Review Article, J Forensic Toxicol Pharmacol Vol: 12 Issue: 1
Review of Alprazolam in Forensic Toxicology
Hilary Hamnett1* and Abdul Aziz Khalfan Al Bahri2
1Department of Forensic Science, School of Chemistry, University of Lincoln, Green Lane, Lincoln, Lincolnshire, UK
2Department of Forensic Toxicologist, Forensic Science Laboratory, PO Box 446, Muscat, 113, Oman
*Corresponding Author:Hilary Hamnett,
Department of Forensic Science, School of Chemistry University of Lincoln, Green Lane, Lincoln Lincolnshire, LN6 7DL, UK
E-mail: hhamnett@lincoln.ac.uk
Received date: 27 February, 2023, Manuscript No. JFTP-23-86687;
Editor assigned date: 01 March, 2023, PreQC No. JFTP-23-86687(PQ);
Reviewed date: 15 March, 2023, QCNo JFTP-23-86687;
Revised date: 22 March 2023, Manuscript No. JFTP-23-86687(R);
Published date: 31 March 2023 DOI: 10.4172/CICR.1000229.
Citation: Hilary H, Al Bahri AK (2023) Review of Alprazolam in Forensic Toxicology. J Forensic Toxicol Pharmacol 12:1.
Abstract
Purpose: Alprazolam (Xanax®) is one of the most popular Benzodiazepines (BEZs) prescribed as a medication worldwide. It has been used for the treatment of anxiety and panic disorder since 1976. It is prescribed in doses up to 4 mg/day for generalized anxiety. While doses of 6–9 mg/day are used for phobic and panic disorders, the alprazolam may be subject to abuse because of its sedative and euphoric effects. Its abuse potential stems from its unique pharmacokinetic properties, i.e., its rapid onset and short duration of action. Alprazolam is often abused in combination with other substances including alcohol, methadone, oxycodone, and cocaine. A comprehensive survey of some of the largest scientific publishing databases such as PubMed, Medline and Science Direct was carried out to collate information on alprazolam. The outcome is a comprehensive overview that focuses on the more basic information needed by forensic toxicologists to help them analyze and interpret alprazolam data in ante and post-mortem biological specimens. It includes a detailed discussion of drug concentrations found in alprazolam-positive case reports, considering factors such as post-mortem redistribution, drug interactions and stability. The review also covers the pharmacology, and methods of analysis of alprazolam in biological fluids.
Keywords: Alprazolam; 4- hydroxyalprazolam; ?-hydroxyalprazolam; Forensic toxicology
Abbreviations
BEZs: Benzodiazepines; AAPCC: American Association of Poison Control Centers National Data Collection System; NHS: National Health Service; CNS: Central Nervous System; GABAA: Gamma-Amino Butyric Acid A; VD: Volume of Distribution; PMR: Post-Mortem Redistribution; GC: Gas Chromatography; HPLC: High Performance Liquid Chromatography; MS: Mass Spectrometer; IA: Immunoassays; EIA: Enzyme Immunoassays; ELISA: Enzyme-Linked Immunosorbent Assays; FPIA: Fluorescent Polarization Immunoassays; CEDIA: Cloned Enzyme Donor Immunoassay; RIA: Radio Immunoassays; SPE: Solid-Phase Extraction; LLE: Liquid–Liquid Extraction; Cmax: Peak Plasma Concentration; AUC: Area Under The Curve; HRMS: High-Resolution Mass Spectrometry.
Introduction
Alprazolam (Xanax®, Kalma®, Alprax®) a triazolobenzodiazepine derivative (1,4-benzodiazepine) is one of the most popular Benzodiazepines (BEZs) prescribed as a medication worldwide, and has been used for the treatment of anxiety and panic disorder since 1976 [1]. It has a relatively short half-life (about 11 h), and clinical doses range from 0.25 mg, three times daily, to a single dose of 4 mg/day for general anxiety [2]. It is extensively used for the control of panic attacks and in the management of anxiety disorders. This is despite the fact that many prescribers consider alprazolam to have high misuse liability and a more severe withdrawal syndrome than other BEZs [3]. The number of prescriptions for alprazolam in the USA was approximately 21 million in 2018 [4].
Although alprazolam is licensed for the treatment of short-term anxiety in the UK, NHS prescriptions in England are very low (20 in 2020/21 at a cost of £7.50 per item) [5]; it can also be obtained via private prescription [6]. Alprazolam is also the most used BEZ in Bangladesh and Taiwan [7]. Data from Australia indicates that alprazolam represents about 41% of total anxiolytics dispensed in 2011, and that this dispensing declined somewhat after it was prescheduled in 2014 [8]. Moreover, reports by the American Association of Poison Control Centers National Data Collection System (AAPCC) highlighted the alarming rise of fatal deliberate self-poisonings with alprazolam [9]. In the UK, alprazolam was the BEZ that saw the biggest increase in use by young people according to National Drug Treatment Monitoring System (NDTMS) statistics report issued in 2018 from 8 alprazolam presentations recorded for young people in 2016/17 to 53 in 2017/18 [10].
The annual UNODC reports like the non-medical use of benzodiazepines report do not indicate the prevalence of alprazolam in the Middle East, which means it, is not popular in this part of the world, especially in Arab countries, although it is controlled in most of these countries [11]. For example, in the sultanate of Oman, alprazolam is controlled under the Narcotic Drugs and Psychotropic Substances (NDPS) Act 1999 [12]. Although it is not prescribed in public hospitals, a few private hospitals are authorized to prescribe this medication. There has been significant interest in alprazolam since it first emerged. However, the literature reviews and reports in the 1980s and early 1990s were mostly focused on a general view of alprazolam such as its pharmacology, toxicity, and dose efficacy, and more recent reviews have been limited to specific topics. These have included alprazolam’s interaction with other drugs and non-BEZ medications, its effects on human behavior and activity through controlled studies, comparing it with placebo for any reduction in symptoms, post-mortem concentration interpretation, efficacy and safety of alprazolam versus other BEZs, withdrawal, clinical studies, alprazolam as an alternative medication, replacing alprazolam, and numerous papers on its determination and quantification in various biological specimens using different sample preparation and analytical instrumental techniques [13-21].
In this review, we have conducted a comprehensive survey of some of the largest scientific publishing databases such as PubMed, Medline and Science Direct to collate information related to alprazolam’s chemistry, appearance, use and misuse, pharmacology, side-effects, interactions with other drugs, interpretation, and analytical methods for detection and quantification in biological samples. This review aims to be a comprehensive overview that focuses on the more basic information needed by forensic toxicologists to help them analyze and interpret alprazolam data in ante and post-mortem biological specimens.
Chemistry, Appearance, Use and Misuse
Alprazolam (Figure 1) belongs to the class of aromatic compounds known as 1,2,4-triazolo (4,3-a)(1,4) benzodiazepines. These contain a 1,4-benzodiazepine fused to and sharing a nitrogen atom with a 1,2,4- triazole ring. The presence of the triazole ring distinguishes the chemical structure of alprazolam from that of classical BEZs such as diazepam [1]. Alprazolam is also known by the trade names Xanax®, Kalma®, Alprax®, Alpravecs, Alprazig, Frontal, NiravamTM and Valeans [22]. It is a white crystalline powder, which is soluble in methanol and ethanol but has no appreciable solubility in water at physiological pH [23]. It is typically supplied as a free base in tablets of 0.25, 0.5, 1.0, 2.0 and 3.0 mg. The pillbox database of the National Library of Medicine (NLM) provides more details about alprazolam tablet imprints, doses, colours and information [24]. The main degradation products of alprazolam are triazolaminoquinoleine, 5- chloro-(5-methyl-4H-1,2,4-triazol-4-yl) benzophenone and 1- methyl-6-phenyl-4H-S-triazolo(4,3-a)-1,4-benzodiazepinone [25].
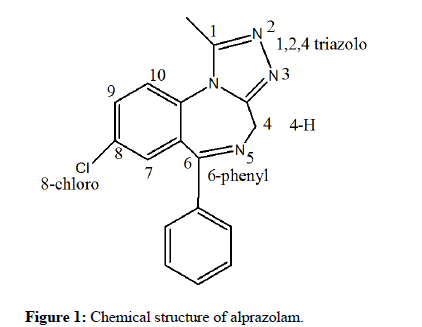
Figure 1: Chemical structure of alprazolam.
