based on current evidence and advised the MS specialists to consider botulinum toxin for MS spasticity [45]. More recently, the Italian botulinum toxin network study confirmed the efficacy and safety of botulinum toxin treatment from the early stages of MS, when spasticity is more localised, and can be continued as the disease progress as monotherapy or combined with commonly used anti-spasticity agents [46].
Intrathecal baclofen has been traditionally used as a last resort for treatment of severe spasticity that does not respond to oral drugs or cannabinoid spray [2]; A recent study demonstrated that the use of intrathecal baclofen in moderate to severe spasticity resulted in the preservation of ambulation for several years in patients with MS [47].
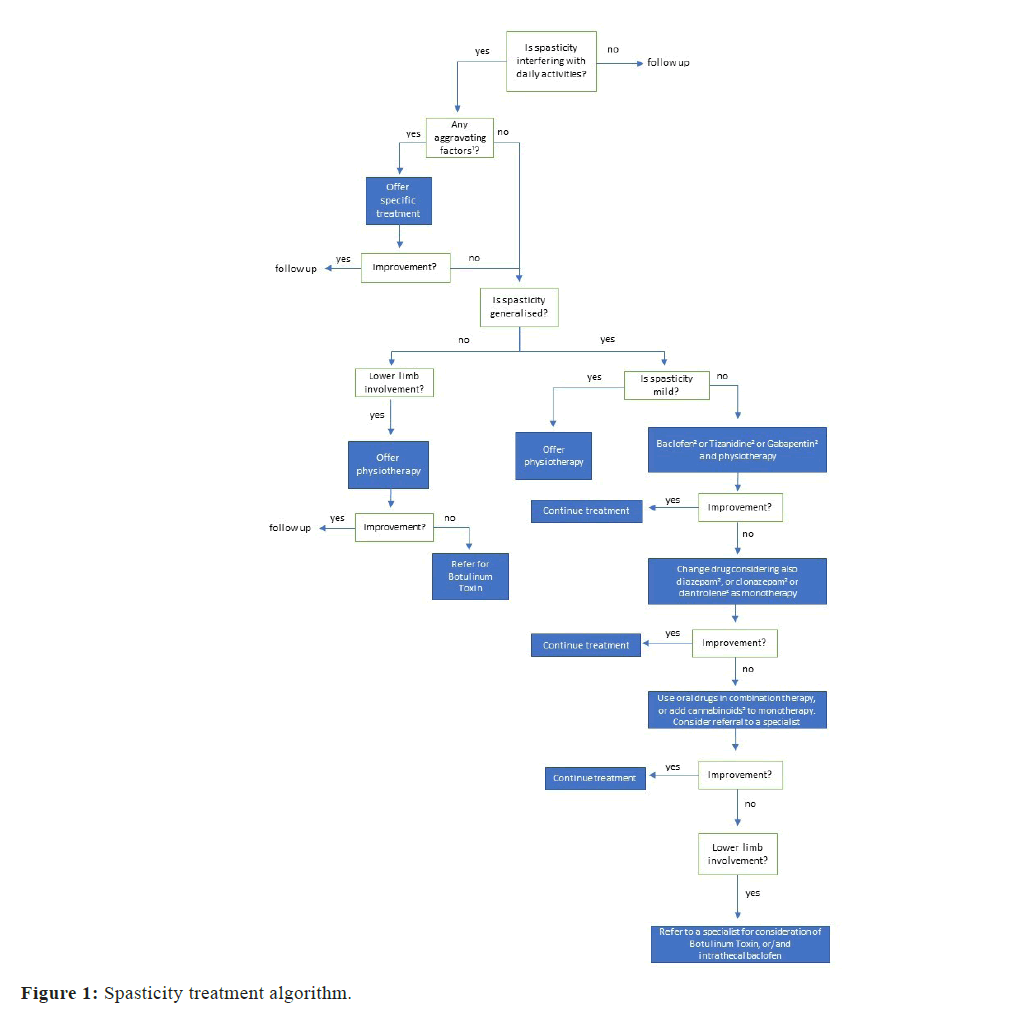
Despite a wide range of non-pharmacological interventions that can potentially help with spasticity in MS, a Cochrane systematic review found some evidence for only physical activity programs used in isolation or in combination with other interventions (pharmacological or non-pharmacological) and for magnetic stimulation with or without adjuvant physical exercise [48]. A systematic review and metaanalysis of repetitive transcranial magnetic stimulation showed some preliminary evidence of improvement in MS-associated spasticity [49]. Figure 1 demonstrates a spasticity treatment algorithm.