Alprazolam is prescribed in doses up to 4.0 mg/day for general anxiety. While doses of 6–9 mg/day are used for phobic and panic disorders. It is also used in the treatment of depression and as an alternative to midazolam for the pre-medication of surgical patients [21]. Alprazolam is available in immediate and extended-release preparations (Xanax-XR available in the USA). The immediaterelease preparation can be used as a short-acting drug given to patients four times a day, or the extended-release preparation can be used to deliver sustained therapeutic concentrations for 24 h after once-daily dosing. The main advantage of the extended-release formulation appears to be its greater tolerability and safety [26,27]. Because of its sedative and euphoric effects, alprazolam may be subject to misuse [28].
Its misuse potential stems from its unique pharmacokinetic properties of rapid absorption, low lipophilicity, short half-life, and high potency. Compared with other BEZs like diazepam, alprazolam is less lipophilic, thus has a smaller Volume of Distribution (VD) 0.9–1.3 L/kg for alprazolam and 0.7–2.6 L/kg for diazepam and is less proteinbound at 68%, compared with 98% for diazepam [19,29,30]. Xanax bars (rectangular alprazolam pills, Figure 2) contain 2 mg, which is four times the amount of a typical pill (0.5 mg). As a result, Xanax bars are a common alternative among recreational drug users because the ‘high’ they experience is stronger and lasts longer [31,32].

Figure 2:Xanax bars 2 mg.
Tablets or bars sold as Xanax on the unregulated drug market may contain little or no alprazolam but instead could contain harmful substances including New Psychoactive Substances (NPS). For example, ongoing drug-checking reports in North America have indicated that counterfeit Xanax tablets could contain a range of substances in a variety of doses, including potent synthetic opioids (e.g., fentanyl and U-47700 in 2019) or NPS benzodiazepines like etizolam and flualprazolam, and often appear by eye to be indistinguishable from the legitimate pharmaceutical drug [33]. In addition to NPS BEZs, between 2017 and 2021, the drug checking service WEDINOS in the UK detected prescribed BEZs such as lorazepam, clonazolam (also known as clonitazolam) and diazepam in products purchased as Xanax, as well as other medications, such as zopiclone, doxepin and gabapentin [34].
Pharmacology
This section summarizes the clinical pharmacology of alprazolam including its basic pharmacodynamics and pharmacokinetics to provide forensic toxicologists with the required information for proper analysis and interpretation of alprazolam-related specimen and samples.
Pharmacodynamics
The pharmacodynamics of all BEZs is qualitatively similar. They are anxiolytic, sedative, hypnotic, muscle relaxant, ataxic and anticonvulsant. Their selectivity, however, seems to differ. Clonazepam, nitrazepam and bromazepam are considered to be more anticonvulsant, and flunitrazepam is more sedative; diazepam and chlordiazepoxide are more anxiolytic than other BEZs [35]. However, alprazolam has been reported to have unusual clinical effects that distinguish it from other BEZs. Alprazolam’s unique clinical profile among BEZs stems from its effectiveness in the treatment of panic disorder, its antidepressive effect and its moderate antipanic activities. In term of antidepressive effect, a large population of depressed patients (906 outpatients) was investigated for six weeks in 1983, who were men or nonpregnant women (18 to 70 years old).
They were suffering from moderate-to-severe symptoms of a unipolar major depressive disorder of at least one month’s duration. Out of 906, 723 patients were suffering from depression and were divided into three groups for treatment. Group one was given alprazolam, group two was given imipramine and group three was given a placebo. The study indicates that the alprazolam was as effective as imipramine in relieving the depression and significantly more effective in relieving somatic symptoms. The study also indicates that drowsiness was the only side-effect reported for alprazolam. More than a decade later, a similar study confirmed the effectiveness of alprazolam as an antidepressant is comparable to that of low-dose tricyclic antidepressants like amitriptyline [36, 37]. In another old study, 526 patients were evaluated for panic attacks or panic disorder by a study psychiatrist.
Capsules containing either 1 mg of alprazolam or lactose (placebo) were administered in equally divided doses (1 mg or 2 mg) up to four times daily (after meals and at bedtime). Medication was gradually increased following a flexible schedule until maximum benefit was achieved, or dose-limiting side-effects occurred. After week 4, a primary comparison of 82% of the patients who completed the medication to this period, rated their panic attacks moderately improved [38]. Also in 2011, a study of the efficacy and safety of alprazolam versus other medications in the treatment of panic disorder, found that the onset of anxiolytic action was notably more rapid for alprazolam compared with amitriptyline, and its antipanic effect was notably more rapid compared with propranolol and imipramine [18]. Smith, et al. studied the pharmacodynamics of alprazolam at 1 mg dose using IV and oral administration routes. The study revealed that, with the exception of the rapidity of onset, the pharmacodynamic profiles of IV and oral administration routes are very similar. Consequently, alprazolam is fully available after oral administration, and kinetic parameters are not affected by the administration route [39].
Mechanism of Action
Due to its lipophilic activity, alprazolam easily crosses the blood– brain barrier and enters the Central Nervous System (CNS). Although the precise mechanism of action for the anxiolytic and antipanic properties of alprazolam has not been fully elucidated, presumably it acts in a manner similar to other BEZs [40,41]. Alprazolam binds with high affinity to non-selective Gamma-Aminobutyric Acid A (GABAA) receptors. By its attachment to these sites, alprazolam enhances GABAA receptor responses to the neurotransmitter, which facilitates the binding of GABA and increases the influx of chloride ions [1]. Thus, the influx of chloride ions causes the depression of the CNS [13]. However, the exact mechanism of action remains unknown. Clinically, all BEZs result in a dose-related CNS depressant activity varying from the mild impairment of task performance to hypnosis [42].
Pharmacokinetics
Absorption: The pharmacokinetics of alprazolam and its major metabolites were studied during the 1980s and 1990s. Following oral administration, approximately 80%–90% of the alprazolam dose is absorbed. The relationship of alprazolam dose to steady-state plasma levels for doses up to 10 mg daily was assessed. The assessment indicated that the absorption rate of alprazolam is independent of the administered dose, and a steady state is reached in 2–3 days, which is also independent of the dosage schedule. The reported steady-state serum concentrations of alprazolam after chronic daily oral administration of 1.5 mg–6 mg are 0.025 mg/L–0.055 mg/L, and doserelated increases in steady-state plasma concentrations were reported with average concentrations of 0.102 mg/L after doses of 9 mg/day [43]. It was demonstrated that there is a predictable, linear relationship between dose and plasma concentration; for each mg of alprazolam, there is an approximate 0.01–0.02 mg/L increase in plasma concentration.
For example, for a single dose of 0.5 mg to 3 mg, the peak plasma concentration (Cmax) ranged between 0.00735 mg/L and 0.0392 mg/L with no significant difference in Cmax among single or multiple doses [44]. Whereas serum concentrations of 0.025–0.055 mg/L were reported in patients taking daily oral doses of 1.5 mg to 6 mg. However, the Cmax of alprazolam is affected if other drugs are simultaneously administered with it. For instance, one study showed that ethanol can significantly increase the concentrations of alprazolam by up to 642% in 120 min, which might increase the toxicity of alprazolam by inhibiting the activity of CYP3A4, but this study was in vivo in a rat model and in vitro in HLM [45], so may not be representative of in vivo human pharmacokinetics. Fluoxetine can prolong the half-life of alprazolam by 16% and increase the Area Under the Curve (AUC) by 32% [46]. Alprazolam is rapidly absorbed after oral administration. A study of the absorption rate of 1 mg alprazolam immediately after a meal and after a 12 h fasting shows no decrease in the extent of absorption in the presence of food [1,47,48].
Distribution: Distribution of alprazolam occurs rapidly and extensively, with the VD in the range 0.8–1.3 L/kg [49]. Comparing with diazepam (98% protein-bound), alprazolam is less protein bound (less lipophilic) (65%–75%) and has a smaller VD. Therefore, it has a faster metabolism rate and shorter duration of action, which would increase its abuse liability compared to diazepam [19,50].
Metabolism and elimination: BEZs are metabolized extensively in the liver by the Cytochrome P450 (CYP) enzymes and excreted by the kidneys. They are mostly converted by N-dealkylation, deamination, and hydroxylation to form hydrophilic, and in some cases, active metabolites [51]. Alprazolam is bio-transformed by hepatic microsomal oxidation, yielding two hydroxylated metabolites: 4- hydroxyalprazolam (4-OH-alprazolam) and α-hydroxyalprazolam (α- OH-alprazolam) (Figure 3). Both hydroxy-metabolites are subsequently converted to the corresponding glucuronides in the liver via uridine 5′-diphospho-glucuronosyltransferase (UDPglucuronosyltransferase (UGT)) before being excreted in the urine [52].
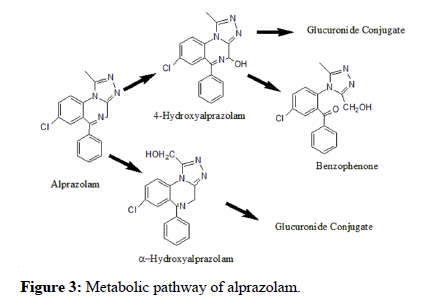
Figure 3:Metabolic pathway of alprazolam.
The two metabolites are active but show less activity than the parent compound. Early studies concluded that 4-hydroxyalprazolam is mainly catalyzed by CYP3A4 and α-hydroxyalprazolam by CYP3A5 [53,54]. However, subsequent studies demonstrated that both metabolites are mainly formed by CYP3A4 and that the role of CYP3A5 is insignificant. This finding also illustrates why alprazolam is used as a probe drug only for CYP3A4. Most studies are consistent in their findings that the plasma concentrations of 4- hydroxyalprazolam and α-hydroxyalprazolam relative to unchanged alprazolam are less than 10% [48,55–58]. Table 1 illustrates the relative percentages of alprazolam and its metabolites excreted in urine [53]. However, 29 metabolites of alprazolam have been identified in urine so far, with only about 20% of the administered dose excreted as unchanged alprazolam. Besides the major metabolite α- hydroxyalprazolam, which accounts for 17% of the total 14C activity recovered in urine samples, the other metabolites found in the urine were 4-hydroxyalprazolam (0.3%), α-4-dihydroxyalprazolam (0.2%), 3-methyltriazolyl (0.9%) and analogues of the chlorobenzophenone (17%) [59]. A benzophenone derived from alprazolam is also found in humans as 3-hydroxymethyl-5-methyltriazolylchlorobenzophenone (3- HMB benzophenone) [60,61].
Table 1: Illustration the relative percentages of alprazolam and its metabolites excreted in urine.
Alprazolam and its metabolites are eliminated via cytochrome P450 3A (CYP3A), and excreted mainly in urine. The mean plasma elimination half-life of alprazolam following administration of an extended-release tablet ranges from 10.7–15.8 h in healthy adults, in some references up to 18 h, which is similar to lorazepam [62]. However, the clearance and other pharmacological parameters of alprazolam are affected when it is administered with other drugs, as demonstrated in the next section. Moreover, alprazolam tends to be transferred into breast milk because of its low molecular weight and low plasma protein binding [63]. The expected detection times of alprazolam after ingestion of single and multiple doses have been studied in various biological specimens.
The median detection times of alprazolam and metabolites after ingestion of 0.5 mg are 12 h (0–27 h) for alprazolam and 36 h (26–61 h) for α-OH-alprazolam in urine, and 26 h (4–37 h) for alprazolam in oral fluid [51]. In a study of oral detection of alprazolam, saliva samples were collected from heavy users admitted to a detoxification facility. The study showed that the maximum detection time for alprazolam in oral fluid was 2.5 days with confirmation concentrations ranging from 0.001 mg/L to 0.022 mg/L (cut-off 0.001 mg/L) [64]. Therefore, oral fluid is a practical sample for alprazolam in living individuals because its detection time is about two days. However, generally, the influence of a variety of disease states on the pharmacokinetic parameters absorption, distribution, metabolism and excretion of alprazolam, including alcoholism and geriatric patients has been reported.
Alprazolam Interactions
Alprazolam, as mentioned above, is primarily eliminated by metabolism via cytochrome P450 3A (CYP3A). Most of the interactions that have been documented with alprazolam are with drugs that inhibit or induce CYP3A4. The interaction of alprazolam with other drugs may increase the risk of serious side-effects, limit its pharmacotherapy, and speed up its elimination from the body. For example, patients are advised to avoid or limit using alcohol while receiving alprazolam because concurrent alcohol use may increase CNS depression [41]. Compounds that are potent inhibitors of CYP3A would be expected to slightly raise alprazolam plasma concentrations. Some of the drugs that have been studied in vivo, along with their effect on raising alprazolam concentrations are ketoconazole (3.98 fold), itraconazole (2.70 fold), nefazodone (1.98 fold), fluvoxamine (1.96 fold), and erythromycin (1.61 fold) [54]. In addition, other medications like rifampin, carbamazepine, and phenytoin were noted to be potent metabolic inducers, and their co-use resulted in a loss of BEZ therapeutic efficacy [65].
It is not recommended to co-administer ritonavir with alprazolam because it may result in the impairment of alprazolam clearance and enhancement of its clinical effects. It has been reported that ritonavir reduces the usual alprazolam therapeutic plasma concentration (2 mg/L) by 50% [66]. Other examples of drugs that can accelerate the removal of alprazolam from the body, which may also affect how it works, are azole antifungals such as itraconazole and ketoconazole, cimetidine, certain antidepressants such as fluoxetine, fluvoxamine and nefazodone, medications to treat HIV (delavirdine and indinavir), macrolide antibiotics (such as erythromycin), rifamycins (rifabutin), venlafaxine and propranolol. While nefazodone and triazolam were found to inhibit the elimination rate of alprazolam [67-71]. In spite of the above negative interaction effects, alprazolam can improve the clinical effects of other medications. For example, it improves the analgesic effect of ibuprofen in post-endodontic pain [72].
Adverse Effects
The adverse effects associated with alprazolam are generally due to the discontinuation of its pharmacotherapy, and tend to be directly related to dosage and plasma level [73]. The most common adverse reactions accompanied by CNS depressant actions are confusion, drowsiness, headache, depression, incoordination, over-sedation, and rare paradoxical reactions like agitation, hallucinations and sleep disturbances. The other side-effects are Gastro-Intestinal (GI) such as constipation, diarrhea, nausea and vomiting, as well as blurred vision. Withdrawal symptoms including delirium and seizures might occur if chronic therapy is discontinued [74].
Due to the adverse effects associated with the use of alprazolam, there have been several attempts to reduce the use of this medication, especially among older people. A recent study was performed to reduce the use of alprazolam among the elderly, 65 years and older, via educational outreach. However, the study concluded that alprazolam continues to be a challenging medication for patients to discontinue [75]. The toxicity of alprazolam is relatively low but considered to be greater than that of other commonly used BEZs, such as diazepam [9]. However, most reported cases indicate that its toxicity is due to its combination with other drugs (see the interpretation section).
Tolerance and Dependence
Tolerance, the decrease in efficacy of a drug over time, and dependence, the need to use a drug to maintain physical function to avoid withdrawal, are among the most commonly encountered problems that occur during long-term BEZ treatment. Tolerance to alprazolam has been reported in sedation treatment; chronic intravenous alprazolam administration (1 mg/kg, every 4 h, 38 days total) was studied in four female rhesus monkeys. The study findings indicate that maximal deep sedation was evident on day 1 but was not significantly different from baseline levels by day 4, and was absent for the remaining days of treatment. Therefore, chronic alprazolam treatment resulted in rapid sedation tolerance, but no tolerance to other behaviors like sleep posture [76]. However, this study is based on in vitro methods and this has not been shown directly in humans to date.
The tolerance of aggressive and social behavior developed during alprazolam treatment was investigated in male mice. Alprazolam (1 mg/kg) was given orally once or twice a day for 8 days for some mice, and for 21 days for other mice, and their behavior was measured based on video analysis. The study measured the interactions of pairs of singly housed male mice with non-aggressive group-housed male mice. The results showed that the administration of alprazolam significantly reduced aggressive activities and increased social investigation without changing locomotion or other behavior. Also, tolerance inhibited the effects of alprazolam on aggressive behavior.
Thus, the conclusion drawn was that tolerance to alprazolam’s effects on aggressive and social behavior developed at different rates, suggesting that they are differentially regulated [77].
It is well recognized that dependence on BEZs develops not only with long-term therapeutic doses but also with high acute doses. A study has shown that alprazolam, with its short elimination half-life, can cause more severe withdrawal syndrome and higher physical dependence liability than BEZs of longer elimination half-lives [78]. Also, the high-potency characteristics and long duration of treatment with alprazolam can increase the susceptibility of users to severe alprazolam dependence compared with BEZs of lower potency [79-81].
Interpretation of Alprazolam in Toxicology Cases
In Table 2 gives a summary of concentrations of alprazolam in different case types and samples.
Table 2: Concentrations of alprazolam reported in different forensic toxicology case types.