Results and Discussion
The current study provides a treatment guide for managing pain and spasticity (that often co-exist) in patients with MS. The evidence base for most available treatments is poor and relies on evidence in other conditions [2,5]; only cannabinoids for the treatment of spasticity have demonstrated their efficacy in randomised controlled trials in patients with MS [2,5].
Symptomatic treatment in MS can be difficult and may lead to polypharmacy which can cause serious side effects and worsening of other MS symptoms such as fatigue, cognitive impairment, and sexual dysfunction [42,50]. Therefore, a sequential approach with regular review of medication efficacy and optimization is required [50]. Individualised treatment for every patient with MS is of crucial importance and patients are encouraged to have an active involvement in the management of their symptoms [6,50]. Moreover, a holistic approach that includes non-pharmacological treatments such as physical exercise, physiotherapy, occupational therapy and cognitive behavioural therapy is suggested [6,50].
There is growing evidence of the benefits of physical exercise in MS [51,52]; based on current evidence and expert opinion, the national MS society has recommended >150 min/week of exercise and/or >150 min/week of lifestyle physical activity for all patients with MS [53]. As disease progresses and engagement in physical activity is more difficult, a referral to a physical or occupational therapist with experience in MS is recommended, to offer an individualised exercise plan, taking into account disability status, comorbidities and symptom fluctuations [53].
It is of great importance to find which specific exercise interventions are the most helpful in patients with MS. At the same time there is an increased need for medicines that can help with more than one symptom to minimize polypharmacy; longerduration randomised controlled trials, to look for medicines (such as cannabinoids) that can be effective in more than one MS symptom are needed. Future trials should also focus on outcome measures that are important from the patients’ perspective.
Conclusion
Symptomatic treatment of pain and spasticity in MS can be difficult and may lead to polypharmacy which can cause serious side effects and worsening of other MS symptoms. An individualised and holistic approach that includes non-pharmacological treatments such as physical exercise, physiotherapy, occupational therapy and cognitive behavioural therapy is suggested. There is an increased need for randomised controlled trials, to find which specific exercise interventions are the most helpful, and to look for medicines that can be effective in more than one MS symptoms.
References
- Filippi M, Bar-Or A, Piehl F, Preziosa P, Solari A et al. (2018) Multiple sclerosis. Nat Rev Dis Primers. 4(1):43.
- Thompson AJ, Toosy AT, Ciccarelli O (2010) Pharmacological management of symptoms in multiple sclerosis: current approaches and future directions. Lancet Neurol. 9(12):1182-1199.
- Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O (2018) Multiple sclerosis. Lancet 391(10130):1622-1636.
- Rommers PS, Eichstädt K, Ellenberger D, Flachenecker P, Friede T et al. (2019) Symptomatology and symptomatic treatment in multiple sclerosis: Results from a nationwide MS registry. Mult Scler. 25(12):1641-1652.
- Feinstein A, Freeman J, Lo AC (2015) Treatment of progressive multiple sclerosis: What works, what does not, and what is needed. Lancet Neurol.14:194-207.
- Crayton H, Heyman RA, Rossman HS. (2004) A multimodal approach to managing the symptoms of multiple sclerosis. Neurology.63(11):S12-18.
- Brochet B, Deloire MS, Ouallet JC, Salort E, Bonnet M et al. (2009) Pain and quality of life in the early stages after multiple sclerosis diagnosis: a 2-year longitudinal study. Clin J Pain.25:211-217.
- Foley PL, Vesterinen HM, Laird BJ, Sena ES, Colvin LA et al. (2013) Prevalence and natural history of pain in adults with multiple sclerosis: systematic review and meta-analysis. Pain. 154:632-642.
- Harrison AM, Bogosian A, Silber E, McCracken LM, Moss-Morris R (2015) ‘It feels like someone is hammering my feet’: Understanding pain and its management from the perspective of people with multiple sclerosis. Mult Scler. 21(4): 466–476.
[GoogleScholar] [PubMed][Crossref]
- Yilmazer C, Lamers I, Solaro C, Feys P (2020) Clinical perspective on pain in multiple sclerosis. Mult Scler. 28(4):502-511.
- O’Connor AB, Schwid SR, Hermann DN, Markman JD, Dworkin RH (2008) Pain associated with multiple sclerosis: systematic review and proposed classification. Pain. 137(1):96-111.
- Alschuler KN, Jensen MP, Ehde DM (2012) Defining mild, moderate, and severe pain in persons with multiple sclerosis. Pain Med. 13(10): 1358-1365.
- Uritis I, Adamian L, Fiocchi J, Hoyt D, Ernst C et al. (2019) Advances in the understanding and management of chronic pain in multiple sclerosis: a comprehensive review. Curr Pain Headache Rep. 23:59.