Post-mortem cases
Alprazolam is most often misused in combination with other substances including alcohol, methadone, oxycodone, and cocaine. Through our literature investigations, we believe that alprazolam misuse alone is rarely responsible for overdose deaths, which occur primarily when it is combined with other drugs or alcohol. Therefore, this creates difficulty in defining a lethal alprazolam dose range, as it might not be possible to determine the actual role of alprazolam as a causal factor when combined with other drugs. In the USA, a study of 143 ecstasy users in Miami in the state of Florida found that alprazolam (82 users, 57.3%) was the most commonly co-ingested prescription drug [80].
A similar study was performed across the state of Florida between 2005 and 2007, which indicated that A total of 1,199 (363 in 2005, 414 in 2006, and 422 in 2007) drug-induced or drug-related deaths occurred between January 2005 and mid-November 2007, 172 deaths involving oxycodone, 443 were attributed to combination with BEZs and, alprazolam was the most frequently encountered (26 in 2005, 62 in 2006 and 116 in 2007) [81]. The new South Wales department of forensic medicine reported that the total number of alprazolam-related deaths from 1997 to 2012 was 412, with 0.08 mg/L alprazolam in post-mortem peripheral blood (femoral or subclavian, preservation status not given) as the median concentration (range: 0.005 mg/L–2.10 mg/L). The highest concentrations were seen amongst deliberate drug overdoses of 0.18 mg/L (median) with about 42% of these recorded cases having a concentration more than 0.2 mg/L [82].
In cases where the cause of death was not due to alprazolam, the non-toxic preserved femoral blood (1% sodium fluoride) concentration range of alprazolam reported in the literature is 0.041 mg/L–0.056 mg/L, and the toxic concentration range is 0.1 mg/L–0.3 mg/L [17,83]. Several fatal overdoses have been reported with alprazolam along with other drugs such as tramadol (peripheral blood alprazolam was 0.2 mg/L, hydroxyalprazolam is not detected in blood but detected in bile and liver) [84], heroin (a statistical study of the number of alprazolam detections in Victoria (Australia) heroin-related deaths from 1996–2010) [85], nortriptyline, methadone, methamphetamine and kratom (blood alprazolam was 0.2 mg/L) [86], butyryl fentanyl, acetyl fentanyl and ethanol (peripheral blood alprazolam was 0.04 mg/L) [87], U-47700 (peripheral blood alprazolam was 0.12 mg/L) [88], and morphine, 6- monoacetylmorphine (6-MAM), fentanyl and acetylfentanyl (femoral blood alprazolam was 0.065 mg/L) [89]. In most case reports, alprazolam metabolites were not targeted in post-mortem blood samples and whether the blood was preserved or unpreserved was not stated.
Driving under the influence cases
The effect of alprazolam on driving ability has been studied in some places. These studies investigated motor control and the interaction of drivers with the traffic environment. For example, approaching vehicles in the adjacent traffic lane in order to overtake and responding to road signaling. Twenty volunteers were subjected to a driving test on a primary highway during normal traffic at a constant speed (90 km/h) one hour after oral administration of 1 mg alprazolam. The study showed significantly impaired driving quality, decreased alertness, decreased mental activation, and increased mental effort during driving [90]. The influence of alprazolam on driving ability was also studied along with alcohol in the Netherlands at 1 mg alprazolam and at the legal blood alcohol limit (0.5 g/L) and well above (1.0 g/L). The study concluded that alcohol as well as alprazolam clearly affected self-perceived performance [91]. The concentrations of alprazolam in the blood of impaired drivers (venous blood samples) were compared with post-mortem blood (femoral blood samples).
The study showed that the mean alprazolam concentrations in blood from living and deceased persons are not very different (0.08 vs. 0.10 mg/L) [83]. In Houston City, 80 drivers were evaluated between 2015 and 2019 that concurrently tested positive for hydrocodone (opioid), alprazolam, and carisoprodol (muscle relaxant). The mean blood concentrations were 0.075 (range: 0.069–0.322) mg/L for hydrocodone, 0.058 (range: 0.0058–0.18) mg/L for alprazolam, and 3.9 (range: 0.3–14) mg/L for carisoprodol [92]. It is illegal for motorists to drive under California’s vehicle code (23152 (f)) while positive for alprazolam if they do not have a prescription for Xanax [93]. Also, it is illegal in all parts of UK to drive with drugs in your body if you’re driving is impaired [94]. In other countries like Gulf Cooperation Council (GCC) countries, the drug acts are applicable to all types of cases related to drugs including driving under the influence of drugs.
Alprazolam in sexual assault cases
Alprazolam has been reported as one of the drugs used in Drug- Facilitated Sexual Assault (DFSA) because it is evident that it may impair performance in a variety of skills in humans. Two DFSA cases were tested positive for alprazolam in hair, one case was a 12-year-old girl who tested two times (2 cm hair segments at 4.9 and 2.4 pg/mg), the second case was for an adolescent who tested four times (1 cm hair segments at 0.4–3.1 pg/mg) [90].
Relevant factors
The Post-Mortem Redistribution (PMR) of alprazolam was studied in 8 rats, which were sacrificed 30 min after alprazolam administration. Blood and tissue samples liver, lung and brain were collected at 0,2,6, and 24 h after death. Analytical results indicated that median post-mortem blood alprazolam concentrations increased approximately two-fold compared with ante-mortem concentrations due to PMR. The highest alprazolam tissue concentrations were found in fat and liver, whereas the lowest levels were observed in the lungs and brain [95]. Another evaluation of alprazolam PMR phenomena using the ratios of cardiac-to-peripheral blood concentrations (C/P ratios) revealed that alprazolam exhibits minimal PMR [96]. Other studies reported C/P ratios of; 1.5 (range: 1.0–2.8) [97], 0.9 (range: 0.5–1.3) [98], 0.9 [43]; and 1.22 in a fatal multidrug intoxication [99].
In the chemistry, appearance, use, and misuse’ section we noted that alprazolam in its pharmaceutical form undergoes photo degradation. However, alprazolam shows more stability in biological samples. For example, 0.003 mg/L alprazolam was spiked in plasma and stored at – 18°C. The stability of alprazolam in the plasma sample was investigated for 1 month in five freeze–thaw cycles (from –18°C to room temperature), and it was found to be stable for five cycles [100]. Also, the freeze–thaw stability of alprazolam along with other BEZs in breast milk and plasma was assessed in the short-term (24 h, at 4°C) and the long term (8 weeks, at −30°C). For short-term stability, the remaining amounts of alprazolam in the breast milk were 111.3% (± 3.8) and in the plasma were 89.4% (± 4.7). While in long-term stability, the remaining amounts of alprazolam in the breast milk were 96.5% (± 4.3) and in the plasma were 114.7% (± 3.1). The results indicated that no significant degradation was observed under these conditions [101].
In many cases, there is a delay of weeks to months between sample collection, drug screening, and quantitation. Therefore, a long-term drug stability study is very important for interpretation in both antemortem (blood and urine) and post-mortem (blood, urine, brain, liver, stomach contents) cases. A long-term stability study (over an 8-month period at 4, −4 and −20°C) of 26 sedative−hypnotics including alprazolam was carried out in six toxicological matrices: ante-mortem blood in potassium oxalate: sodium fluoride (1:10), post-mortem blood in potassium oxalate: sodium fluoride (1:10), post-mortem urine in sodium fluoride, homogenized liver (20 g diluted 1:3 with deionized water), homogenized brain (20 g diluted 1:2 with deionized water), and homogenized gastric contents (1:100 with deionized water). Table 1 displays the time it took for alprazolam and α- hydroxyalprazolam to lose 20% of their initial value in each sample type. Table 1 Time taken for alprazolam and α-hydroxyalprazolam to lose 20% of their initial value in each sample type [102].
Laboratory detection and methodology
The wide prevalence of alprazolam and its potential for misuse or dependence from the early 1980s has contributed to the development of numerous analytical methods to detect alprazolam and its metabolites in biological samples and pharmaceutical formulations. Some are preliminary screening methods like immunoassay, while others are confirmatory methods such as single chromatography (GC, HPLC) or coupled with a mass spectrometer(s) (MS, MS/MS). However, most of these analytical methods are comprehensive methods for the detection of a group of BEZs. Due to the rapid development in analytical techniques over time, it is recommended that clinical and toxicological laboratories apply the most recently developed methods due to their higher accuracy, sensitivity, selectivity and throughput. Therefore, this review focuses only on presumptive and confirmed methods published since the year 2000, as older methods may no longer be deemed reliable.
Preliminary screening methods
Many different Immunoassay (IA) screening methods are suitable for the detection of BEZs and their metabolites in body fluids, including alprazolam and its metabolites in urine, blood and oral fluid. These techniques include Enzyme Immunoassays (EIA, e.g., EMIT [103] or Enzyme-Linked Immunosorbent Assay (ELISA) [104]), Fluorescence Polarization Immunoassay (FPIA, e.g., SBENZ, Abbott TDx and ADx [105]) micro particle immunoassay (e.g., TRIAGE and ONLINE [106]), and cloned enzyme donor immunoassay (CEDIA) [83,107]). However, these techniques do not have the same sensitivity or selectivity for all BEZs, causing false-negative results, especially for low-dose substances. Thus, comparative studies have been conducted to evaluate different IAs including for detecting alprazolam and its metabolites alone or along with other BEZs. They found that not all techniques are able to identify all BEZs, due to the wide variation in potency, structure, metabolism, elimination as well as the variable immunoreactivity of the antibodies to group members [108].
The sensitivity and selectivity of EMIT II have been determined for a battery of BEZs including alprazolam and OH-alprazolam in urine (0.3 mg/L cut-off) and oral fluid (0.1 mg/L cut-off) [109]. The cut-off levels of 0.3 and 0.1 mg/L are high for alprazolam in both urine and oral fluid and are not fit-for-purpose for forensic toxicology applications, as they should report even trace amounts. However, the sensitivity of LC-MS/MS and IAs techniques has been improved over time and alprazolam has been recently detected by IA and confirmed by LC-MS/MS techniques in oral fluid [51]. Generically speaking, the sensitivity problems with some immunoassay techniques have been solved by using high-resolution mass spectrometric methods as a screening test for drugs in biological samples. References from 110– 112 demonstrate the screening of alprazolam along with other drugs in a variety of body fluids [110-112].
Confirmation methods
Identification and quantitation methods for alprazolam and α- hydroxyalprazolam in various biological matrices like urine, blood, plasma, breast milk, oral fluid, hair and meconium using liquid chromatography-electrospray ionization MS/MS have been reported since the 1990s, and recently by UPLC-MS(/MS) and LCTOF- MS, with only a few studies employing Raman spectroscopy. Some of these methods, which are specific for alprazolam, are designed to support clinical monitoring of patients receiving alprazolam, while others are selective for a group of BEZs including alprazolam, and are designed to increase the throughput of forensic toxicology laboratories [63,113–118].
Discussion
It was mentioned in the ‘metabolism and elimination’ section that only trace amounts of parent alprazolam are present in the urine due to extensive metabolism and conjugation, as is consistent with most BEZs. Thus, hydrolysis of glucuronides is necessary for improved detection. Enzyme hydrolysis is preferred to retain identification specificity, but can be costly and time-consuming. Several investigators have evaluated the efficacy of BEZ enzymatic hydrolysis including alprazolam with different enzymes of different sources and activities like β-glucuronidase from Helix pomatia, Escherichia coli and Patella vulgata.
In previous stuides [25,119] report that the recombinant IMCSzyme β-glucuronidase has higher hydrolysis efficiency than other β- glucuronidases for most BEZ compounds including alprazolam and its metabolites, such that over 95% recovery of alprazolam and its hydroxyl metabolites was observed. As a result, enzymatic hydrolysis is more convenient for alprazolam analysis in biological samples as it does not form benzophenones during acidic hydrolysis [120]. Acidic and enzymatic hydrolysis, which is considered sample pre-treatment methods, is followed by extraction such as Liquid–Liquid Extraction (LLE) and Solid-Phase Extraction (SPE). Various commercially available SPE cartridges have been employed in alprazolam extraction. Some of these SPE cartridges are Hydrophilic–Lipophilic Balance (HLB) [113], Trace-B columns [121], and ChemElut columns [122]. LLE is employed frequently in drugs of abuse testing in biological samples including for alprazolam [123].
However, LLE techniques involve lengthy extraction times, large sample volumes and excess use of organic solvents. Thus, there are attempts to find an alternative to LLE such as Dispersive Liquid– Liquid Micro Extraction (DLLME), which was introduced for first time by Rezaee, et al. [124]. In previous studies [125,126] illustrate the use of DLLME and study by Fernández [127] illustrates the Ultrasound-Assisted Dispersive Liquid–Liquid Micro Extraction (UA-DLLME) method for the determination of BEZs including alprazolam in biological samples. Furthermore, several derivatizing agents have been used in the analysis of BEZs, like tetramethylammonium hydroxide and propyliodide (propylation), as well as a mixture of triethylamine:propionican hydride (propionylation) [128].
Conclusion
Alprazolam is a BEZ derivative that is currently used in the treatment of anxiety, panic disorder and depression. It exhibits rapid onset and short duration of action and relief. Its metabolism is catalyzed by CYP3A4 and potentially CYP3A5 too. The main metabolites are 4-hydroxyalprazolam and α-hydroxyalprazolam. No significant effect on AUC blood concentration related to these metabolites has been reported and thus, presumably, on clinical efficacy. Also, age does not seem to have a significant effect on the main pharmacokinetic parameters, such as Cmax, VD and apparent clearance. Because of its sedative and euphoric effects, alprazolam may be subject to abuse or misuse. Its misuse potential stems from its unique pharmacokinetic properties like rapid absorption, low lipophilicity, short half-life and high potency. Chronic alprazolam treatment results in rapid sedation tolerance.
Alprazolam is significantly more toxic than other BEZs like diazepam and should be avoided in patients with increased risk of suicide, or who are also using alcohol, opioids, or other sedating drugs. The most frequently observed post-mortem cases reveal that alprazolam is ingested in combination with other substances including alcohol, methadone, oxycodone, and cocaine. PMR phenomena using the ratios of cardiac-to-femoral-blood concentrations (C/P ratios) indicate that alprazolam exhibits minimal PMR. There are many different IA screening methods that are suitable for the detection BEZs and their metabolites in body fluids including alprazolam like EIA, ELISA, FPIA, CEDIA and RIA. The most currently used confirmation and quantification techniques are GC-MS and LC-MS/MS, which provide wide applicability and good analytical performance granting high precision, accuracy and feasibility.
Funding
This study received no funding from government, commercial or not-for-profit sectors.
Data Availability Statement
The data underlying this article are available in the article.
Conflict of Interest
None
References
- Verster JC, Volkerts ER. (2004) Clinical pharmacology, clinical efficacy, and behavioral toxicity of alprazolam: A review of the literature. CNS Drug Rev 10:45–76.
- Nudelman NS, Cabrera CG (2002) Isolation and structural elucidation of degradation products of alprazolam: Photostability studies of alprazolam tablets. J Pharm Sci 91:1274–1286.
- Yan Li, Gaotong Lin, Bingbao Chen, Jing Zhang, Lingtian Wang, et al. (2017) Effect of alprazolam on rat serum metabolic profiles. Biomed Chromatogr 31:e3956.
- Kane S (2021) Alprazolam drug usage statistics, United States, 2013–2020.
- NHS Business Services Authority (2016) Prescription Cost Analysis (PCA) Data.
- Hockenhull J, Amioka E, Black JC, Colleen MH, Paul D, et al. (2019) Nonmedical use of alprazolam in the UK: Results from a nationally representative survey. Br J Clin Pharmacol 85:1841–1845.
- Chowdhury ZS, Morshed MM, Shahriar M, et al (2016) The effect of chronic alprazolam intake on memory, attention, and psychomotor performance in healthy human male volunteers. Behav Neurol 2016:3730940.
- Sutherland R, Peacock A, Nielsen S, Bruno R (2020) Alprazolam use among a sample of Australians who inject drugs: Trends up to six years post regulatory changes. Int J Drug Policy 79:1–9.
- Isbister GK, O’Regan L, Sibbritt D, Whyte IM (2004) Alprazolam is relatively more toxic than other benzodiazepines in overdose. Br J Clin Pharmacol 58:88–95.
- Public Health England (2018) Alcohol and drug treatment for young people: Statistics summary 2017 to 2018.
- United Nations Office on Drug and Crime (2017) Non-medical use of benzodiazepines: A growing threat to public health.
- Ministry of Law Affairs (2014) Narcotic drugs and psychotropic substances. 407–470
- Mondal S, Chakradhar T (2019) Review on drug interactions of alprazolam on pharmacodynamic. Eur J Biomed Pharm Sci 6:657–662.
- Hindmarch I, Dawson J, Stanley N (2005) A double-blind study in healthy volunteers to assess the effects on sleep of pregabalin compared with alprazolam and placebo. Sleep 28:187–193.