- Neuropathic pain in adults: Pharmacological management in non-specialist settings. National Institute for Health and Care Excellence (2013).
[PubMed]
- Van Kleef M, van Genderen WE, Narouze S, Nurmikko TJ, van Zundert J, et al. (2009) Trigeminal neuralgia. Pain Pract. 9:252-259.
- Chisari CG, Sgarlata E, Arena S, D’Amico E, Toscano S et al. (2020) An update on the pharmacological management of pain in patients with multiple sclerosis. Expert Opin Pharmacother. 28:1-15.
- Broggi G, Ferroli P, Franzini A, Nazzi V, Farina L et al. (2004) Operative findings and outcomes of microvascular decompression for trigeminal neuralgia in 35 patients affected by multiple sclerosis. Neurosurgery. 55:830-838.
[Google Scholar] [PubMed] [Crossref]
- Attal N, Cruccu G, Baron R, Haanpaa M, Hansson P et al. (2010) EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 Revision. Eur J Neurol. 17(9):1113-e88.
- Nielsen S, Germanos R, Weier M, Pollard J, Degenhardt L et al. (2018) The use of cannabis and cannabinoids in treating symptoms of multiple sclerosis: A systematic review of reviews. Curr Neurol Neurosci Rep. 18(2):8.
- Yadav V, Bever C Jr, Bowen J, Bowling A, Weinstock-Guttman B et al. (2014) Summary of evidence-based guideline: complementary and alternative medicine in multiple sclerosis: Report of the guideline development subcommittee of the American Academy of Neurology. Neurology. 82:1083-1092.
- Safapour Y, Mousavi T, Jabbari B (2017) Botulinum Toxin Treatment in Multiple Sclerosis-a Review. Curr Treat Options Neurol. 19(10):33.
[Google Scholar] [PubMed] [Crossref]
- Hadoush H, Alawneh A, Kassab M, Al-Wardat M, Al-Jarrah M (2022) Effectiveness of non-pharmacological rehabilitation interventions in pain management in patients with multiple sclerosis: Systematic review and meta-analysis. Neurorehabilitation. 50(4):347-365.
- Khoo E-L, Small R, Cheng W, Hatchard T, Glynn B et al. (2019) Comparative evaluation of group-based mindfulness-based stress reduction and cognitive behavioural therapy for the treatment and management of chronic pain: A systematic review and network meta-analysis. Evid Based Ment Health. 22(1):26-35.
- McCracken LM, Yu L, Vowles KE (2022) New generation psychological treatments in chronic pain. BMJ. 376:e057212.
- Amatya B, Khan F, Young J (2018) Non-pharmacological interventions for chronic pain in multiple sclerosis. Cochrane Database of Systematic Reviews. 12:CD012622.
- Demaneuf T, Aitken Z, Karahalios A, leng Leong T, De Livera AM et al. (2019) Effectiveness of exercise interventions for pain reduction in people with multiple sclerosis: a systematic review and meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 100(1):128-139.
- Khodaie F, Abbasi N, Kazemi Motlagh AH, Zhao B, Moghadasi AN (2022) Acupuncture for multiple sclerosis: A literature review. Mult Scler Relat Disord. 60:103715.
- Milinis K, Tennant A, Young CA (2016) Spasticity in multiple sclerosis: Associations with impairments ad overall quality of life. Mult Scler Relat Disord. 5:34-39.
- Rizzo MA, Hadjimichael OC, Preiningerova J, Vollmer TL (2014) Prevalence and treatment of spasticity reported by multiple sclerosis patients. Mult Scler. 10:589-595.
- Platz T, Eickhof C, Nuyens G, Vuadens P (2005) Clinical scales for the assessment of spasticity, associated phenomena, and function: a systematic review of the literature. Disabil Rehabil. 27:7-18.
- Hobart JC, Riazi A, Thompson AJ, Styles IM, Ingram W et al. (2006) Getting the measure of spasticity in multiple sclerosis: The Multiple Sclerosis Spasticity Scale (MSSS-88). Brain.129:224-234.
- Multiple sclerosis in adults: Management. (2019) NICE guideline CG186.
[Google Scholar] [PubMed][Crossref]
- Comi G, Solari A, Leocani L, Centonze D, Otero-Romero S (2020) Italian Consensus Group on treatment of spasticity in multiple sclerosis. Eur J Neurol. 27(3):445-453.
- Shakespeare DT, Boggild M, Young C (2003) Anti-spasticity agents for multiple sclerosis. Cochrane Database Syst Rev.4:CD001332.