- Riba J, Rodríguez-Fornells A, Münte TF, Barbanoj MJ (2005) A neurophysiological study of the detrimental effects of alprazolam on human action monitoring. Brain Res Cogn 25:554–565.
- Stein DJ, Hollander E, Mullen LS, Decaria CM, Liebowitz MR (1992) Comparison of clomipramine, alprazolam and placebo in the treatment of obsessive compulsive disorder. Hum Psychopharmacol Clin Exp 7:389–395.
- Skov L, Holm KMD, Johansen SS, Linnet K (2016) Postmortem brain and blood reference concentrations of alprazolam, bromazepam, chlordiazepoxide, diazepam, and their metabolites and a review of the literature. J Anal Toxicol 40:529–536.
- Moylan S, Staples J, Ward SA, et al (2011) The efficacy and safety of alprazolam versus other benzodiazepines in the treatment of panic disorder. J Clin Psychopharmacol 31:647–652.
- Ait-Daoud N, Hamby AS, Sharma S, Blevins D (2018) A review of alprazolam use, misuse, and withdrawal. J Addict Med 12:4–10.
- Straw R (1985) Brief review of published alprazolam clinical studies. Br J Clin Pharmacol 19:57–59.
- Kim D, Lee S, Pyeon T, Jeong S (2015) Use of triazolam and alprazolam as premedication for general anesthesia. Korean J Anesthesiol 68:346–351.
- Mandrioli R, Mercolini L, Augusta Raggi M (2008) Benzodiazepine metabolism: An analytical perspective. Curr Drug Metab 9:827–844.
- Ashnagar A, Kouchak M, Soltani M, Salimi A (2007) In vitro evaluation of some different brands of alprazolam tablets. J Chem 4:563–573.
- National Institutes of Health (2020) NLM to retire Pillbox on January 29, 2021. NLM Tech Bull 435:e2
- Kang MG, Lin HR (2019) Systematic evaluation and validation of benzodiazepines confirmation assay using LC-MS-MS. J Anal Toxicol 43:96–103.
- Schulz M, Schmoldt A, Andresen-Streichert H, Iwersen-Bergmann S (2020) Revisited: Therapeutic and toxic blood concentrations of more than 1100 drugs and other xenobiotics. Crit Care 24:195.
- Rickels K (2004) Alprazolam extended-release in panic disorder. Expert Opin Pharmacother 5:1599–1611.
- Forrester MB (2006) Alprazolam abuse in Texas, 1998-2004. J Toxicol Environ Heal A 69:237–243.
- Ellinwood EH, Heatherly DG, Nikaido AM, et al (1985) Comparative pharmacokinetics and pharmacodynamics of lorazepam, alprazolam and diazepam. Psychopharmacology (Berl) 86:392–399.
- Randall CB (2020) Disposition of toxic drugs and chemicals in man. (12th edn) Biomedical Publication, California.
- TruVida Recovery (2021) What are Xanax bars?
- MedWorks Media (2019) Alprazolam Xanax.
- Tobias S, Shapiro AM, Grant CJ, et al. (2021) Drug checking identifies counterfeit alprazolam tablets. Drug Alcohol Depend 218:108300.
- Clinical pharmacology, clinical efficacy, and behavioral toxicity of alprazolam: A review of the literature.
- Griffin CE, Kaye AM, Rivera Bueno F, et al. (2013) Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J 13:214–223. [Crossref]
- Srisurapanont M, Boonyanaruthee V (1997) Alprazolam and standard antidepressants in the treatment of depression: A meta-analysis of the antidepressant effect. J Med Assoc Thail 80:182–188.
- Feighner JP, Aden GC, Fabre LF, et al (1983) Comparison of alprazolam, imipramine, and placebo in the treatment of depression. J Amer Med Assoc 249:3057–3064.
- Noyes R, DuPont RL, Pecknold JC, et al. (1988) Alprazolam in panic disorder and agoraphobia: Results from a multicenter trial: II. Patient acceptance, side effects, and safety. Arch Gen Psychiatry 45:423–428.
- Smith RB, Kroboth PD, Vanderlugt JT, et al. (1984) Pharmacokinetics and pharmacodynamics of alprazolam after oral and IV administration. Psychopharmacology (Berl) 84:452–456.
- Mericle BP (1996) Current Psychotherapeutic Drugs. (2nd edn). American Psychiatric Press, Washington.
- Pagliaro LA, Pagliaro AM (2020) Psychologists’ Psychotropic Drug Reference. (1st edn). Brunner, London.
- Lippi G, Sanchis-Gomar F, Banfi G (2012) Anti-negative-doping testing: A new perspective in anti-doping research? Eur J Appl Physiol 112:2383–2384.
- Jenkins AJ, Levine B, Locke JL, Smialek JE (1997) A fatality due to alprazolam intoxication. J Anal Toxicol 21:218–220.
- Fleishaker JC, Phillips JP, Eller MG, Smith RB (1989) Pharmacokinetics and pharmacodynamics of alprazolam following single and multiple oral doses of a sustainedâ?release formulation. J Clin Pharmacol 29:543–549.
- Huang Z, Xu Z, Wang H, Zhao ZQ, Rao Y, et al. (2018) Influence of ethanol on the metabolism of alprazolam. Expert Opin Drug Metab Toxicol 14:551–559.
- Hall J, Naranjo CA, Sproule BA, Herrmann N (2003) Pharmacokinetic and pharmacodynamic evaluation of the inhibition of alprazolam by citalopram and fluoxetine. J Clin Psychopharmacol 23:349–357.
- Wolff K (2002) Benzodiazepines and GHB detection and pharmacology. Addiction 97:1229–1230.
- Ciraulo DA, Antal EJ, Smith RB, et al (1990) The relationship of alprazolam dose to steady-state plasma concentrations. J Clin Psychopharmacol 10:27–32.
- Greenblatt DJ, Wright CE (1993) Clinical pharmacokinetics of alprazolam: Therapeutic implications. Clin Pharmacokinet 24:453–471.
- VonVoigtlander PF, Straw RN (1985) Alprazolam: Review of pharmacological, pharmacokinetic, and clinical data. Drug Dev Res 6:1–12.
- Temte V, Kjeldstadli K, Bruun LD, Morris B, Liliana B, et al. (2019) An experimental study of diazepam and alprazolam kinetics in urine and oral fluid following single oral doses. J Anal Toxicol 43:104–111.
- Kampfrath T, Peng P, Vairavan V, Lee D (2015) Benzodiazepine in a urine specimen without drug metabolites. Lab Med 46:164–167.
- Hirota N, Ito K, Iwatsubo T, Green CE, Tyson CA, et al. (2001) In vitro/in vivo scaling of alprazolam metabolism by CYP3A4 and CYP3A5 in humans. Biopharm Drug Dispos 22:53–71.
- Allqvist A, Miura J, Bertilsson L, Mirghani RA (2007) Inhibition of CYP3A4 and CYP3A5 catalyzed metabolism of alprazolam and quinine by ketoconazole as racemate and four different enantiomers. Eur J Clin Pharmacol 63:173–179.
- Smith RB, Kroboth PD (1987) Influence of dosing regimen on alprazolam and metabolite serum concentrations and tolerance to sedative and psychomotor effects. Psychopharmacology (Berl) 93:105–112.
- Dehlin O, Kullingsjö H, Lidén A, Agrell B, Moser G, et al. (1991) Pharmacokinetics of alprazolam in geriatric patients with neurotic pepression. Pharmacol Toxicol 68:121–124.
- Kroboth PD, McAuley JW, Smith RB (1990) Alprazolam in the elderly: Pharmacokinetics and pharmacodynamics during multiple dosing. Psychopharmacology (Berl) 100:477–484.
- Molanaei H, Stenvinkel P, Qureshi AR, et al (2012) Metabolism of alprazolam (a marker of CYP3A4) in hemodialysis patients with persistent inflammation. Eur J Clin Pharmacol 68:571–577.
- Fraser AD, Bryan W, Isner AF (1991) Urinary screening for alprazolam and its major metabolites by the Abbott ADx and TDx analyzers with confirmation by GC/MS. J Anal Toxicol 15:25–29.
- Garzone PD, Kroboth PD (1989) Pharmacokinetics of the newer benzodiazepines. Clin Pharmacokinet 16:337–364.
- Fraser AD (1987) Urinary screening for alprazolam, triazolam and their metabolites with the EMIT d.a.u.TM benzodiazepine metabolite assay. J Anal Toxicol 11:263–266.
- Derendorf H, Hochhaus G (1995) Handbook of pharmacokinetic/pharmacodynamic Correlation. (1st edn). CRC Press, Boca Raton.
- Furugen A, Nishimura A, Kobayashi M, et al (2019) Quantification of eight benzodiazepines in human breast milk and plasma by liquid-liquid extraction and liquid-chromatography tandem mass spectrometry: Application to evaluation of alprazolam transfer into breast milk. J Pharm Biomed Anal 168:83–93.
- Nordal K, Øiestad EL, Enger A, et al (2015) Detection times of diazepam, clonazepam, and alprazolam in oral fluid collected from patients admitted to detoxification, after high and repeated drug intake. Ther Drug Monit 37:451–460.
- Yuan R, Flockhart DA, Balian JD (1999) Pharmacokinetic and pharmacodynamic consequences of metabolism-based drug interactions with alprazolam, midazolam, and triazolam. J Clin Pharmacol 39:1109–1125.
- Greenblatt DJ, Von Moltke LL, Harmatz JS, Durol AL, Daily JP, et al. (2000) Alprazolam-ritonavir interaction: Implications for product labeling. Clin Pharmacol Ther 67:335–341.
- Ohno Y, Hisaka A, Suzuki H (2007) General framework for the quantitative prediction of CYP3A4-mediated oral drug interactions based on the AUC increase by coadministration of standard drugs. Clin Pharmacokinet 46:681–696.
- Greenblatt DJ, Preskorn SH, Cotreau MM, Horst WD, Harmatz JS, et al. (1992) Fluoxetine impairs clearance of alprazolam but not of clonazepam. Clin Pharmacol Ther 52:479–486.
- Suzuki Y, Shioiri T, Muratake T, Yoshiaki K, Satoshi S, et al. (2003) Effects of concomitant fluvoxamine on the metabolism of alprazolam in Japanese psychiatric patients: Interaction with CYP2c19 mutated alleles. Eur J Clin Pharmacol 58:829–833.
- Kroboth PD, Folan MM, Lush RM, et al (1995) Coadministration of nefazodone and benzodiazepines: I. Pharmacodynamic assessment. J Clin Psychopharmacol 15:306–319.
- Ashraf Mozani LR (2004) Handbook of Drug Interactions: A Clinical and Forensic Guide. Springer, New York.
- Baradaran M, Hamidi MR, Firoozabad MRM, Sohrab K, Manouchehr A, et al. (2014) Alprazolam role in the analgesic effect of ibuprofen on postendodontic pain. Casp J Intern Med 5:196–201. [Crossref]
- Ciraulo DA, Barnhill JG, Boxenbaum HG, et al (1986) Pharmacokinetics and clinical effects of alprazolam following single and multiple oral doses in patients with panic disorder. J Clin Pharmacol 26:292–298. doi: 10.1002/j.1552-4604.1986.tb03526.x
- Breier A, Charney DS, Nelson JC (1984) Seizures induced by abrupt discontinuation of alprazolam. Am J Psychiatry 141: 1606-1607.
[Crossref] [Google Scholar] [Indexed]
- Navy HJ, Weffald L, Delate T, Patel RJ, Dugan JP (2018) Clinical pharmacist intervention to engage older adults in reducing use of alprazolam. Consult Pharm 33:711-722.
[Crossref] [Google Scholar] [Indexed]
- Duke AN, Platt DM, Rowlett JK (2020) Tolerance and dependence following chronic alprazolam treatment: Quantitative observation studies in female rhesus monkeys. Psychopharmacology (Berl) 237: 1183-1194.
[Crossref] [Google Scholar] [Indexed]
- Votava M, Krsiak M, Podhorna J, Miczek KA (2001) Alprazolam withdrawal and tolerance measured in the social conflict test in mice. Psychopharmacology (Berl) 157: 123-130.
[Crossref] [Google Scholar] [Indexed]
- Wolf B, Griffiths RR (1991) Physical dependence on benzodiazepines: Differences within the class. Drug Alcohol Depend 29: 153-156.
[Crossref] [Google Scholar] [Indexed]
- Chen TT, Ko CH, Chen ST, Yen CN, Su PW, et al. (2015) Severity of alprazolam dependence and associated features among long-term alprazolam users from psychiatric outpatient clinics in Taiwan. J Formos Med Assoc 114: 1097-1104.
[Crossref] [Google Scholar] [Indexed]
- Kurtz SP, Inciardi JA, Surratt HL, Cottler L (2005) Prescription drug abuse among ecstasy users in Miami. J Addict Dis 24: 1-16.
[Crossref] [Google Scholar] [Indexed]
- Shah NA, Abate MA, Smith MJ, Kaplan JA, Kraner JC, et al. (2012) Characteristics of alprazolam-related deaths compiled by a centralized state medical examiner. Am J Addict 21: S27-S34.
[Crossref] [Google Scholar] [Indexed]
- Darke S, Torok M, Duflou J (2014) Circumstances and toxicology of sudden or unnatural deaths involving alprazolam. Drug Alcohol Depend 138: 61-66.
[Crossref] [Google Scholar] [Indexed]
- Jones AW, Holmgren A (2013) Concentrations of alprazolam in blood from impaired drivers and forensic autopsies were not much different but showed a high prevalence of co-ingested illicit drugs. J Psychopharmacol 27: 276-281.
[Crossref] [Google Scholar] [Indexed]
- Michaud K, Augsburger M, Romain N, Giroud C, Mangin P (1999) Fatal overdose of tramadol and alprazolam. Forensic Sci Int 105: 185-189.
[Crossref] [Google Scholar] [Indexed]
- Rintoul AC, Dobbin MDH, Nielsen S, Degenhardt L, Drummer OH (2013) Recent increase in detection of alprazolam in Victorian heroin-related deaths. Med J Aust 198: 206-209.
[Crossref] [Google Scholar] [Indexed]
- Tungtananuwat W, Lawanprasert S (2010) Fatal 4×100; home-made kratom juice cocktail. J Heal Res 24: 43-47
- Poklis J, Poklis A, Wolf C, et al (2016) Two fatal intoxications involving butyryl fentanyl. J Anal Toxicol 40: 703-708.
[Crossref] [Google Scholar] [Indexed]
- McIntyre IM, Gary RD, Joseph S, Stabley R (2017) A fatality related to the synthetic opioid U-47700: Postmortem concentration distribution. J Anal Toxicol 41:158–160.
[Crossref] [Google Scholar] [Indexed]
- Fagiola M, Hahn T, Avella J (2019) Evaluation of acetylfentanyl following suspected heroin overdose when complicated by the presence of toxic fentanyl and alprazolam concentrations. Acad Forensic Pathol 9:191–199.
[Crossref] [Google Scholar] [Indexed]
- Verster JC, Volkerts ER, Verbaten MN (2002) Effects of alprazolam on driving ability, memory functioning and psychomotor performance: A randomized, placebo-controlled study. Neuropsychopharmacology 27:260–269.
[Crossref] [Google Scholar] [Indexed]
- Huizinga CRH, Zuiker RG, de Kam ML, Dimitrios Z, Jorrit K, et al. (2019) Evaluation of simulated driving in comparison to laboratory-based tests to assess the pharmacodynamics of alprazolam and alcohol. J Psychopharmacol 33:791–800.
[Crossref] [Google Scholar] [Indexed]
- Lee D, Stout P, Egdorf D (2021) Houston Cocktail: Driving under influence of hydrocodone, alprazolam, and carisoprodol. Forensic Sci Int 323:1–7.
[Crossref] [Google Scholar] [Indexed]
- Shouse Law Group (2022) Driving under the influence of drugs in California.
- HM Government (2022) Drugs and driving: The law.
- HoÅ?ínková J, Kozlík P, KÅ?ížek T, Danica M, Martin S, et al. (2020) Post-mortem redistribution of alprazolam in rats. Prague Med Rep 121:244–253.
[Crossref] [Google Scholar] [Indexed]
- Han E, Kim E, Hong H, Sujin J, Jihyun K, et al. (2012) Evaluation of postmortem redistribution phenomena for commonly encountered drugs. Forensic Sci Int 219:265–271.
[Crossref] [Google Scholar] [Indexed]
- Degouffe M, Drost M (1995) A comparison of drug concentrations in postmortem cardiac and peripheral blood in 320 cases. J Can Soc Forensic Sci 28:113–121.
- Hepler B, Isenschmid D, Schmid C (2004) Postmortem redistribution: Practical considerations in death investigation. In: Annual Meeting of the American Academy of Forensic Sciences. Dallas, Texas.
- Geracea E, Salomone A, Corcia D Di, et al (2015) Postmortem redistribution of triazolam, alprazolam, delorazepam (chlordesmethyldiazepam) and zolpidem in a suicide case. Toxicol Anal Clin 27:233–238.
- Samanidou V, Pechlivanidou A, Papadoyannis I (2007) Development of a validated HPLC method for the determination of four 1,4-benzodiazepines in human biological fluids. J Sep Sci 30:679–687.
[Crossref] [Google Scholar] [Indexed]
- Furugen A, Nishimura A, Kobayashi M, et al (2019) Quantification of eight benzodiazepines in human breast milk and plasma by liquid-liquid extraction and liquid-chromatography tandem mass spectrometry: Application to evaluation of alprazolam transfer into breast milk. J Pharm Biomed Anal 168:83–93.
[Crossref] [Google Scholar] [Indexed]
- Mata DC (2016) Stability of 26 sedative hypnotics in six toxicological matrices at different storage conditions. J Anal Toxicol 40:663–668.
[Crossref] [Google Scholar] [Indexed]
- Schwenzer KS, Pearlman R, Tsilimidos M, et al (2000) New fluorescence polarization immunoassays for analysis of barbiturates and benzodiazepines in serum and urine: performance characteristics. J Anal Toxicol 24:726–732.
[Crossref] [Google Scholar] [Indexed]
- Marin SJ, Keith L, Merrell M, McMillin GA (2009) Evaluation of a new ELISA kit for the detection of benzodiazepines in meconium. J Anal Toxicol 33:177–181.
[Crossref] [Google Scholar] [Indexed]
- Spinelli E, Menezes Filho JA, Dourado SB, (2003) Evaluation of structure-affinity relationship for 28 benzodiazepines on EMITd.a.u.® immunoassay at 200 ng/mL cutoff. Rev Bras Toxicol 16:27–34.
- Klette KL, Wiegand RF, Horn CK, Peter RS, Joseph M, et al. (2005) Urine benzodiazepine screening using Roche Online® KIMS immunoassay with β-glucuronidase hydrolysis and confirmation by gas chromatography-mass spectrometry. J Anal Toxicol 29:193–200.
[Crossref] [Google Scholar] [Indexed]
- DeRienz RT, Holler JM, Manos ME, et al (2008) Evaluation of four immunoassay screening kits for the detection of benzodiazepines in urine. J Anal Toxicol 32:433–437.
[Crossref] [Google Scholar] [Indexed]
- Bertol E, Vaiano F, Borsotti M, et al (2013) Comparison of immunoassay screening tests and LC-MS-MS for urine detection of benzodiazepines and their metabolites: Results of a national proficiency test. J Anal Toxicol 37:659–664.
[Crossref] [Google Scholar] [Indexed]
- Smink BE, Mathijssen MPM, Lusthof KJ, et al (2006) Comparison of urine and oral fluid as matrices for screening of thirty-three benzodiazepines and benzodiazepine-like substances using immunoassay and LC-MS(-MS). J Anal Toxicol 30:478–485.
[Crossref] [Google Scholar] [Indexed]
- Fagiola M, Hahn T, Avella J (2018) Screening of novel psychoactive substances in postmortem matrices by liquid chromatography–tandem mass spectrometry (LC–MS-MS). J Anal Toxicol 42:562–569.
[Crossref] [Google Scholar] [Indexed]
- Kintz P, Villain M, Concheiro M, Cirimele V (2005) Screening and confirmatory method for benzodiazepines and hypnotics in oral fluid by LC-MS/MS. Forensic Sci Int 150:213–220.
[Crossref] [Google Scholar] [Indexed]
- Jagerdeo E, Schaff JE (2016) Rapid screening for drugs of abuse in biological fluids by ultra-high performance liquid chromatography/Orbitrap mass spectrometry. J Chromatogr B 1027:11–18.
[Crossref] [Google Scholar] [Indexed]
- Allqvist A, Wennerholm A, Svensson JO, Mirghani RA (2005) Simultaneous quantification of alprazolam, 4- and α-hydroxyalprazolam in plasma samples using liquid chromatography mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci 814:127–131.
[Crossref] [Google Scholar] [Indexed]
- Kintz P, Villain M, Chèze M, Pépin G (2005) Identification of alprazolam in hair in two cases of drug-facilitated incidents. Forensic Sci Int 153:222–226.
[Crossref] [Google Scholar] [Indexed]
- Vindenes V, Lund HME, Andresen W, et al (2012) Detection of drugs of abuse in simultaneously collected oral fluid, urine and blood from Norwegian drug drivers. Forensic Sci Int 219:165–171.
[Crossref] [Google Scholar] [Indexed]
- Marchi I, Schappler J, Veuthey JL, Rudaz S (2009) Development and validation of a liquid chromatography-atmospheric pressure photoionization-mass spectrometry method for the quantification of alprazolam, flunitrazepam, and their main metabolites in haemolysed blood. J Chromatogr B Anal Technol Biomed Life Sci 877:2275–2283.
[Crossref] [Google Scholar] [Indexed]
- ElSohly MA, Gul W, Murphy TP, et al (2007) LC-(TOF) MS analysis of benzodiazepines in urine from alleged victims of drug-facilitated sexual assault. J Anal Toxicol 31: 505-514.
[Crossref] [Google Scholar] [Indexed]
- Lv J, Zhang W, Feng J, Liu Y (2014) Characterization and discrimination of diazepam, alprazolam, clorazepate, temazepam and bromazepam with confocal Raman microscopy. J Adv Microsc Res 9: 29-33.
- Morris AA, Chester SA, Strickland EC, McIntire GL (2014) Rapid enzymatic hydrolysis using a novel recombinant Β-glucuronidase in benzodiazepine urinalysis. J Anal Toxicol 38:610–614.
[Crossref] [Google Scholar] [Indexed]
- Thangaduraia S, Dhanalakshmia A, Kannan MS (2013) Separation and detection of certain benzodiazepines by thin-layer chromatography. Malaysian J Forensic Sci 4:47–53
- Marin SJ, Coles R, Merrell M, McMillin GA (2008) Quantitation of benzodiazepines in urine, serum, plasma, and meconium by LC-MS-MS. J Anal Toxicol 32: 491-498.
[Crossref] [Google Scholar] [Indexed]
- Sauve EN, Langødegård M, Ekeberg D, Øiestad AML (2012) Determination of benzodiazepines in ante-mortem and post-mortem whole blood by solid-supported liquid-liquid extraction and UPLC-MS/MS. J Chromatogr B Anal Technol Biomed Life Sci 883–884: 177-188.
[Crossref] [Google Scholar] [Indexed]
- Song A (2016) Determination of 13 organic toxicants in human blood by liquid-liquid extraction coupling high-performance liquid chromatography tandem mass spectrometry. Anal Sci 32: 645–652.
[Crossref] [Google Scholar] [Indexed]
- Rezaee M, Assadi Y, Milani Hosseini MR, et al (2006) Determination of organic compounds in water using dispersive liquid-liquid microextraction. J Chromatogr A 1116:1–9.
[Crossref] [Google Scholar] [Indexed]
- Vardini MT, Mashayekhi HA, Saber-Tehrani M (2012) Dispersive liquid-liquid microextraction followed by high-performance liquid chromatography as an efficient and sensitive technique for the simultaneous determination of alprazolam, oxazepam, and diazepam in human urine samples. J Liq Chromatogr Relat Technol 35:988–999.
- Mashayekhi HA, Khalilian F (2016) Development of solid-phase extraction coupled with dispersive liquid-liquid microextraction method for the simultaneous determination of three benzodiazepines in human urine and plasma. J Chromatogr Sci 54:1068–1073.
[Crossref] [Google Scholar] [Indexed]
- Fernández P, González C, Teresa Pena M, et al (2013) A rapid ultrasound-assisted dispersive liquid-liquid microextraction followed by ultra-performance liquid chromatography for the simultaneous determination of seven benzodiazepines in human plasma samples. Anal Chim Acta 767: 88-96.
[Crossref] [Google Scholar] [Indexed]
- Papoutsis II, Athanaselis SA, Nikolaou PD, Pistos CM, Spiliopoulou CA, et al (2010) Development and validation of an EI-GC-MS method for the determination of benzodiazepine drugs and their metabolites in blood: applications in clinical and forensic toxicology. J Pharm Biomed Anal 52: 609-614.
[Crossref] [Google Scholar] [Indexed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 