- Otero-Romero S, Sastre-Garriga J, Comi G, Hartung HP, Sorensen PS et al. (2016) Pharmacological management of spasticity in multiple sclerosis: Systematic review and consensus paper. Mult Scler. 22(11):1386-1396.
- Zajicek J, Fox P, Sanders H, Wright D, Vickery J et al. (2003) Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): Multicentre randomized placebo-controlled trial. Lancet. 362:1517-1526.
[Google Scholar] [PubMed] [Crossref]
- Zajicek JP, Sanders HP, Wright DE, Vickery PJ, Ingram WM et al. (2005) Cannabinoids in multiple sclerosis (CAMS) study: Safety and efficacy data for 12 months follow up. J Neurol Neurosurg Psychiatry. 76:1664-1669.
- Fragoso YD, Carra A, Macias MA (2020) Cannabis and Multiple Sclerosis. Expert Rev Neurother. 20(8):849-854.
- Conte A, Vila Silvan C (2021) Review of available data for the efficacy and effectiveness of nabiximols oromucosal spray (Sativex) in multiple sclerosis patients with moderate to severe spasticity. Neurodegener Dis. 21(3-4):55-62.
- Fernandez O, Costa-Frossard L, Martinez-Ginez M, Montero P, Prieto JM et al. (2020) The Broad Concept of ‘’Spasticity-Plus Syndrome’’ in Multiple Sclerosis: A Possible New Concept in the Management of Multiple Sclerosis Symptoms. Front Neurol.11:152.
- Fernandez O, Costa-Frossard L, Martinez-Ginez ML, Montero P, Prieto-Gonzalez JM et al. (2021) Integrated Management of Multiple Sclerosis Spasticity and Associated Symptoms Using the Spasticity-Plus Syndrome Concept: Results of a Structured Specialists’ Discussion Using the Workmat Methodology. Front Neurol. 12:722801.
- Bruno A, Dolcetti E, Centonze D (2022) Theoretical and Therapeutic Implications of the Spasticity-Plus Syndrome Model in Multiple Sclerosis. Front Neurol.12:802918.
- Filippini G, Minozzi S, Borrelli F, Cinquini M, Dwan K (2022) Cannabis and cannabinoids for symptomatic treatment for people with multiple sclerosis. Cochrane Database Syst Rev. CD013444.
- Hyman N, Barnes M, Bhakta B, Cozens A, Bakheit M et al. (2000) Botulinum toxin (Dysport) treatment of hip adductor spasticity in multiple sclerosis: a prospective, randomized, double blind, placebo controlled, dose ranging study. J Neurol Neurosurg Psychiatry. 68:707-712.
- D Dressler, R Bhidayasiri, S Bohlega, A Chahidi, TM Chung et al. (2017) Botulinum toxin therapy for treatment of spasticity in multiple sclerosis: Review and reccomendations of the IAB-Interdisciplinary Working Group for Movement Disorders task force. J Neurol. 264:112-120.
- Moccia M, Frau J, Carotenuto A, Butera C, Coghe G et al. (2020) Botulinum toxin for the management of spasticity in multiple sclerosis: the Italian botulinum toxin network study. Neurol Sci. 41(10):2781-2792.
- Sammaraiee Y, Stevenson VL, Keenan E, Buchanan K, Lee H et al. (2020) Evaluation of the impact of intrathecal baclofen on the walking ability of people with Multiple Sclerosis related spasticity. Mult Scler Relat Disord. 46:102503.
- Amatya B, Khan F, La Mantia L, Demetrios M, Wade DT (2013) Non pharmacological interventions for spasticity in multiple sclerosis. Cochrane Database Syst Rev. CD009974.
- Chen X, Yin L, An Y, Yan H, Zhang T et al. (2022) Effects of repetitive transcranial magnetic stimulation in multiple sclerosis: A systematic review and meta-analysis. Mult Scler Relat Disord. 59:103564.
- Papathanasiou A, Saunders L, Sare G (2021) Symptom management of patients with multiple sclerosis in primary care: focus on overlooked symptoms. Br J Gen Pract. 71(704):139-141.
- Edwards T, Pilutti LA (2017) The effect of exercise training in adults with multiple sclerosis with severe mobility disability: A systematic review and future research directions. Mult Scler Relat Disord. 16:31-39.
- Motl RW, Sandroff BM, Kwakkel G, Dalgas U, Feinstein E et al. (2017) Exercise in patients with multiple sclerosis. Lancet Neurol. 16:848-856.
- Kalb R, Brown TR, Coote S, Costello K, Dalgas U et al. (2020) Exercise and lifestyle physical activity recommendations for people with multiple sclerosis throughout the disease course. Mult Scler. 26(12):1459-1469.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi